Abstract
The hepatokine fibroblast growth factor 21 (FGF21) is a novel polypeptide ligand, which is involved in glucose and lipid metabolism, and contributes significantly to lowering body weight and enhancing insulin sensitivity. A large number of pre-clinical and clinical results demonstrate that FGF21 is a potential drug target for treating obesity and type 2 diabetes mellitus. In the present review, the tissue specific actions and molecular mechanisms of FGF21 are discussed with a focus on the liver, adipose tissue and nervous system, as well as investigating the outcomes of clinical trials of FGF21, with the aim of interpreting and delineating the complexity physiology of FGF21.
Keywords: fibroblast growth factor 21, obesity, metabolic, diabetes
1. Introduction
Fibroblast growth factor 21 (FGF21) is a member of the family of fibroblast growth factors (FGFs). Since the discovery of the first FGF (FGF1 or FGF-a) in 1976 (1), 22 members of this family have been identified (1). FGFs exhibit diverse biological and physiological activities. Specifically, FGFs are involved in metabolic regulation and cell differentiation, proliferation and metabolism (1–6). FGF21 was first cloned by Nishimura et al (7). In 2005, FGF21 was reported as a novel metabolic regulator with biological activities (8). Subsequent studies revealed that FGF21 is a key factor secreted by the liver and is a signaling molecule involved in important metabolic regulation. Therefore, the current study presents a summary of the molecular mechanisms underlying the metabolic regulation of FGF21 in different types of tissue, including liver, adipose and nervous system tissues. The findings may serve as a reference for drug development of FGF21.
2. Biological actions of FGF 21
Kharitonenkov et al (8) first demonstrated the effects of FGF21 on metabolic regulation and found that FGF21 regulates the expression of glucose transporter 1, and promotes the intake of glucose in 3T3-L1 cells and primary lipocytes in the human body. Subsequent experiments indicated that FGF21 significantly reduced the blood sugar levels and body weights of mice with genetic control- or diet-induced obesity by reducing the contents of triglycerides in the liver and serum; however, the glucose intake of the mice demonstrated no significant change (8–10). In addition, FGF21 influences the insulin sensitivity of the mice. In mice with diet-induced obesity, FGF21 may reverse hepatic steatosis and enhance hepatic insulin sensitivity by suppressing glucose production in the liver and increasing hepatic glycogen content, thereby improving systemic glucose intolerance and insulin resistance (10–12). The regulatory effects of FGF21 in glycolipid metabolism are influenced by hepatic metabolism (12); however, the specific underlying mechanism remains to be elucidated. FGF21 was shown to exert the same metabolic effects in monkeys with diabetes mellitus. FGF21 reduced the concentration of low-density lipoprotein cholesterol (LDL-C) and increased that of high-density lipoprotein cholesterol (HDL-C) (13). These findings indicate that FGF21 is significant in metabolic regulation in rodents and non-human primates with obesity.
3. Receptors mediating the biological effects of FGF21
FGF21 participates in signal transduction by triggering FGF receptors (FGFRs) (8). Unlike conventional FGFs, FGF21 does not directly bind FGFRs, but exerts the biological functions by binding the accessory receptor βKlotho and FGFRs. This conclusion has been confirmed by the following: i) βKlotho restores ththe biological functions by binding the accessory receptor βKlotho and FGFRs. This conclusion has been confirmed by the following: i) βKlotho restores the activity of FGF21 in cells in which FGF21 is ineffective (14–16); ii) the expression of the induced receptor βKlotho increases susceptibility to FGF21 (17); and iii) downregulation of βKlotho mediated by small-interfering RNA weakens the activity of FGF21. Therefore, the receptor of FGF21 consists of the following two components in terms of structure: FGFR and βKlotho (15). Neither βKlotho nor FGFR activate the signaling of FGF21, and FGF21 only acts when the two are combined.
The N- and C-termini of FGF21 are closely associated with the bioactivity of FGF21; after the C-terminus combines with the transmembrane protein of βKlotho, the N-terminus binds with FGFR. Subsequent to forming a stable FGF21/βKlotho/FGFR complex, the associated downstream signaling molecules are activated to exert their biological effects (18,19). Previous studies have confirmed that FGFR1c is a key receptor of FGF21, and that other receptors mediating FGF21 signaling include FGFR2 and FGFR4 (14,18). βKlotho is abundant in various types of metabolically active tissue, including the liver, heart, white adipose tissue (WAT), brown adipose tissue (BAT), and the central nervous system (20). FGFRs are widely expressed in various tissue and the selective expression of βKlotho determines the tissue-specific biological functions of FGF21 (21).
4. Regulatory effects of FGF21 in various tissues of the body
Effects of FGF21 in the liver
FGF21 is expressed in the liver, adipose tissue, central nervous system, and other tissues (20). Under normal physiological us system, and other tissues (20). Under normal physiological conditions, FGF21 in the blood circulation is derived from hepatic secretion (22). In the liver, long-time fasting may induce the expression of FGF21, although gain-of-function experimental results indicated that FGF21 itself also causes various effects of fasting; specifically, FGF21 stimulates the oxidation of fatty acids and the production of ketone bodies in the liver and inhibits lipogenesis (9,23–25). In addition, loss-of-function experimental results showed that mice with FGF21 knockout exhibit serious weight gains after ketogenic diets, with hepatic steatosis and functional impairments in the synthesis of ketone bodies, as compared with wild-type mice (26).
Transcriptional mechanisms of FGF21 in the liver
The complex network of nuclear receptors, nutritive stimuli and hormones involves the gene regulation of FGF21 in different tissues, thereby influencing its effects in regulating hunger stimuli and systemic energy balance. Badman et al (23) and Inagaki et al (24) found that the nuclear receptor peroxisome proliferator-activated receptor α (PPARα) induces FGF21 expression. This findiuli and systemic energy balance. Badman et al (23) and Inagaki et al (24) found that the nuclear receptor peroxisome proliferator-activated receptor α (PPARα) induces FGF21 expression. This finding indicates that various effects of PPARα in the liver are mediated by FGF21. PPARα induces FGF21 transcription in mice after long-time fasting, ketogenic diets, and treatment of primary hepatocytes treated with PPARα by combining peroxisome proliferator response elements (PPRE) transcriptional response elements on the FGF21 promoter (23,24). However, in the liver of mice with PPARα knockout, hunger stimuli induced a five-fold increase in the RNA level of FGF21 (24). This finding indicates that FGF21 transcription is regulated by additional cytokines. Furthermore, the results confirmed that FGF21 is the direct target of the retinoic acid receptor β (RARβ). RARβ overexpression increases the generation and secretion of FGF21 in the liver, thereby performing a regulatory role in metabolism (27). The NAD+-dependent histone deacetylase, sirtuin 1 alleviates the fasting-induced hepatic fat deposition in mice by inducing FGF21 expression in the liver (28,29). Recent gain-of-function and loss-of-function experimental results demonstrated that the transcription of FGF21 in primary hepatocytes in the liver is also regulated by cAMP response element binding protein (CREB) H (30), PPARγ coactivator1α (31) and retinoic acid receptor-related orphan receptor α (32). In addition, the carbohydrate response element binding protein, activated by high-carbohydrate diets, upregulates FGF21 in the liver (33). FGF21 is regulated by the inositol-requiring enzyme 1α-X-box binding protein 1 signaling pathway of the unfolded protein response (34). Thus, FGF21 exerts a wide range of effects on the hepatic response to nutritive stimuli and regulates energy metabolism.
Regulatory mechanisms of FGF21 in glycolipid metabolism in the liver
FGF21 exerts significant effects on hepatic metabolism; specifically, it refines hepatic insulin resistance and steatosis in mice with diet-induced obesity, and regulates glycogen synthesis and ketone body production (9,23–25). In mice injected with FGF21 extracellular signal-regulated kinase (ERK)1/2 phosphorylation is induced in the liver and immediately regulates the expression of early genes in the liver. FGF21 also induces the expression of peroxisome proliferator-activated receptor gamma coactivator 1α and its downstream genes, and enhances the oxidation of fatty acids in the liver (25). However, FGF21 exerted no effect in the liver of isolated mice or in the primary hepatocytes of rats, which indicates that the effects of FGF21 in the liver are indirectly mediated by other signaling pathways of the liver or by other tissues, such as adipose tissues (35). Lin et al (35) found that adiponectin (APN) mediates the regulatory effects of FGF21 on systemic metabolism. The authors also found that the capacity of FGF21 to ameliorate plasma triglycerides, hepatic steatosis, and liver injuries disappears in mice with obesity and diet-induced APN knockout (35). However, FGF21 may still significantly reduce the blood sugar level of mice with APN knockout. This result demonstrates that APN mediates the lipid-lowering effects of FGF21, although the specific glucose-lowering mechanisms of FGF21 remain unidentified. In addition, FGF21 and tissue-specific accessory receptor, βKlotho are highly expressed in the liver (33). This indicates that FGF21 directly regulates hepatic metabolism using βKlotho/FGFR. Thus, mice with liver-specific βKlotho knockout or silencing are used in experiments to elucidate the direct effect of FGF21 on the liver and its relative contribution to the balance of systemic glycolipid metabolism. Various studies have evaluated the upstream regulation of FGF21; however, the downstream regulation mediated by FGF21 in the liver remains to be elucidated. Therefore, this is currently a research hotspot.
Effects of FGF21 in adipose tissues
In vivo and in vitro experiments on the metabolic regulation of FGF21 revealed that either the overexpressed FGF21 transgene or the exogenous FGF21 significantly reduce glucose and lipid levels in the blood of mice (8,10). However, FGF21 increased the intake of glucose in mature 3T3-L1 cells and the primary lipocytes in the human body (36). Subsequent experiments revealed that FGF21 regulates the intake of glucose in WAT, the generation and decomposition of adipose tissues, and lipid metabolism by mediating the expression of associated genes (8,9,36). FGF21 induces the expression of uncoupling protein 1 (UCP1) in WAT (37). UCP1 is a typical protein in BAT that is involved in uncoupling respiration and thermogenesis. This phenomenon is the so-called ‘browning’ of WAT, usually occurring in subcutaneous WAT dominated by the sympathetic nerve; FGF21 is able to regulate thermogenesis using this mechanism (37). In addition, FGF21 activates the expression and secretion of APN in lipocytes by autocrine or paracrine signaling (35,38), thereby regulating the glycolipid metabolism in adipose tissues and further improving insulin sensitivity. In vivo and in vitro experiment results also showed that FGF21 stimulates the expression of genes associated with glucose intake and thermogenesis in BAT, thereby regulating energy metabolism (10,37,39,40).
Transcriptional regulation mechanism of FGF21 in adipose tissues
In WAT, the nuclear receptor, PPARγ induces the expression of FGF21 (41). FGF21 acts in synergy with the PPARγ stimulant, rosiglitazone, thereby promoting the differentiation of lipocytes and the intake of glucose (17,41–43). Unlike the hunger-induced regulation of FGF21 in the liver, the feeding-induced regulation of FGF21 in WAT further activates the activity of PPARγ in the form of feedforward regulation (44). The PPARγ signaling pathway is impaired in mice with FGF21 knockout through the downregulation of genes depending on body fat and PPARγ expression. In addition, the PPARγ stimulant, rosiglitazone has side effects, including insulin sensitization, weight gain and edema; however, such side effects do not occur in mice with FGF21 knockout. These results demonstrate that FGF21 is a major carrier of the physiological and pharmacological effects of PPARγ in WAT (44). In addition, FGF21 is induced by cold stimuli in BAT, and it regulates thermogenesis via activating transcription factor 2 (ATF2)-mediated β3-adrenaline receptor (37,39,45). FGF21 also directly regulates UCP1 transcription by increasing the transcription factor AMP response elements and activating the phosphorylation of CREB (46). FGF21 also increases the phosphorylation of fatty acid transport protein 3, thereby regulating mitochondrial respiration (46). In a cold stimulation experiment, FGF21 knockout mice exhibited low body temperatures, elevated serum creatinine kinase levels and trembling (37). Thus, FGF21 regulates energy metabolism by promoting thermogenesis in BAT.
Regulatory mechanism of FGF21 in the glycolipid metabolism and energy metabolism in adipose tissues
In vivo loss-of-function experiment results confirmed the significant effects of FGF21 in adipose tissues. After the specific receptor βKlotho of FGF21 is knockout from adipose tissues, the acute effects of FGF21 on insulin sensitivity and glucose intake disappeared from the adipose tissues of the mice with the gene knockout compared with those of the control mice fed with high fat diets (40). Similarly, in diet-induced obesity mice with selective deletion of FGFR1c in adipose tissues, FGF21 exerts no effects on the weights or on the levels of plasma glucose, insulin, triglycerides, or APN (47). Thus, βKlotho and FGFR mediate the effects of FGF21 in regulating the glycolipid metabolism in adipose tissues. The browning and thermogenesis of WAT reveal the important regulatory effect of FGF21 on the energy metabolism and body weight of the mice. Furthermore, the signal transmission and cross-talk mechanisms between the liver and adipose tissues mediate the regulatory effects of FGF21 on energy metabolism. The endocrine signaling pathway of FGF21 in hepatocytes increases the energy expenditure in mice by regulating the browning of WAT, thereby enhancing the energy metabolism and reducing body weight (28). Additionally, Fisher et al (37) found that the in vivo and in vitro thermogenesis of FGF21 is closely associated with peroxisome proliferator-activated receptor gamma coactivator 1α (PGC1α), the key factor regulating thermogenesis and energy metabolism (37). In addition, FGF21 regulates the browning of WAT by elevating the level of protein rather than gene expression of PGC1α, thereby promoting thermogenesis (37).
Effects of FGF21 in the brain
Although it is not expressed in the brain (20), FGF21 passes through the blood brain barrier (BBB) by simple diffusion, thereby entering the human cerebrospinal fluid (48) and the hypothalami of fasting mice (49), and further activating ERK1/2 phosphorylation (50). Administering FGF21 in the cerebral ventricles increases energy expenditure and improves the insulin sensitivity in obese rats (51), thereby triggering the FGF21-mediated signal interaction between the brain and its peripheral tissues (52). Thus, FGF21 directly acts on the brain.
Regulatory mechanisms of FGF21 in the brain
In the hypothalamus, FGF21 affects the expression levels of two important neuropeptides, namely, corticotrophin releasing factor (CRF) and arginine vasopressin (AVP) (49,53–55) in the brain. FGF21 induces CRF expression in the hypothalamic (49,53,55). Consequently, CRF increases the secretion of CRF in the pituitary gland, thereby stimulating the secretion of CRF. CRF-induced sympathetic excitation also passes onto BAT (56). Notably, CRF receptor inhibitors completely block FGF21-induced sympathetic excitation (53). Therefore, its inducing action on CRF is deemed as a mechanism by which FGF21 promotes glucocorticoids, thermogenesis and weight loss. Conversely, long-term treatment of lean mice (except for those with CRF-induced weight loss) with FGF21 may also inhibit AVP expression in the suprachiasmatic nucleus (SCN) (54). In the hypothalami of hungry mice with βKlotho knockout, the mRNA expression level of AVP is elevated. In female mice, the downregulation of AVP may decrease the level of luteinizing hormone secreted in the pituitary gland and reduce ovulation, thereby inhibiting reproduction.
However, the specific site of the CRF-induced action of FGF21 in the hypothalamus remains unknown. A recent study demonstrated that βKlotho is expressed in the CRF neurons of the paraventricular nucleus (PVN) (49). At this site, FGF21 induces CRF expression by activating the transcription factor, CREB (49). This result is consistent with the expression of UCP1 in FGF21-induced BAT (46). Therefore, FGF21 in the PVN also directly or indirectly induces the actions of CRF.
Effects of FGF21 in signal transmission and cross-talk in the brain and its peripheral tissues
Four types of FGFRs extensively exist in the nervous system; however, the expression of βKlotho in the nervous system is limited (20). In the hypothalamus, βKlotho is expressed in the SCN and PVN (49,55), in the nucleus tractus solitarius of the back polar region and hindbrain, and in the nodose ganglion root of the peripherals (55). These regions constitute the dorsal vagal complex, which is a major integrated aspect of the autonomic nervous system.
The brain mediates certain roles of FGF21 in the systemic blood circulation system. In mice, various chronic effects of FGF21 on the production of ketone bodies, circadian rhythms, and the fertility of female mice require the involvement of the βKlotho protein in the central nervous system (54,55). Further research confirmed that FGF21 stimulates the release of CRF from the nervous system to activate the sympathetic nervous system, thereby causing WAT browning, fatty acid oxidation in BAT, thermogenesis and lipolysis accompanying hepatic ketogenesis (53). These results demonstrate why certain effects of FGF21 disappear in the absence of βKlotho from the nervous system of mice (53,55). Notably, the glucose-lowering roles of the FGF21 transgene are intact in mice with brain-specific βKlotho knockout (55), but are completely inhibited in mice with βKlotho systemic knockout (40,57). This result reveals that other metabolic tissues mediate the glucose-lowering roles of FGF21.
Effects of FGF21 in other tissues
FGF21 is also expressed in the endocrine-specific pancreatic α and β cells, and the expression of FGF21 is exposed to chemical-induced pancreatic trauma (58). However, the physiological functions of FGF21 in chemical-induced pancreatic trauma remain unknown (58). In isolated islet cells, FGF21 increases the quantity of insulin and the secretion of insulin induced by glucose stimulation, thereby inhibiting glucagon secretion (8) and exerting protective effects on mice with pancreatitis (58). Furthermore, studies have reported that FGF21 modulates inflammation and damage induced by experimental pancreatitis. FGF21-null mice develop more damage than wild-type mice, whereas mice overexpressing human FGF21 exhibit an attenuated phenotype (58). Further studies identified the transcription factor, basic helix-loop-helix family member a15 (BHLHA15; also termed, MIST1) as an upstream regulator of FGF21 and showed that deletion of the MIST1 gene leads to a marked reduction in pancreatic FGF21 levels by epigenetic silencing, resulting in increased susceptibility to pancreatitis (59). In addition, FGF21 may be involved in enhanced islet transplant survival, which was demonstrated in a model of streptozotocin-induced diabetes (60). FGF21 may promote β-cell survival, and protect isolated rat islets and insulin-producing INS cells from glucolipotoxicity and cytokine-induced apoptosis (61). However, human FGF21 fails to alter insulin and glucagon secretion from islets isolated from healthy mice (62), although FGF21 stimulates insulin secretion in ex vivo islets isolated from diabetic mice (61). Islets from obese diabetic db/db mouse failed to respond to FGF21, possibly as a consequence of reduced βKlotho expression levels (63).
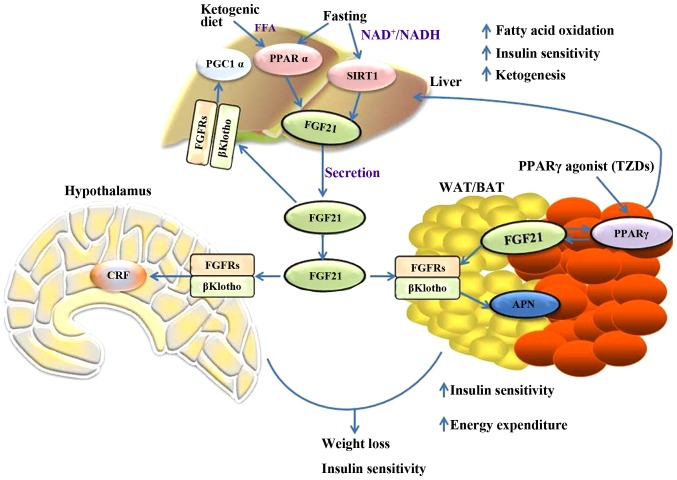
In addition, the dysfunction of the mitochondria in the skeletal muscle induces the expression of FGF21 by activating ATF4 (64,65). Thus, FGF21 is important in the metabolic network. Fig. 1 briefly outlines the metabolic regulations of FGF21 in different tissues and the interactions between them.
Figure 1.
Summary of the metabolic actions of FGF21 in the liver, adipose, and nervous system tissues. FGF21, fibroblast growth factor 21; PGC1α, peroxisome proliferator-activated receptor gamma coactivator 1α; PPARα, peroxisome proliferator-activated receptor α; SIRT1, sirtuin 1; FGFR, fibroblast growth factor receptor; APN, adiponectin; CRF, corticotrophin releasing factor; WAT, white adipose tissue; BAT, brown adipose tissue; TZD, thiazolidinediones.
5. Clinical applications of FGF21
Manifestations of FGF21 in the human body
Similar to the findings in experiments with mice, PPARα stimulants (such as fibrates) increase the circulating level of FGF21 in the human body (66–68); however, the concentration of FGF21 is significantly increased in patients with rheumatoid arthritis after 7 days of fasting or in patients with obesity and diets low in calories within 3 weeks (66). These results demonstrate that FGF21 performs similar metabolic regulation and clinical applications in different species; however, the effects of PPARα stimulant and fasting on FGF21 expression are relatively weaker in the human body than in mice. Notably, neither short duration fasting nor anorexia nervosa increases the circulating level of FGF21 in the human body (68). Ketogenic diets do not elevate FGF21 expression levels in the human body (69). This discrepancy may be caused by the low content of protein in the ketogenic diet used in the experiment with mice (70). Therefore, the regulatory roles of FGF21 in rodents differ from those in the human body. Notably, certain studies detected FGF21 mRNA expression in human WAT (43,71), although Dushay et al (69) reported that FGF21 is not expressed in human WAT. In addition, clinical studies revealed that the circulating levels of FGF21 in patients with obesity or in those with accompanying diabetes mellitus (type 2), insulin resistance, or nonalcoholic fatty liver disease are considerably elevated (71–73). This result may be attributed to the increased content of FGF21 in the liver induced by diets containing high levels of fat and carbohydrates. Similarly, the concentration of FGF21 is increased in the plasma of rodents with obesity, and this phenomenon is similar to hyperinsulinism and hyperleptinemia; therefore, introducing the concept of FGF21 resistance (11,74,75). However, when the concentration of FGF21 in the blood of mice with diet-induced obesity is increased, FGF21 effectively exerts pharmacodynamic effects, with a marked increase in insulin sensitivity. Therefore, the hypothesis on FGF21 resistance remains controversial (74,76).
FGF21 significantly reduced body weight and improved insulin sensitivity in obese rodents and primates (76). Prolonged fasting or feeding with a ketogenic diet results in hepatic production and secretion of FGF21. In the liver, FGF21 increases fatty acid oxidation and ketogenesis, and decreases hepatic lipid biosynthesis, resulting in improved insulin sensitivity (75). FGF21 induces production and secretion of adiponectin through PPARγ in adipose tissue, which in turn potentiates insulin-sensitizing effects of PPARγ and stimulates browning by inducing PGC1α (75,76). FGF21 stimulates sympathetic nerve activity via CRF, leading to improved whole body metabolism. These effects of FGF21 require signaling via the liver- and adipose-enriched coreceptor, βKlotho and one of the FGFRs, such as FGFR1, FGFR2 or FGFR4.
Clinical trials on FGF21
The significant curative effects of FGF21 in the animal model are of interest. However, inadequacies in its physical and biological properties rendered the natural FGF21 protein hard to apply to molecule drugs for clinical settings (77). The main causes include the following: First, the FGF21 protein stored in solution is easily degraded. In addition, FGF21 is conformationally unstable. In addition, the large-scale expression and purification of FGF21 are difficult. Considering the above factors, Kharitonenkov et al (78) developed a drug, LY2405319 (LY), which avoids the biopharmaceutical defects of FGF21; the engineered drug is an FGF21 isomer that retains the curative effects of FGF21 on various metabolic disorders, but avoids the defects of the wild-type FGF21 (78). For patients with obesity and type 2 diabetes mellitus, LY is administered by daily injection, and clinical test results indicated that injected LY significantly improves the profiling levels of four types of lipids (i.e., total cholesterol, LDL-C, HDL-C and triglycerides) and normalizes the body weights of these patients. These results are consistent with those of FGF21 or LY in Rhesus monkeys with obesity (13).
The triglyceride level declines quickly after fasting and LY treatment. This phenomenon is accompanied by a reduction in apolipoprotein C III and aesult indicates that LY regulates the oxidation level of fatty acids in the human body (26). Furthermore, the insulin level in the human body subsequent to fasting and LY treatment is reduced markedly, the APNesult indicates that LY regulates the oxidation level of fatty acids in the human body (79–81). Furthermore, the insulin level in the human body subsequent to fasting and LY treatment is reduced markedly, the APN level in the plasma is elevated appreciably (79), and the APN of high molecular weight is associated with the treatment efficiency of type 2 diabetes mellitus. However, the continuous glucose-lowering effect of LY on the subject is insignificant compared with that of FGF21 in rodents and monkeys with diabetes mellitus, which is potentially due to individual differences and small sample sizes (8,13,82). The potential effect and glucose regulation of exogenous FGF21 in obese patients may be elucidated further using a large sample size, employing long-term regimens, or treating patients with other diseases, such as impaired fasting glucose or type 2 diabetes mellitus of various severities (79).
6. Conclusion
The current review primarily summarizes the metabolic functions and regulatory mechanisms of FGF21 in the liver, adipose, brain and other tissues. Given its consistent and reproducible efficacy in animals, FGF21 is a candidate drug for curing obesity and diabetes mellitus. This drug is now entering the pre-clinical research stage. Clinical trials with a large sample size are required to validate the potential therapeutic effects of FGF21 on metabolic diseases. Although wild-type FGF21 may have side effects on bone mass and fertility, population studies showed that the content of FGF21 in the blood of healthy women positively correlates with bone density (61). This result suggests that the effect of FGF21 in mice with osteoporosis may differ from that in human bones. However, the inhibitory actions of FGF21 on fertility via the central nervous system are eliminated by synthesizing FGF21 isomers, which could not pass through the BBB, by genetic modification (61).
To date, the mechanism of action of FGF21 has not been fully elucidated, and studies on the biological target organs and molecular mechanisms of FGF21 remain incomplete. There are few studies discussing whether FGF21 as a secretory hormone regulates systemic metabolism in different tissues. In addition, thiazolidinedione drugs affect the expression of FGF21 and its receptor βKlotho (17,41,42), as well as enhancing the sensitivity of FGF21 (17). This result indicates that these drugs act against diabetes mellitus by partially affecting the FGF21 signaling pathway. Therefore, intensive studies on the signaling pathway, regulatory mechanisms and biological functions of FGF21 in different tissues are significant for the treatment of human-associated metabolic disorders, such as diabetes mellitus and obesity. With the number of patients with metabolic syndrome increasing annually, the development of drugs for this disease has been receiving considerable attention (61). With further animal experiments and clinical trials covering the improvements in the function and survival of β cells, FGF21 is expected to become a novel candidate for insulin in treating diabetes mellitus. However, animal experiments are inconsistent with clinical trials in certain aspects, and FGF21 requires further investigation. The elevated expression level of FGF21 in the plasma indicates that FGF21 resistance probably compensates for glucolipotoxicity and requires an FGF21 dose exceeding the normal to exert the necessary biological effects. Given the elevated level of FGF21 in the plasma of patients with metabolic disorders, whether FGF21 will be developed into a molecular marker for the early diagnosis and assessment of associated diseases requires further investigation.
Acknowledgements
The present study was supported by the Natural Science Foundation of China (grant no. 81600342), the Medical Foundation of Hui Zhou (grant no. 2015Y134), the Medical Research Foundation of Guangdong Province (grant no. A2015620) and the Graduate Student Research Innovation Project of Hunan Province (grant no. CX2013B396).
References
- 1.Itoh N. Hormone-like (endocrine) Fgfs: Their evolutionary history and roles in development, metabolism, and disease. Cell Tissue Res. 2010;342:1–11. doi: 10.1007/s00441-010-1024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelleher FC, O'Sullivan H, Smyth E, Mc Dermott R, Viterbo A. Fibroblast growth factor receptors, developmental corruption and malignant disease. Carcinogenesis. 2013;34:2198–2205. doi: 10.1093/carcin/bgt254. [DOI] [PubMed] [Google Scholar]
- 3.Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, Lopez JF, Thompson RC, Meng F, Stead JD, et al. Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci USA. 2004;101:15506–15511. doi: 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutley L, Shurety W, Newell F, Mc Geary R, Pelton N, Grant J, Herington A, Cameron D, Whitehead J, Prins J. Fibroblast growth factor 1: A key regulator of human adipogenesis. Diabetes. 2004;53:3097–3106. doi: 10.2337/diabetes.53.12.3097. [DOI] [PubMed] [Google Scholar]
- 5.Yamagata H, Chen Y, Akatsu H, Kamino K, Ito J, Yokoyama S, Yamamoto T, Kosaka K, Miki T, Kondo I. Promoter polymorphism in fibroblast growth factor 1 gene increases risk of definite Alzheimer's disease. Biochem Biophys Res Commun. 2004;321:320–323. doi: 10.1016/j.bbrc.2004.06.142. [DOI] [PubMed] [Google Scholar]
- 6.van der Walt JM, Noureddine MA, Kittappa R, Hauser MA, Scott WK, McKay R, Zhang F, Stajich JM, Fujiwara K, Scott BL, et al. Fibroblast growth factor 20 polymorphisms and haplotypes strongly influence risk of Parkinson disease. Am J Hum Genet. 2004;74:1121–1127. doi: 10.1086/421052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 2000;1492:203–206. doi: 10.1016/S0167-4781(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 8.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in dietinduced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berglund ED, Li CY, Bina HA, Lynes SE, Michael MD, Shanafelt AB, Kharitonenkov A, Wasserman DH. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology. 2009;150:4084–4093. doi: 10.1210/en.2009-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, Stanislaus S, Chinookoswong N, Lau YY, Hager T, Patel J, Ge H, Weiszmann J, Lu SC, Graham M, et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models-association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab. 2009;297:E1105–E1114. doi: 10.1152/ajpendo.00348.2009. [DOI] [PubMed] [Google Scholar]
- 13.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, Eliseenkova AV, Mohammadi M, Kuro-o M. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci USA. 2007;104:7432–7437. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kharitonenkov A, Dunbar JD, Bina HA, Bright S, Moyers JS, Zhang C, Ding L, Micanovic R, Mehrbod SF, Knierman MD, et al. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J Cell Physiol. 2008;215:1–7. doi: 10.1002/jcp.21357. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki M, Uehara Y, Motomura-Matsuzaka K, Oki J, Koyama Y, Kimura M, Asada M, Komi-Kuramochi A, Oka S, Imamura T. betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol Endocrinol. 2008;22:1006–1014. doi: 10.1210/me.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moyers JS, Shiyanova TL, Mehrbod F, Dunbar JD, Noblitt TW, Otto KA, Reifel-Miller A, Kharitonenkov A. Molecular determinants of FGF-21 activitysynergy and cross-talk with PPARgamma signaling. J Cell Physiol. 2007;210:1–6. doi: 10.1002/jcp.20847. [DOI] [PubMed] [Google Scholar]
- 18.Yie J, Hecht R, Patel J, Stevens J, Wang W, Hawkins N, Steavenson S, Smith S, Winters D, Fisher S, et al. FGF21 N- and C-termini play different roles in receptor interaction and activation. FEBS Lett. 2009;583:19–24. doi: 10.1016/j.febslet.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Micanovic R, Raches DW, Dunbar JD, Driver DA, Bina HA, Dickinson CD, Kharitonenkov A. Different roles of N- and C- termini in the functional activity of FGF21. J Cell Physiol. 2009;219:227–234. doi: 10.1002/jcp.21675. [DOI] [PubMed] [Google Scholar]
- 20.Tacer Fon K, Bookout AL, Ding X, Kurosu H, John GB, Wang L, Goetz R, Mohammadi M, Kuro-o M, Mangelsdorf DJ, Kliewer SA. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol. 2010;24:2050–2064. doi: 10.1210/me.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams AC, Coskun T, Rovira AR, Schneider MA, Raches DW, Micanovic R, Bina HA, Dunbar JD, Kharitonenkov A. Fundamentals of FGF19 & FGF21 action in vitro and in vivo. PLo S One. 2012;7:e38438. doi: 10.1371/journal.pone.0038438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M, Potthoff MJ. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63:4057–4063. doi: 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology. 2009;150:4931–4940. doi: 10.1210/en.2009-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Wong K, Walsh K, Gao B, Zang M. Retinoic acid receptor β stimulates hepatic induction of fibroblast growth factor 21 to promote fatty acid oxidation and control whole-body energy homeostasis in mice. J Biol Chem. 2013;288:10490–10504. doi: 10.1074/jbc.M112.429852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Wong K, Giles A, Jiang J, Lee JW, Adams AC, Kharitonenkov A, Yang Q, Gao B, Guarente L, Zang M. Hepatic SIRT1 attenuates hepatic steatosis and controls energy balance in mice by inducing fibroblast growth factor 21. Gastroenterology. 2014;146:539–549.e7. doi: 10.1053/j.gastro.2013.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim H, Mendez R, Zheng Z, Chang L, Cai J, Zhang R, Zhang K. Liver-enriched transcription factor CREBH interacts with peroxisome proliferator-activated receptor α to regulate metabolic hormone FGF21. Endocrinology. 2014;155:769–782. doi: 10.1210/en.2013-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Estall JL, Ruas JL, Choi CS, Laznik D, Badman M, Flier Maratos E, Shulman GI, Spiegelman BM. PGC-1alpha negatively regulates hepatic FGF21 expression by modulating the heme/Rev-Erb (alpha) axis. Proc Natl Acad Sci USA. 2009;106:22510–22515. doi: 10.1073/pnas.0912533106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Solt LA, Burris TP. Regulation of FGF21 expression and secretion by retinoic acid receptor-related orphan receptor alpha. J Biol Chem. 2010;285:15668–15673. doi: 10.1074/jbc.M110.102160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iizuka K, Takeda J, Horikawa Y. Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS Lett. 2009;583:2882–2886. doi: 10.1016/j.febslet.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 34.Shao M, Shan B, Liu Y, Deng Y, Yan C, Wu Y, Mao T, Qiu Y, Zhou Y, Jiang S, et al. Hepatic IRE1α regulates fasting-induced metabolic adaptive programs through the XBP1s-PPARalpha axis signalling. Nat Commun. 2014;5:3528. doi: 10.1038/ncomms4528. [DOI] [PubMed] [Google Scholar]
- 35.Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, Itoh N, Wang Y, Bornstein SR, Xu A, Li X. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013;17:779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-o M. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem. 2007;282:26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, Bauer SM, Wade M, Singhal E, Cheng CC, et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, Villarroya F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem. 2011;286:12983–12990. doi: 10.1074/jbc.M110.215889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding X, Boney-Montoya J, Owen BM, Bookout AL, Coate KC, Mangelsdorf DJ, Kliewer SA. βKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 2012;16:387–393. doi: 10.1016/j.cmet.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muise ES, Azzolina B, Kuo DW, El-Sherbeini M, Tan Y, Yuan X, Mu J, Thompson JR, Berger JP, Wong KK. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol Pharmacol. 2008;74:403–412. doi: 10.1124/mol.108.044826. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Qiang L, Farmer SR. Identification of a domain within peroxisome proliferator-activated receptor gamma regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes. Mol Cell Biol. 2008;28:188–200. doi: 10.1128/MCB.00992-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, Wong RL, Chow WS, Tso AW, Lam KS, Xu A. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 44.Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor-21 regulate PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148:556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chartoumpekis DV, Habeos IG, Ziros PG, Psyrogiannis AI, Kyriazopoulou VE, Papavassiliou AG. Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol Med. 2011;17:736–740. doi: 10.2119/molmed.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu AL, Kolumam G, Stawicki S, Chen Y, Li J, Zavala-Solorio J, Phamluong K, Feng B, Li L, Marsters S, et al. Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Sci Transl Med. 2011;3:113ra126. doi: 10.1126/scitranslmed.3002669. [DOI] [PubMed] [Google Scholar]
- 47.Adams AC, Yang C, Coskun T, Cheng CC, Gimeno RE, Luo Y, Kharitonenkov A. The breadth of FGF21's metabolic actions are governed by FGFR1 in adipose tissue. Mol Metab. 2012;2:31–37. doi: 10.1016/j.molmet.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan BK, Hallschmid M, Adya R, Kern W, Lehnert H, Randeva HS. Fibroblast growth factor 21 (FGF21) in human cerebrospinal fluid: Relationship with plasma FGF21 and body adiposity. Diabetes. 2011;60:2758–2762. doi: 10.2337/db11-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang Q, Zhong L, Zhang J, Wang Y, Bornstein SR, Triggle CR, Ding H, Lam KS, Xu A. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes. 2014;63:4064–4075. doi: 10.2337/db14-0541. [DOI] [PubMed] [Google Scholar]
- 50.Yang C, Jin C, Li X, Wang F, Mc Keehan WL, Luo Y. Differential specificity of endocrine FGF19 and FGF21 to FGFR1 and FGFR4 in complex with KLB. PLoS One. 2012;7:e33870. doi: 10.1371/journal.pone.0033870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarruf DA, Thaler JP, Morton GJ, German J, Fischer JD, Ogimoto K, Schwartz MW. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes. 2010;59:1817–1824. doi: 10.2337/db09-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsuchou H, Pan W, Kastin AJ. The fasting polypeptide FGF21 can enter brain from blood. Peptides. 2007;28:2382–2386. doi: 10.1016/j.peptides.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, Rahmouni K, Kliewer SA, Mangelsdorf DJ. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 2014;20:670–677. doi: 10.1016/j.cmet.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Owen BM, Bookout AL, Ding X, Lin VY, Atkin SD, Gautron L, Kliewer SA, Mangelsdorf DJ. FGF21 contributes to neuroendocrine control of female reproduction. Nat Med. 2013;19:1153–1156. doi: 10.1038/nm.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, Kliewer SA. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med. 2013;19:1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arase K, York DA, Shimizu H, Shargill N, Bray GA. Effects of corticotropin-releasing factor on food intake and brown adipose tissue thermogenesis in rats. Am J Physiol. 1988;255:e255–e259. doi: 10.1152/ajpendo.1988.255.3.E255. [DOI] [PubMed] [Google Scholar]
- 57.Adams AC, Cheng CC, Coskun T, Kharitonenkov A. FGF21 requires βklotho to act in vivo. PLoS One. 2012;7:e49977. doi: 10.1371/journal.pone.0049977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson CL, Weston JY, Chadi SA, Fazio EN, Huff MW, Kharitonenkov A, Köester A, Pin CL. Fibroblast growth factor 21 reduces the severity of cerulein-induced pancreatitis in mice. Gastroenterology. 2009;137:1795–1804. doi: 10.1053/j.gastro.2009.07.064. [DOI] [PubMed] [Google Scholar]
- 59.Johnson CL, Mehmood R, Laing SW, Stepniak CV, Kharitonenkov A, Pin CL. Silencing of the fibroblast growth factor 21 gene is an underlying cause of acinar cell injury in mice lacking MIST1. Am J Physiol Endocrinol Metab. 2014;306:E916–E928. doi: 10.1152/ajpendo.00559.2013. [DOI] [PubMed] [Google Scholar]
- 60.Uonaga T, Toyoda K, Okitsu T, Zhuang X, Yamane S, Uemoto S, Inagaki N. FGF-21 enhances islet engraftment in mouse syngeneic islet transplantation model. Islets. 2010;2:247–251. doi: 10.4161/isl.2.4.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kharitonenkov A, Adams AC. Inventing new medicines: The FGF21 story. Mol Metab. 2013;3:221–229. doi: 10.1016/j.molmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu J, Stanislaus S, Chinookoswong N, Lau YY, Hager T, Patel J, Ge H, Weiszmann J, Lu SC, Graham M, et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin resistant mouse models-association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab. 2009;297:E1105–E1114. doi: 10.1152/ajpendo.00348.2009. [DOI] [PubMed] [Google Scholar]
- 63.So WY, Cheng Q, Chen L, Evans-Molina C, Xu A, Lam KS, Leung PS. High glucose represses β-klotho expression and impairs fibroblast growth factor 21 action in mouse pancreatic islets: Involvement of peroxisome proliferator-activated receptor γ signaling. Diabetes. 2013;62:3751–3759. doi: 10.2337/db13-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tyynismaa H, Carroll CJ, Raimundo N, Ahola-Erkkilä S, Wenz T, Ruhanen H, Guse K, Hemminki A, Peltola-Mjøsund KE, Tulkki V, et al. Mitochondrial myopathy induces a starvationlike response. Hum Mol Genet. 2010;19:3948–3958. doi: 10.1093/hmg/ddq310. [DOI] [PubMed] [Google Scholar]
- 65.Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, Kim SS, Kim DH, Hur KY, Kim HK, et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med. 2013;19:83–92. doi: 10.1038/nm.3014. [DOI] [PubMed] [Google Scholar]
- 66.Gälman C, Lundåsen T, Kharitonenkov A, Bina HA, Eriksson M, Hafström I, Dahlin M, Amark P, Angelin B, Rudling M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab. 2008;8:169–174. doi: 10.1016/j.cmet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 67.Christodoulides C, Dyson P, Sprecher D, Tsintzas K, Karpe F. Circulating fibroblast growth factor 21 is induced by peroxisome proliferator-activated receptor agonists but not ketosis in man. J Clin Endocrinol Metab. 2009;94:3594–3601. doi: 10.1210/jc.2009-0111. [DOI] [PubMed] [Google Scholar]
- 68.Mráz M, Lacinová Z, Kaválková P, Haluzíková D, Trachta P, Drápalová J, Hanušová V, Haluzík M. Serum concentrations of fibroblast growth factor 19 in patients with obesity and type 2 diabetes mellitus: The influence of acute hyperinsulinemia, very-low calorie diet and PPAR-α agonist treatment. Physiol Res. 2011;60:627–636. doi: 10.33549/physiolres.932099. [DOI] [PubMed] [Google Scholar]
- 69.Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, Badman MK, Martinez-Chantar ML, Maratos-Flier E. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139:456–463. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Münzberg H, Hutson SM, Gettys TW, Schwartz MW, Morrison CD. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124:3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mraz M, Bartlova M, Lacinova Z, Michalsky D, Kasalicky M, Haluzikova D, Matoulek M, Dostalova I, Humenanska V, Haluzik M. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol (Oxf) 2009;71:369–375. doi: 10.1111/j.1365-2265.2008.03502.x. [DOI] [PubMed] [Google Scholar]
- 72.Chen WW, Li L, Yang GY, Li K, Qi XY, Zhu W, Tang Y, Liu H, Boden G. Circulating FGF-21 levels in normal subjects and in newly diagnose patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2008;116:65–68. doi: 10.1055/s-2007-985148. [DOI] [PubMed] [Google Scholar]
- 73.Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, Defronzo RA, Tripathy D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care. 2009;32:1542–1546. doi: 10.2337/dc09-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, Maratos-Flier E. Obesity is a fibroblast growth factor 21 (FGF21) resistant state. Diabetes. 2010;59:2781–2789. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lundasen T, Hunt MC, Nilsson LM, Sanyal S, Angelin B, Alexson SE, Rudling M. PPARalpha is a key regulator of hepatic FGF21. Biochem Biophys Res Commun. 2007;360:437–440. doi: 10.1016/j.bbrc.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 76.Hale C, Chen MM, Stanislaus S, Chinookoswong N, Hager T, Wang M, Véniant MM, Xu J. Lack of overt FGF21 resistance in two mouse models of obesity and insulin resistance. Endocrinology. 2012;153:69–80. doi: 10.1210/en.2010-1262. [DOI] [PubMed] [Google Scholar]
- 77.Maruthur NM. The growing prevalence of type 2 diabetes: Increased incidence or improved survival? Curr Diab Rep. 2013;13:786–794. doi: 10.1007/s11892-013-0426-4. [DOI] [PubMed] [Google Scholar]
- 78.Kharitonenkov A, Beals JM, Micanovic R, Strifler BA, Rathnachalam R, Wroblewski VJ, Li S, Koester A, Ford AM, Coskun T, et al. Rational design of a fibroblast growth factor 21-based clinical candidate, LY2405319. PLoS One. 2013;8:e58575. doi: 10.1371/journal.pone.0058575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, Moller DE. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013;18:333–340. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 80.Fisher FM, Estall JL, Adams AC, Antonellis PJ, Bina HA, Flier JS, Kharitonenkov A, Spiegelman BM, Maratos-Flier E. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology. 2011;152:2996–3004. doi: 10.1210/en.2011-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mai K, Bobbert T, Groth C, Assmann A, Meinus S, Kraatz J, Andres J, Arafat AM, Pfeiffer AF, Möhlig M, Spranger J. Physiological modulation of circulating FGF21: Relevance of free fatty acids and insulin. Am J Physiol Endocrinol Metab. 2010;299:E126–E130. doi: 10.1152/ajpendo.00020.2010. [DOI] [PubMed] [Google Scholar]
- 82.Adams AC, Halstead CA, Hansen BC, Irizarry AR, Martin JA, Myers SR, Reynolds VL, Smith HW, Wroblewski VJ, Kharitonenkov A. LY2405319, an engineered FGF21 variant, improves the metabolic status of diabetic monkeys. PLoS One. 2013;8:e65763. doi: 10.1371/journal.pone.0065763. [DOI] [PMC free article] [PubMed] [Google Scholar]