Abstract
Insulin-like growth factor binding protein-3 (IGFBP-3) has previously been identified as a putative tumor suppressor gene. The present study investigated the clinical and prognostic significance of IGFBP-3 expression levels in patients with hepatocellular carcinoma (HCC). Immunohistochemistry (IHC) probing for IGFBP-3 was performed on paraffin-embedded tissue samples obtained from 120 patients with HCC, including tissue samples from 120 primary cancer sites and 50 matched adjacent non-malignant sites. Receiver-operator curve (ROC) analysis was used to determine the cut-off scores for the presence of IGFBP-3-positive tumor cells and to estimate the survival time of the patients. The threshold for marking the positive expression of IGFBP-3 was 65%, based on the area under the ROC. Positive expression of IGFBP-3 was observed in 65/120 (54.2%) of the HCC tissues, and in 36/50 (72%) of the adjacent non-malignant liver tissues. Low levels of IGFBP-3 expression were correlated with tumor size (P=0.003), tumor multiplicity (P=0.044), node (P=0.008), metastasis (P=0.001) and clinical stage (P=0.001), as well as reduced survival time (P=0.015). Using univariate survival analysis, a significant direct correlation between high and low IGFBP-3 expression levels, and patient survival time (mean survival time high IGFBP-3, 39.4 vs. low IGFBP-3, 18.7 months) was identified. Kaplan-Meier analysis demonstrated that IGFBP-3 expression levels and patients survival time were significantly correlated (P<0.001). Multivariate analysis revealed IGFBP-3 expression to be an independent parameter (P=0.003). Therefore, low levels of IGFBP-3 expression are associated with advance clinicopathological classification and may be a predictor of poor survival in patients with HCC. Furthermore, these findings suggest that IGFBP-3 may serve as an independent molecular marker for the evaluation of prognosis in patients with HCC.
Keywords: hepatocellular carcinoma, insulin-like growth factor binding protein-3, insulin-like growth factor, immunohistochemistry
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent types of human malignancies worldwide, as well as the primary cause of mortality in patients with chronic liver disease (1). Despite recent advances in therapeutics and surveillance programs for high-risk populations (2,3), the long-term prognosis for patients with HCC remains poor due to the high incidence of intrahepatic recurrence (4). Therefore, it is important to elucidate the molecular mechanisms underlying HCC progression and to identify the risk factors for tumor recurrence following curative treatment, in order to select appropriate therapies and accurately evaluate patient prognosis.
The insulin-like growth factor (IGF) signaling pathway is associated with HCC cell growth and is important during the development and progression of HCC (5). IGF-I, IGF-II and their corresponding receptors mediate the biological functions of the IGF signaling pathway through the activation of the mitogen-activated protein kinase signaling pathway, which is involved in cell growth and metabolism (6,7). The ligands IGF-I and IGF-II, as well as the abnormal stimulation of their receptors, have been implicated in the early stages of human hepatocarcinogenesis (8,9). IGF binding protein (IGFBP) 3 is a member of the IGFBP family, which regulates components of the IGF signaling pathway (10). IGFBP-3 has been demonstrated to inhibit cell proliferation, independently of its effects on IGF-stimulated growth, in numerous types of malignancies (11,12). Additionally, it has been reported that the expression levels of IGFBP-3 correlate with the response of the patient to radiotherapy and may serve as a marker for the chemosensitivity of glioblastoma to semustine (13). A previous study demonstrated that IGFBP-3 expression level in patients with esophageal squamous cell carcinoma (ESCC) was a risk factor for poor patient survival, as well as a marker for evaluating patient prognosis (14). However, the significance of IGFBP-3 expression levels, and their association with the prognosis of patients with HCC, has yet to be investigated.
The present study was conducted in order to investigate the clinical and prognostic implications of IGFBP-3 expression levels in patients with HCC. Immunohistochemistry (IHC) was used to examine whether the expression of IGFBP-3 in human clinical tissue samples was associated with the clinicopathological characteristics of HCC. Furthermore, the levels of IGFBP-3 expression and the clinical and prognostic factors of patients with HCC were evaluated to determine if any correlations were present, as well as whether the expression levels of IGFBP-3 are able to accurately predict patient survival.
Materials and methods
Patients and tissue specimens
In the present retrospective study, a total of 120 HCC tissue samples and 50 matched adjacent non-malignant tissues were obtained from 120 patients with HCC, following the receipt of informed patient consent and ethical approval from The Institute Research Ethics Committee of Xi'an Medical College Affiliated Hospital (Xi'an, China). The tissue samples were collected between January 2003 and December 2009 at The Xi'an Medical College Affiliated Hospital. The HCC cases (summarized in Table I) were selected based on pathological diagnosis and the availability of follow-up data. The diagnosis of HCC was determined based on the 2002 American Joint Committee on Cancer/International Union Against Cancer Tumor-Node-Metastasis (TNM) classification system guidelines (15). The original clinical assessment records were available and the tissue samples were assessed histologically. Curative treatment was defined as the complete removal of the tumor tissue, with no residual tumor visible in three-phase dynamic computed tomography scans or gadoxetic acid-enhanced magnetic resonance imaging scans at one month following surgery. The follow-up period extended for >60 months.
Table I.
Correlations between the IGFBP-3 expression levels in tumor tissue samples and the clinicopathological characteristics of patients with hepatocellular carcinoma.
| IGFBP-3 protein | ||||
|---|---|---|---|---|
| Characteristics | Number of cases | Low expression (%) | High expression (%) | P-value |
| Age, years | 0.436 | |||
| ≥50b | 63 | 31 (49.2)) | 32 (50.8 | |
| <50b | 57 | 24 (42.1) | 33 (57.9) | |
| Gender | 0.677 | |||
| Male | 103 | 48 (46.6) | 55 (53.4) | |
| Female | 17 | 7 (41.1) | 10 (58.9) | |
| AFP, ng/ml | 0.291 | |||
| ≤20 | 42 | 20 (47.6) | 22 (52.4) | |
| >20 | 78 | 35 (44.9) | 43 (54.1) | |
| Liver cirrhosis | 0.369 | |||
| Yes | 75 | 32 (42.7) | 43 (57.3) | |
| No | 45 | 23 (51.1) | 22 (48.9) | |
| Tumor size, cm | 0.003a | |||
| ≥5 | 52 | 32 (61.5) | 20 (39.5) | |
| <5 | 68 | 23 (33.8) | 45 (66.2) | |
| Tumor multiplicity | 0.044a | |||
| Single | 78 | 41 (52.6) | 37 (47.4) | |
| Multiple | 42 | 14 (33.3) | 28 (66.7) | |
| N status | 0.008a | |||
| N0 | 33 | 21 (63.6) | 12 (36.4) | |
| N1 | 87 | 32 (36.8) | 55 (63.2) | |
| M status | 0.001a | |||
| M0 | 57 | 35 (61.4) | 22 (38.6) | |
| M1 | 63 | 20 (31.7) | 43 (68.3) | |
| Clinical stage | 0.001a | |||
| I | 13 | 10 (76.9) | 3 (23.1) | |
| II | 47 | 28 (65.9) | 19 (34.1) | |
| III | 39 | 10 (51.5) | 29 (48.5) | |
| IV | 21 | 7 (33.3) | 14 (66.7) | |
| Survival status | 0.015a | |||
| Alive | 31 | 20 (64.5) | 11 (35.5) | |
| Succumbed | 89 | 35 (39.3) | 54 (61.7) | |
P<0.05 was considered to indicate a statistically significant difference (χ2 test).
Median age=50 years. IGFBP-3, insulin-like growth factor binding protein-3; AFP, α-fetoprotein; N, node; M, metastasis.
IHC
IGFBP-3 expression was evaluated using a previously established standard two-step IHC technique (14). Briefly, the tissue slides cut by microtome (5-µm thick) were dried using a dryer overnight at 37°C, dewaxed in xylene, rehydrated with graded ethanol and immersed in 3% hydrogen peroxide for 20 min at room temperature to block endogenous peroxidase activity. For antigen retrieval, the tissue slides were heated at 100°C in Tris (hydroxymethyl) aminomethane-EDTA buffer (pH 8.0) in a pressure cooker for 10 min. Subsequently, the tissue slides were incubated with 10% normal rabbit serum (catalog no. 18140; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at room temperature for 20 min to reduce nonspecific interactions. Tissue sections were then incubated with a 1:50 dilution of anti-IGFBP-3 polyclonal antibody (directed against amino acids 113–210 of human IGFBP-3; catalog no. sc-9028; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h at 37°C in a humidified chamber. Following washing five times with 0.01 mol/l PBS (pH 7.4) for 10 min, the slides were incubated with a secondary rabbit anti-mouse antibody (catalog no. F0232; Envision; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) at a dilution of 1:100 for 30 min at 37°C. This was followed by washing 3 times with PBS and staining with 50% 3,3-diaminobenzidine for 20 sec at room temperature. Nuclei were counterstained with Meyer's hematoxylin. PBS alone was used as a negative control.
Evaluation of IGFBP-3 expression using IHC
IGFBP-3 expression levels were evaluated using IHC by randomly selecting and counting the percentage of positive cells in five fields under a light microscope (DM2000; Leica Microsystems GmbH, Wetzlar, Germany) (x400) from each tissue slide, and then calculating the mean score for each slide from these values. The nuclear immunoreactivity of the IGFBP-3 was scored semi-quantitatively by comparing the number of IGFBP-3 positive tumor cells with the total number of tumor cells. Scores were assigned in 5% increments (0–100%). The reproducibility of this scoring method between pathologists has been described previously (16,17). Three independent pathologists who were blinded to the clinical follow-up data evaluated IGFBP-3 expression levels. Their conclusions were concordant in 85% of the cases, suggesting that this scoring method is reproducible.
Selection of cut-off scores
ROC analysis was used to determine the cut-off scores for positive IGFBP-3 expression in tissue specimens using the 0, 1 positivity-criterion (18). To determine the IGFBP-3 score, the sensitivity and specificity for each outcome (clinicopathological features) was plotted, generating an ROC curve. In ROC analysis, the optimal cut-off score was chosen as the value with the most similar maximum sensitivity and specificity [the point (0.0, 1.0) on the curve]. According to the ROC analysis, the threshold value was defined 65%. Tissue specimens designated as ‘negative’ for the protein were those with scores below or equal to the threshold value, whereas ‘positive’ specimens were those with scores above the threshold (19,20). In order to use ROC analysis, the clinicopathological features were categorized as follows: α-fetoprotein (AFP) level (≥20 or <20), liver cirrhosis (serious hepatocyte necrosis or no hepatocyte necrosis), tumor size (tumor diameter, ≥5 or <5 cm), tumor multiplicity (single or multiple), N stage (N0, no lymph node involvement; N1, lymph node involvement), M stage (M0, absence of metastasis; M1, presence of metastasis), clinical stage (low, I+II; high, III+IV) and length of survival [mortality due to HCC or censored (lost to follow-up, alive, or mortality due to other causes)]. IGFBP-3 immunoreactivity was classified using ROC curve analysis, with a low expression rate defined as <65% IGFBP-3 positive cells and a high expression rate defined as ≥65% IGFBP-3 positive cells.
Western blot analysis
Protein concentration was determined by bicinchoninic acid protein quantification. Total protein (20 µg) was isolated from 10 paired HCC and adjacent non-malignant tissue samples using TRIzol® buffer (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) (21) as previously described (22). Briefly, equal quantities of whole cell lysate and tissue lysate were separated by 8% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Pall Life Sciences, Port Washington, NY, USA) and blocked in 5% milk powder. The membranes were washed three times with PBS and incubated with primary mouse monoclonal antibodies against human IGFBP-3 (dilution, 1:200; catalog no. sc-9028; Santa Cruz Biotechnology, Inc.) at 4°C overnight. Subsequent to washing three times with PBS, blots were incubated with secondary rabbit anti-mouse antibody (catalog no. F0232; Envision; Dako; Agilent Technologies, Inc.) at a dilution of 1:5,000 for 2 h at room temperature. Immunoreactivity was then detected using an Amersham enhanced chemiluminescence western blotting detection kit (GE Healthcare Life Sciences, Uppsala, Sweden) and analyzed using Image lab Software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). All experiments were conducted according to the manufacturer's protocol where applicable.
Statistical analysis
All statistical analyses were performed using SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA). Measurement data are presented by continuous variable and ranked data are presented by classified variable. The correlation between the protein expression levels of IGFBP-3 and the clinicopathological data from patients with HCC was evaluated using the χ2 test. ROC analysis was performed to determine the cut-off score for positive IGFBP-3 expression. The association between the length of patient survival and each variable was evaluated using the log-rank test. Multiple Cox proportional hazards regression analysis was performed to determine whether IGFBP-3 expression levels were an independent predictor of patient survival. P<0.05 was considered to indicate a statistically significant difference.
Results
IGFBP-3 expression levels in HCC and non-malignant patient tissue samples
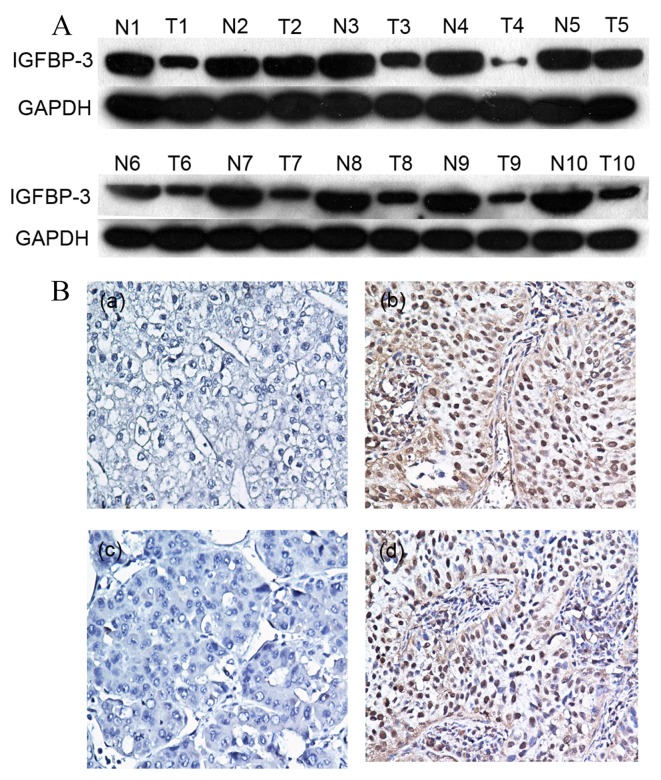
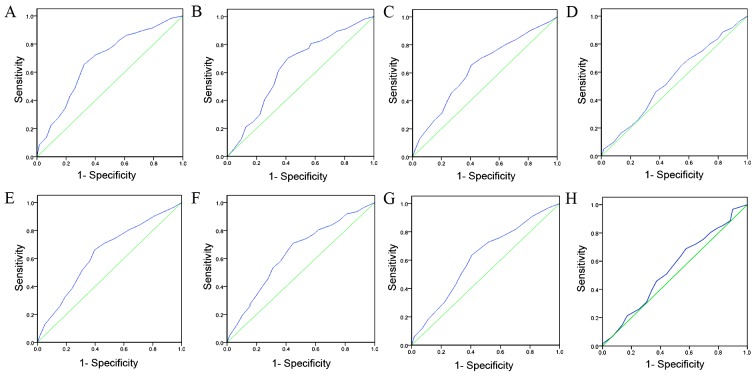
Initially, IGFBP-3 expression was examined in 10 pairs of fresh HCC and adjacent non-malignant tissue specimens obtained from fresh tissue database using western blot analysis. In the primary HCC tissue samples, 7/10 (70%) exhibited markedly reduced levels of IGFBP-3 expression compared with in the adjacent non-malignant liver tissue samples (Fig. 1A). Protein expression was subsequently evaluated using IHC in 120 pairs of HCC and 50 adjacent non-malignant tissue specimens (Fig. 1B). The immunoreactivity scores ranged from 0 to 100%. According to ROC analysis, tissues with IGFBP-3 expression levels that were above the critical threshold of 65% were defined as positive (Fig. 2). High expression levels of IGFBP-3 were detected in 65/120 (54.2%) of the HCC tissue samples, and in 36/50 (72%) of the adjacent non-malignant liver tissues. Therefore, IHC analysis demonstrated that IGFBP-3 expression levels were significantly decreased in the primary cancer tissues, as compared with in the matched adjacent non-malignant tissues (P=0.031).
Figure 1.
Immunohistochemical analysis of IGFBP-3 expression levels in patients with HCC. (A) Low expression levels of IGFBP-3 were detected using western blot analysis in 7/10 pairs of fresh HCC tissues (T1-10) and adjacent non-malignant specimens from patients with HCC (N1-10). (B) (a and c) Two HCC cases exhibited low levels of IGFBP-3 staining (magnification, ×200), and (b and d) two HCC cases exhibited high levels of IGFBP-3 staining (magnification, ×200). IGFBP-3, insulin-like growth factor binding protein-3; HCC, hepatocellular carcinoma.
Figure 2.
Receiver-operator curves for determining the cut-off score for evaluating insulin-like growth factor binding protein-3 expression. The sensitivity and specificity for each of the following outcomes were plotted: (A) survival time, (B) clinical stage, (C) metastasis stage, (D) liver cirrhosis, (E) node stage, (F) tumor size, (G) tumor multiplicity and (H) α-fetoprotein.
Associations between the levels of IGFBP-3 expression and the clinicopathological parameters
The expression levels of IGFBP-3 in patients with HCC, with respect to several standard clinicopathological features, are presented in Table I. Analysis of 120 patients with HCC revealed that the expression levels of IGFBP-3 were correlated with the following clinicopathological parameters: T stage, N stage, M stage, tumor multiplicity, clinical stage and the length of survival (χ2 test, P<0.05; Table I). No significant differences were identified between IGFBP-3 expression levels and patient age, gender, AFP levels or liver cirrhosis (P>0.05; Table I). These results suggest a correlation between decreased IGFBP-3 expression levels and clinical progression in patients with HCC.
Associations between clinicopathological variables and IGFBP-3 expression levels and survival in patients with HCC
Univariate Cox regression analyses and Kaplan-Meier survival curves were evaluated using a log-rank test. The χ2 analysis indicated a correlation between the protein expression levels of IGFBP-3 in tumor tissues and the survival time of patients with HCC (P=0.015; Table I). Kaplan-Meier curves revealed that, within the primary HCC category, patients with high levels of IGFBP-3 expression exhibited a longer overall survival time (median, 39.4 months), as compared with patients with low levels of IGFBP-3 expression (median, 18.7 months; log-rank test, P<0.001; Fig. 3A). In addition, patient survival was analyzed to determine the associations between survival time and IGFBP-3 expression levels in adjacent non-malignant tissues. As presented in Fig. 3B, the overall survival time was greater (median, 57.4 months) for patients with high basal expression levels of IGFBP-3, compared with for patients expressing low levels of IGFBP-3 (median, 37.6 months; log-rank test, P<0.001; Fig. 3B). In addition, Kaplan-Meier analysis demonstrated the significant impact of established clinicopathological prognostic parameters, including tumor size (P=0.003), T stage (P=0.002), N stage (P=0.001) and clinical stage (P<0.001), on patient survival. Furthermore, IGFBP-3 expression levels in the analyzed patient tissue specimens were directly correlated with survival time (P<0.001), even following patient stratification based on clinicopathological classifications (Table II).
Figure 3.
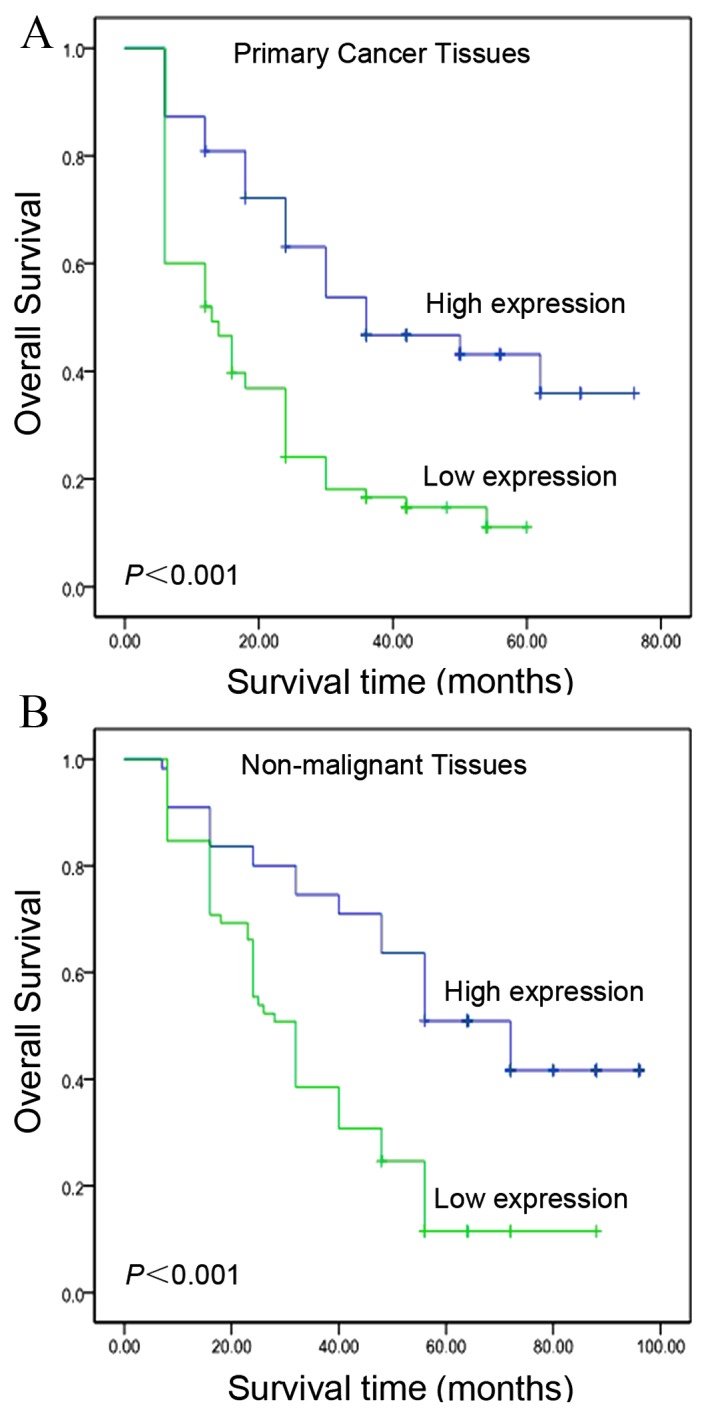
Kaplan-Meier survival analysis based on IGFBP-3 expression in HCC tissue samples and matched adjacent non-malignant tissues (log-rank test). (A) Kaplan-Meier curves revealed that patients with low levels of IGFBP-3 expression exhibited poor overall survival (analysis of 120 primary HCC tissues; P<0.001). (B) Patients with high levels of IGFBP-3 expression exhibited improved overall survival (analysis of 50 matched adjacent non-malignant HCC tissues; P<0.001). IGFBP-3, insulin-like growth factor binding protein-3; HCC, hepatocellular carcinoma.
Table II.
Univariate Cox regression analysis (log-rank test) of the association between IGFBP-3 expression levels and clinicopathological features.
| Patient characteristics | Relative risk (95% CI) | P-value |
|---|---|---|
| Age, years | 0.656 | |
| ≤51.2b | 1.000 | |
| >51.2b | 1.086 (0.698–1.698) | |
| Gender | 0.542 | |
| Male | 1.000 | |
| Female | 0.886 (0.732–1.732) | |
| AFP, ng/ml | 0.124 | |
| ≤20 | 1.000 | |
| >20 | 1.853 (0.987–2.987) | |
| Liver cirrhosis | 0.952 | |
| Yes | 1.000 | |
| No | 1.008 (0.558–1.558) | |
| Tumor size, cm | 0.003a | |
| ≥5 | 1.000 | |
| <5 | 3.689 (1.856–7.856) | |
| T status | 0.002a | |
| T2–3 | 1.000 | |
| T4 | 2.864 (1.862–4.862) | |
| N status | 0.001a | |
| N0 | 1.000 | |
| N1 | 3.869 (1.684–5.684) | |
| Clinical stage | <0.001a | |
| I/II | 1.000 | |
| III/IV | 3.965 (2.589–8.589) | |
| IGFBP-3 expression | <0.001a | |
| High | 1.000 | |
| Low | 3.542 (1.622–7.622) |
P<0.05 was considered to indicate a statistically significant difference.
Median age=51.2 years. IGFBP-3, insulin-like growth factor binding protein-3; CI, confidence interval; T, tumor; N, node; AFP, α-fetoprotein.
Independent prognostic factors for HCC according to multivariate Cox regression analysis
A multivariate progression analysis based on the Cox proportional hazards model was applied to determine the independent value of certain clinical parameters when predicting the overall survival of patients with HCC. As presented in Table III, IGFBP-3 expression levels and other clinicopathological features that were identified as significant by univariate analysis, including tumor size, T stage, N stage and clinical stage, were included in the multivariate analysis. Decreased levels of IGFBP-3 expression were determined to be an independent prognostic factor for favorable overall survival (95% confidence interval, 3.642–13.568; P=0.003). Of the other parameters evaluated, T stage (P=0.015), N stage (P=0.006) and clinical stage (P=0.002) were also demonstrated to be independent prognostic factors for overall survival.
Table III.
Multivariate Cox regression analysis of potential prognostic factors for patients with hepatocellular carcinoma.
| Patient characteristics | Relative risk (95%CI) | P-value |
|---|---|---|
| Tumor size, cm | 0.042a | |
| ≥5 | 1.000 | |
| <5 | 2.346 (0.817–5.817) | |
| T status | 0.015a | |
| T2–3 | 1.000 | |
| T4 | 3.568 (1.325–6.325) | |
| N status | 0.006a | |
| N0 | 1.000 | |
| N1 | 2.359 (1.234-73694) | |
| Clinical stage | 0.002a | |
| I/II | 1.000 | |
| III/IV | 5.367 (2.689–11.689) | |
| IGFBP-3 expression | 0.003a | |
| High | 1.000 | |
| Low | 3.568 (3.642–13.642) |
P<0.05 was considered to indicate a statistically significant difference. IGFBP-3, insulin-like growth factor binding protein-3; CI, confidence interval; T, tumor; N, node.
Discussion
Disease progression and survival in patients with HCC with similar clinicopathological classifications often exhibits considerable variability, and the conventional grading system may have reached its limits for providing information regarding patient prognosis and treatment strategies (23,24). Therefore, it is important to establish criteria to allow the development of novel diagnostics and risk assessments. IGFBP-3 was initially first recognized as a protein carrier and cell signaling pathway messenger, and has an established association with the growth of HCC cells (25). IGFBP-3 is able to bind to IGF-I and IGF-II and regulate the concentrations of these proteins in circulation; therefore, it has been demonstrated to inhibit cell proliferation, promote apoptosis and reduce growth in numerous types of solid tumor, including breast cancer and prostate cancer (25–28). In addition, IGFBP-3 has been reported to possess pro-apoptotic and anti-proliferative functions, via its interactions with other signaling receptors or proteins, to regulate cell apoptosis, cell proliferation and the bioavailability of insulin and IGFs (29). A previous study revealed that IGFBP-3 exerts no direct effect on Hs578T breast cancer cells, but is able enhance apoptosis induced by the physiological trigger ceramide in an IGF-independent manner (30). In addition, increased protein expression levels of IGFBP-3 are associated with the upregulation of the pro-apoptotic proteins B-cell lymphoma 2 (Bcl-2) -associated death promoter and Bcl-2-associated X-protein (Bax), as well as increased apoptosis through the modulation of the Bax/Bcl-2 protein ratio in response to quercetin (a flavonoid present in food products, including onion, grapes and green vegetables) in human prostate cancer cells (31). Another previous study demonstrated that IGFBP-3 is able to increase ceramide-induced apoptosis in breast cancer cells, and enhance tumor protein (p)53-dependent and p53-independent apoptosis in various cancer cell lines, including MCF7 and A549 (32). These observations collectively suggest that IGFBP-3 may function as a tumor suppressor.
Additionally, a previous study revealed that EGF-induced EGFR activation is able to reduce IGFBP-3 levels (33). Therefore, IGFBP-3, an EGFR downstream target molecule, may serve as a radiosensitizer to enhance the sensitivity of ESCC to radiotherapy in primary and immortalized human esophageal epithelial cells (33). A previous study identified a potential association between IGFBP-3 levels in EGFR-overexpressing ESCC cells and the increased chemosensitivity of cells to nimotuzumab (34). In addition, reduced levels of IGFBP-3 expression may be a risk factor for advanced clinicopathological classification and poor prognosis in patients with ESCC and may, therefore, serve as a useful marker for prognostic evaluation (14). Furthermore, Adamek et al (35) reported that an estimation of the IGF-1:IGFBP-3 ratio may provide additional non-invasive markers for hepatitis C virus (HCV)-associated liver injury. Aleem et al (36) demonstrated that serum IGFBP-3 levels are reduced as hepatic dysfunction progresses, and may be correlated with the development of HCC in patients with chronic HCV and liver cirrhosis. Taken together, these findings suggest that IGFBP-3 expression may be a key mediator of HCC tumorigenesis.
In the present study, IHC probing for IGFBP-3 was performed on a large cohort of HCC tumor samples, totaling 120 cases with complete clinicopathological and follow-up data. IGFBP-3 immunoreactivity was evaluated using a scoring system based on the proportion of IGFBP-3 positive tumor cells present in each tissue sample. This method was assessed independently by three pathologists and observed to be reproducible, resulting in a more complete evaluation of the prognostic or predictive value of various markers in liver cancer. In order to avoid the use of predetermined and often arbitrarily set values when selecting IHC cut-off scores for positive IGFBP-3 expression, ROC analysis was performed for each of the clinicopathological parameters, including AFP levels, liver cirrhosis, tumor size, tumor multiplicity, N stage, M stage, clinical stage and survival time. The IHC results for IGFBP-3 expression revealed that the majority of matched non-malignant tissues (72.0%) stained intensely for cytoplasmic IGFBP-3, whereas only 54.2% of primary HCC tissues exhibited intense cytoplasmic IGFBP-3 staining. The IGFBP-3 expression level in HCC cells also demonstrated a correlation with tumor size, tumor multiplicity, N stage, M stage, clinical stage and survival time. Overall, these results suggest that IGFBP-3 may be a novel prognostic marker for HCC.
Univariate Cox proportional hazards analysis revealed that T, N, M and clinical stage classifications may be risk factors for cancer-associated mortality. IGFBP-3 expression levels were significantly correlated with survival time (mean, 39.4 months vs. 18.7 months; P<0.001). Furthermore, decreased levels of IGFBP-3 expression in patients with HCC were demonstrated to be an independent predictor of shorter survival time, as evaluated by multivariable Cox proportional hazards regression analysis. These results suggest that the reduction of IGFBP-3 expression levels in HCC cells may facilitate cancer cell invasion and metastasis. By contrast, patients that retained higher levels of IGFBP-3 expression exhibited a significantly more favorable prognosis. These findings demonstrate the importance of IGFBP-3 expression levels for the survival and prognosis of patients with HCC. The results also raise the possibility that IGFBP-3 possesses an important function within the underlying biological mechanisms that promote the growth and development of human cancer. Therefore, further studies must investigate the correlation between the expression levels of IGFBP-3 and the treatment outcomes following chemotherapy and radiofrequency ablation therapy in patients with HCC. Although further investigation is required, an evaluation of the IGFBP-3 expression profile may be useful when assessing the prognosis of patients with HCC.
In conclusion, the present study demonstrated that low levels of IGFBP-3 expression correlate with certain clinicopathological features and the poor overall survival of patients with HCC. Therefore, evaluation of IGFBP-3 expression patterns using IHC may be used as a novel approach for identification of those patients with HCC who have increased risk of tumor invasion and progression. Overall, these results suggest that IGFBP-3 may be used as a novel marker to aid the assessment of prognosis for patients with HCC.
Acknowledgements
This study was supported by the National Nature Science Foundation of China (grant nos. 81225018, 81172340 and 30901769), the 973 Project of China (grant nos. 2010CB912802 and 2010CB529404) and the Ph.D. Programs Foundation of The Ministry of Education of China (grant no. 20110171110078).
References
- 1.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, Piscaglia F, Gramantieri L, Zanetti M, Sherman M. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: A cost effectiveness analysis. Gut. 2001;48:251–259. doi: 10.1136/gut.48.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuen MF, Cheng CC, Lauder IJ, Lam SK, Ooi CG, Lai CL. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology. 2000;31:330–335. doi: 10.1002/hep.510310211. [DOI] [PubMed] [Google Scholar]
- 4.Mikami E, Kanno N, Ueno Y, Shimosegawa T. Retrospective evaluation of tumor-mass-reduction therapy for the prognosis of recurrent hepatocellular carcinoma. Hepatol Int. 2007;1:460–468. doi: 10.1007/s12072-007-9021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho EJ, Lee JH, Yoo JJ, Choi WM, Lee MJ, Cho Y, Lee DH, Lee YB, Kwon JH, Yu SJ, et al. Serum insulin-like growth factor-I level is an independent predictor of recurrence and survival in early hepatocellular carcinoma: A prospective cohort study. Clin Cancer Res. 2013;19:4218–4227. doi: 10.1158/1078-0432.CCR-12-3443. [DOI] [PubMed] [Google Scholar]
- 6.Gao L, Wang X, Wang X, Zhang L, Qiang C, Chang S, Ren W, Li S, Yang Y, Tong D, et al. IGF-1R, a target of let-7b, mediates crosstalk between IRS-2/Akt and MAPK pathways to promote proliferation of oral squamous cell carcinoma. Oncotarget. 2014;5:2562–2574. doi: 10.18632/oncotarget.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin C, Guo J, Qiu X, Ma K, Xiang M, Zhu X, Guo J. IGF-1 induces iNOS expression via the p38 MAPK signal pathway in the anti-apoptotic process in pulmonary artery smooth muscle cells during PAH. J Recept Signal Transduct Res. 2014;34:325–331. doi: 10.3109/10799893.2014.903417. [DOI] [PubMed] [Google Scholar]
- 8.Yan XD, Yao M, Wang L, Zhang HJ, Yan MJ, Gu X, Shi Y, Chen J, Dong ZZ, Yao DF. Overexpression of insulin-like growth factor-I receptor as a pertinent biomarker for hepatocytes malignant transformation. World J Gastroenterol. 2013;19:6084–6092. doi: 10.3748/wjg.v19.i36.6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sedlaczek N, Hasilik A, Neuhaus P, Schuppan D, Herbst H. Focal overexpression of insulin-like growth factor 2 by hepatocytes and cholangiocytes in viral liver cirrhosis. Br J Cancer. 2003;88:733–739. doi: 10.1038/sj.bjc.6600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jogie-Brahim S, Min HK, Oh Y. Potential of proteomics towards the investigation of the IGF-independent actions of IGFBP-3. Expert Rev Proteomics. 2005;2:71–86. doi: 10.1586/14789450.2.1.71. [DOI] [PubMed] [Google Scholar]
- 11.Akturk M, Arslan M, Altinova A, Ozdemir A, Ersoy R, Yetkin I, Ayvali E, Gonen S, Toruner F. Association of serum levels of IGF-I and IGFBP-1 with renal function in patients with type 2 diabetes mellitus. Growth Horm Igf Res. 2007;17:186–193. doi: 10.1016/j.ghir.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Jeschke MG, Barrow RE, Suzuki F, Rai J, Benjamin D, Herndon DN. IGF-I/IGFBP-3 equilibrates ratios of pro- to anti-inflammatory cytokines, which are predictors for organ function in severely burned pediatric patients. Mol Med. 2002;8:238–246. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Z, Liu Y, He H, Chen X, Chen J, Lu YC. Candidate genes influencing sensitivity and resistance of human glioblastoma to Semustine. Brain Res Bull. 2011;86:189–194. doi: 10.1016/j.brainresbull.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Zhao L, He LR, Zhang R, Cai MY, Liao YJ, Qian D, Xi M, Zeng YX, Xie D, Liu MZ. Low expression of IGFBP-3 predicts poor prognosis in patients with esophageal squamous cell carcinoma. Med Oncol. 2012;29:2669–2676. doi: 10.1007/s12032-011-0133-4. [DOI] [PubMed] [Google Scholar]
- 15.Varotti G, Ramacciato G, Ercolani G, Grazi GL, Vetrone G, Cescon M, Del Gaudio M, Ravaioli M, Ziparo V, Lauro A, Pinna A. Comparison between the fifth and sixth editions of the AJCC/UICC TNM staging systems for hepatocellular carcinoma: Multicentric study on 393 cirrhotic respected patients. Eur J Surg Oncol. 2005;31:760–767. doi: 10.1016/j.ejso.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Xie D, Zeng YX, Wang HJ, Wen JM, Tao Y, Sham JS, Guan XY. Expression of cytoplasmic and nuclear Survivin in primary and secondary human glioblastoma. Br J Cancer. 2006;94:108–114. doi: 10.1038/sj.bjc.6602904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zlobec I, Steele R, Terracciano L, Jass JR, Lugli A. Selecting immunohistochemical cut-off scores for novel biomarkers of progression and survival in colorectal cancer. J Clin Pathol. 2007;60:1112–1116. doi: 10.1136/jcp.2006.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai MY, Zhang B, He WP, Yang GF, Rao HL, Rao ZY, Wu QL, Guan XY, Kung HF, Zeng YX, Xie D. Decreased expression of PinX1 protein is correlated with tumor development and is a new independent poor prognostic factor in ovarian carcinoma. Cancer Sci. 2010;101:1543–1549. doi: 10.1111/j.1349-7006.2010.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mesa MS, Marrodán MD, Lomaglio DB, López-Ejeda N, Moreno-Romero S, Bejarano JI, Dipierri JE, Pacheco JL. Anthropometric parameters in screening for excess of adiposity in argentinian and spanish adolescents: Evaluation using receiver operating characteristic (ROC) methodology. Ann Hum Biol. 2013;40:396–405. doi: 10.3109/03014460.2013.788210. [DOI] [PubMed] [Google Scholar]
- 20.Hanley JA. Receiver operating characteristic (ROC) methodology: The state of the art. Crit Rev Diagn Imaging. 1989;29:307–335. [PubMed] [Google Scholar]
- 21.Taylor SC, Posch A. The design of a quantitative western blot experiment. Biomed Res Int. 2014;2014:361590. doi: 10.1155/2014/361590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshino K, Motoyama S, Koyota S, Shibuya K, Usami S, Maruyama K, Saito H, Minamiya Y, Sugiyama T, Ogawa J. IGFBP3 and BAG1 enhance radiation-induced apoptosis in squamous esophageal cancer cells. Biochem Biophys Res Commun. 2011;404:1070–1075. doi: 10.1016/j.bbrc.2010.12.115. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka H, Iijima H, Higashiura A, Yoh K, Ishii A, Takashima T, Sakai Y, Aizawa N, Iwata K, Ikeda N, et al. New malignant grading system for hepatocellular carcinoma using the Sonazoid contrast agent for ultrasonography. J Gastroenterol. 2014;49:755–763. doi: 10.1007/s00535-013-0830-1. [DOI] [PubMed] [Google Scholar]
- 24.Han DH, Choi GH, Kim KS, Choi JS, Park YN, Kim SU, Park JY, Ahn SH, Han KH. Prognostic significance of the worst grade in hepatocellular carcinoma with heterogeneous histologic grades of differentiation. J Gastroenterol Hepatol. 2013;28:1384–1390. doi: 10.1111/jgh.12200. [DOI] [PubMed] [Google Scholar]
- 25.Shahjee HM, Bhattacharyya N. Activation of various downstream signaling molecules by IGFBP-3. J Cancer Ther. 2014;5:830–835. doi: 10.4236/jct.2014.59091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tas F, Karabulut S, Bilgin E, Tastekin D, Duranyildiz D. Clinical significance of serum insulin-like growth factor-1 (IGF-1) and insulin-like growth factor binding protein-3 (IGFBP-3) in patients with breast cancer. Tumour Biol. 2014;35:9303–9309. doi: 10.1007/s13277-013-1405-8. [DOI] [PubMed] [Google Scholar]
- 27.Ingermann AR, Yang YF, Han J, Mikami A, Garza AE, Mohanraj L, Fan L, Idowu M, Ware JL, Kim HS, et al. Identification of a novel cell death receptor mediating IGFBP-3-induced anti-tumor effects in breast and prostate cancer. J Biol Chem. 2010;285:30233–30246. doi: 10.1074/jbc.M110.122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butt AJ, Williams AC. IGFBP-3 and apoptosis-a license to kill? Apoptosis. 2001;6:199–205. doi: 10.1023/A:1011388710719. [DOI] [PubMed] [Google Scholar]
- 29.Huang XP, Zhou WH, Zhang YF. Genetic variations in the IGF-IGFR-IGFBP axis confer susceptibility to lung and esophageal cancer. Genet Mol Res. 2014;13:2107–2119. doi: 10.4238/2014.January.24.17. [DOI] [PubMed] [Google Scholar]
- 30.Gill ZP, Perks CM, Newcomb PV, Holly JM. Insulin-like growth factor-binding protein (IGFBP-3) predisposes breast cancer cells to programmed cell death in a non-IGF-dependent manner. J Biol Chem. 1997;272:25602–25607. doi: 10.1074/jbc.272.41.25602. [DOI] [PubMed] [Google Scholar]
- 31.Vijayababu MR, Kanagaraj P, Arunkumar A, Ilangovan R, Dharmarajan A, Arunakaran J. Quercetin induces p53-independent apoptosis in human prostate cancer cells by modulating Bcl-2-related proteins: A possible mediation by IGFBP-3. Oncol Res. 2006;16:67–74. doi: 10.3727/000000006783981224. [DOI] [PubMed] [Google Scholar]
- 32.Butt AJ, Firth SM, King MA, Baxter RC. Insulin-like growth factor-binding protein-3 modulates expression of Bax and Bcl-2 and potentiates p53-independent radiation-induced apoptosis in human breast cancer cells. J Biol Chem. 2000;275:39174–39181. doi: 10.1074/jbc.M908888199. [DOI] [PubMed] [Google Scholar]
- 33.Takaoka M, Harada H, Andl CD, Oyama K, Naomoto Y, Dempsey KL, Klein-Szanto AJ, El-Deiry WS, Grimberg A, Nakagawa H. Epidermal growth factor receptor regulates aberrant expression of insulin-like growth factor-binding protein 3. Cancer Res. 2004;64:7711–7723. doi: 10.1158/0008-5472.CAN-04-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao L, He LR, Xi M, Cai MY, Shen JX, Li QQ, Liao YJ, Qian D, Feng ZZ, Zeng YX, et al. Nimotuzumab promotes radiosensitivity of EGFR-overexpression esophageal squamous cell carcinoma cells by upregulating IGFBP-3. J Transl Med. 2012;10:249. doi: 10.1186/1479-5876-10-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adamek A, Kasprzak A, Mikoś H, Przybyszewska W, Seraszek-Jaros A, Czajka A, Sterzyńska K, Mozer-Lisewska I. The insulin-like growth factor-1 and expression of its binding protein-3 in chronic hepatitis C and hepatocellular carcinoma. Oncol Rep. 2013;30:1337–1345. doi: 10.3892/or.2013.2546. [DOI] [PubMed] [Google Scholar]
- 36.Aleem E, Elshayeb A, Elhabachi N, Mansour AR, Gowily A, Hela A. Serum IGFBP-3 is a more effective predictor than IGF-1 and IGF-2 for the development of hepatocellular carcinoma in patients with chronic HCV infection. Oncol Lett. 2012;3:704–712. doi: 10.3892/ol.2011.546. [DOI] [PMC free article] [PubMed] [Google Scholar]