Abstract
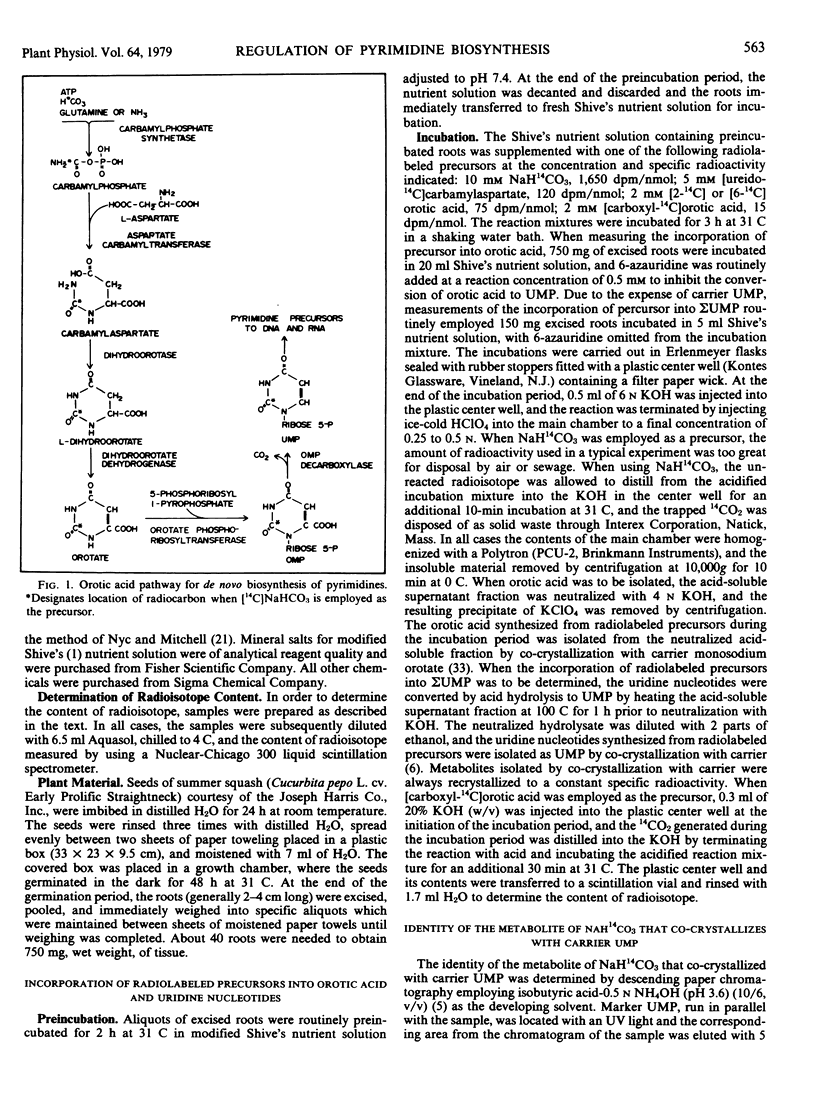
The occurrence of the complete orotic acid pathway for the biosynthesis de novo of pyrimidine nucleotides was demonstrated in the intact cells of roots excised from summer squash (Cucurbita pepo L. cv. Early Prolific Straightneck). Evidence that the biosynthesis of pyrimidine nucleotides proceeds via the orotate pathway in C. pepo included: (a) demonstration of the incorporation of [14C]NaHCO3, [14C]carbamylaspartate, and [14C]orotic acid into uridine nucleotides; (b) the isolation of [14C]orotic acid when [14C]NaHCO3 and [14C]carbamylaspartate were used as precursors; (c) the observation that 6-azauridine, a known inhibitor of the pathway, blocked the incorporation of early precursors into uridine nucleotides while causing a concomitant accumulation of orotic acid; and (d) demonstration of the activities of the component enzymes of the orotate pathway in assays employing cell-free extracts.
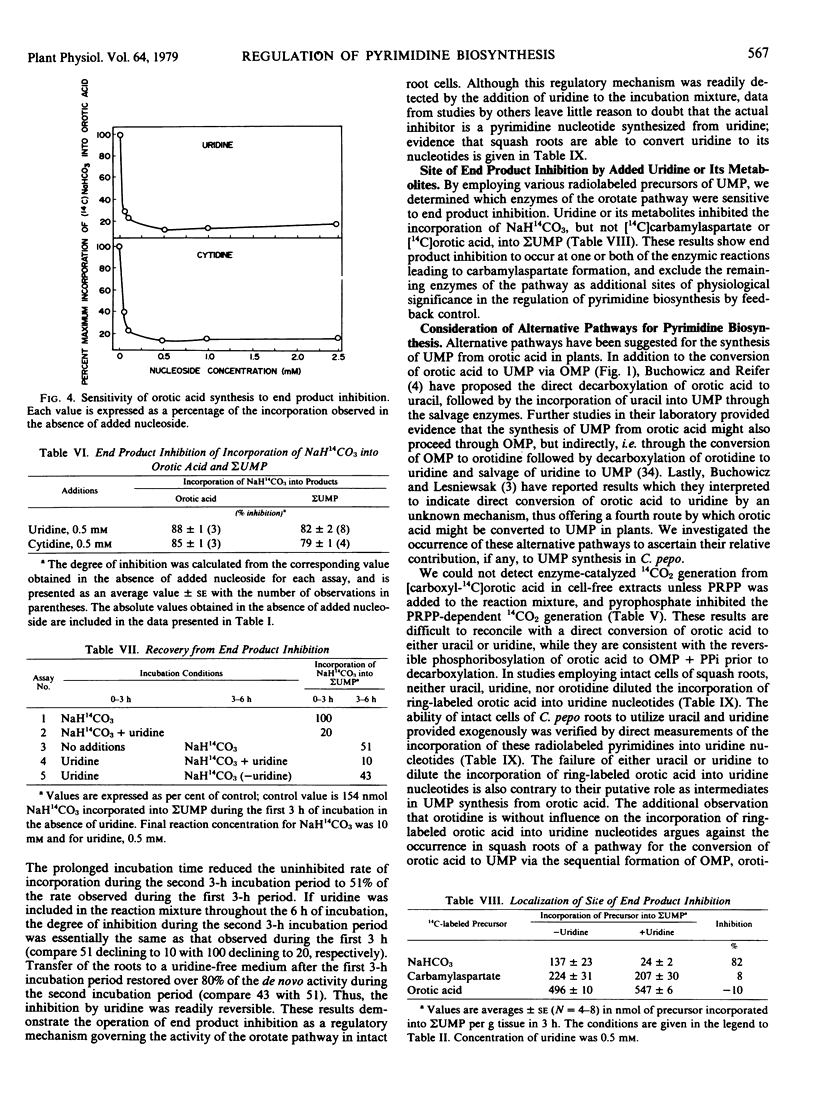
Regulation of the activity of the orotate pathway by end product inhibition was demonstrated in the intact cells of excised roots by measuring the influence of added pyrimidine nucleosides on the incorporation of [14C]NaHCO3 into uridine nucleotides. The addition of either uridine or cytidine inhibited the incorporation of [14C]NaHCO3 into uridine nucleotides by about 80%. The observed inhibition was demonstrated to be readily reversible upon transfer of the roots to a nucleoside-free medium. Experiments employing various radiolabeled precursors indicated that one or both of the first two enzymes in the orotate pathway are the only site(s) of regulation of physiological importance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert L. S., Wilson C. M. Effect of boron on elongation of tomato root tips. Plant Physiol. 1961 Mar;36(2):244–251. doi: 10.1104/pp.36.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardi J. E., Anderson E. P. Separation of purine and pyrimidine derivatives by thin-layer chromatography. Anal Biochem. 1968 Mar;22(3):398–408. doi: 10.1016/0003-2697(68)90282-0. [DOI] [PubMed] [Google Scholar]
- Crandall D. E., Lovatt C. J., Tremblay G. C. Regulation of pyrimidine biosynthesis by purine and pyrimidine nucleosides in slices of rat tissue. Arch Biochem Biophys. 1978 May;188(1):194–206. doi: 10.1016/0003-9861(78)90372-7. [DOI] [PubMed] [Google Scholar]
- Diederich D., Ramponi G., Grisolia S. On the mechanism of enzymatic hydrolysis of carbamyl phosphate. FEBS Lett. 1971 Jun 2;15(1):30–32. doi: 10.1016/0014-5793(71)80072-8. [DOI] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Distinct subunits for the regulation and catalytic activity of aspartate transcarbamylase. Biochemistry. 1965 Jun;4(6):1054–1062. doi: 10.1021/bi00882a012. [DOI] [PubMed] [Google Scholar]
- Grisolia S., Hood W. High reactivity of kidney preparations from man, pig, beef, dog and rat for carbamyl phosphate hydrolysis. FEBS Lett. 1974 Jun 15;42(3):246–248. doi: 10.1016/0014-5793(74)80737-4. [DOI] [PubMed] [Google Scholar]
- HANDSCHUMACHER R. E., PASTERNAK C. A. Inhibition of orotidylic acid decarboxylase, a primary site of carcinostasis by 6-azauracil. Biochim Biophys Acta. 1958 Nov;30(2):451–452. doi: 10.1016/0006-3002(58)90088-x. [DOI] [PubMed] [Google Scholar]
- Herzfeld A., Knox W. E. Carbamyl phosphate hydrolysis in rat tissues and tumors by an alkaline phosphatase. Cancer Res. 1972 Sep;32(9):1837–1841. [PubMed] [Google Scholar]
- Johnson L. B., Niblett C. L., Shively O. D. Asparate transcarbamylase activity in etiolated cowpea hypocotyls treated with 2,4-dichlorophenoxyacetic Acid. Plant Physiol. 1973 Feb;51(2):318–321. doi: 10.1104/pp.51.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWENSTEIN J. M., COHEN P. P. Studies on the biosynthesis of carbamylaspartic acid. J Biol Chem. 1956 May;220(1):57–70. [PubMed] [Google Scholar]
- Mazuś B., Buchowicz J. Purification and properties of dihydro-orotase from pea plants. Acta Biochim Pol. 1968;15(4):317–325. [PubMed] [Google Scholar]
- NEUMANN J., JONES M. E. Aspartic transcarbamylase from lettuce seedings: case of end-product inhibition. Nature. 1962 Aug 18;195:709–710. doi: 10.1038/195709a0. [DOI] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal D., Naylor A. W. Purine and pyrimidine nucleotide inhibition of carbamyl phosphate synthetase from pea seedlings. Biochem Biophys Res Commun. 1968 May 10;31(3):322–327. doi: 10.1016/0006-291x(68)90478-6. [DOI] [PubMed] [Google Scholar]
- O'neal T. D., Naylor A. W. Some regulatory properties of pea leaf carbamoyl phosphate synthetase. Plant Physiol. 1976 Jan;57(1):23–28. doi: 10.1104/pp.57.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong B. L., Jackson J. F. Aspartate transcarbamoylase from Phaseolus aureus. Partial purification and properties. Biochem J. 1972 Sep;129(3):571–581. doi: 10.1042/bj1290571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REIFER I., BUCHOWICZ J., TOCZKO K. The synthesis of the pyrimidine ring from 1-carbamylaspartic acid in excised blades of wheat seedlings. Acta Biochim Pol. 1960;7:29–38. [PubMed] [Google Scholar]
- RUBIN R. J., REYNARD A., HANDSCHUMACHER R. E. AN ANALYSIS OF THE LACK OF DRUG SYNERGISM DURING SEQUENTIAL BLOCKADE OF DE NOVO PYRIMIDINE BIOSYNTHESIS. Cancer Res. 1964 Jul;24:1002–1007. [PubMed] [Google Scholar]
- Rybicka H., Buchowicz J., Reifer I. [Carbamoyl-14-C]citrulline in pyrimidine synthesis in wheat seedlings. Acta Biochim Pol. 1967;14(2):249–254. [PubMed] [Google Scholar]
- STEIN L. I., COHEN P. P. CORRELATION OF GROWTH AND ASPARTATE TRANSCARBAMYLASE ACTIVITY IN HIGHER PLANTS. Arch Biochem Biophys. 1965 Mar;109:429–433. doi: 10.1016/0003-9861(65)90386-3. [DOI] [PubMed] [Google Scholar]
- Wasilewska L. D., Reifer I. Comparison of anabolism of 14C-labelled orotic acid, uracil and uridine in excised pea plants. Acta Biochim Pol. 1967;14(1):57–62. [PubMed] [Google Scholar]
- Wolcott J. H., Ross C. Orotidine-5'-phosphate decarboxylase and pyrophosphorylase of bean leaves. Plant Physiol. 1967 Feb;42(2):275–279. doi: 10.1104/pp.42.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]


