Abstract
Decreased expression of human chemokine-like factor-like MARVEL transmembrane domain-containing 3 (CMTM3) has been identified in a number of human tumors and tumor cell lines, including gastric and testicular cancer, and PC3, CAL27 and Tca-83 cell lines. However, the association between CMTM3 expression and the clinicopathological features and prognosis of esophageal squamous cell carcinoma (ESCC) patients remains unclear. The aim of the present study was to investigate the correlation between CMTM3 expression and clinicopathological parameters and prognosis in ESCC. CMTM3 mRNA and protein expression was analyzed in ESCC and paired non-tumor tissues by quantitative real-time polymerase chain reaction, western blotting and immunohistochemical analysis. The Kaplan-Meier method was used to plot survival curves and the Cox proportional hazards regression model was also used for univariate and multivariate survival analysis. The results revealed that CMTM3 mRNA and protein expression levels were lower in 82.5% (30/40) and 75% (30/40) of ESCC tissues, respectively, when compared with matched non-tumor tissues. Statistical analysis demonstrated that CMTM3 expression was significantly correlated with lymph node metastasis (P=0.002) and clinical stage (P<0.001) in ESCC tissues. Furthermore, the survival time of ESCC patients exhibiting low CMTM3 expression was significantly shorter than that of ESCC patients exhibiting high CMTM3 expression (P=0.01). In addition, Kaplan-Meier survival analysis revealed that the overall survival time of patients exhibiting low CMTM3 expression was significantly decreased compared with patients exhibiting high CMTM3 expression (P=0.010). Cox multivariate analysis indicated that CMTM3 protein expression was an independent prognostic predictor for ESCC after resection. This study indicated that CMTM3 expression is significantly decreased in ESCC tissues and CMTM3 protein expression in resected tumors may present an effective prognostic biomarker.
Keywords: chemokine-like factor-like MARVEL transmembrane domain-containing 3, esophageal squamous cell carcinoma, tumor suppressor, immunohistochemistry, prognostic marker
Introduction
Esophageal cancer is a common malignant tumor that represents the sixth leading cause of cancer-associated mortality, worldwide (1). Esophageal squamous cell carcinoma (ESCC) accounts for >90% of all esophageal cancer cases (2). ESCC is a potentially fatal malignancy that arises from esophageal epithelial cells, that is common in China, with a high global incidence (3). Although diagnosis and treatment of ESCC have improved in recent years, ESCC is frequently diagnosed in patients with advanced stage disease and subsequently, tumors are unresectable (4,5). The identification of early diagnostic and prognostic evaluation markers are required to improve diagnosis and prognosis of ESCC.
The human chemokine-like factor (CKLF)-like MARVEL transmembrane domain-containing (CMTM) family is a novel gene family, consisting of nine genes, CKLF and CMTM1-8 (6,7). CMTM proteins exhibit critical functions in the immune system, male reproductive system and tumorigenesis (8–16). CMTM3, which is a member of the chemokine-like factor gene superfamily, is similar to the chemokine and transmembrane 4 superfamily (TM4SF) of signaling molecules in that they both have 4 transmembrane regions (7). The TM4SF family contains a number of tumor-associated genes, including, TM4SF/plasmolipin, MAL and BENE, which exhibit strong tumor-suppressive functions in esophageal and prostate cancer (17,18). CMTM3, which is located at 16q22.1, has frequently been identified in a number of carcinomas, including prostate tumors, oral squamous cell carcinoma and testicular cancer (11,19,20). Therefore, the present study hypothesized that CMTM3 may act as a tumor suppressor gene (TSG). A previous study demonstrated that CMTM3 was downregulated in 7/18 esophageal cell lines (21). However, the association between CMTM3 expression and the prognosis and clinicopathological features of ESCC patients remains unclear.
The aim of the present study was to investigate association between CMTM3 expression and prognosis in ESCC. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and western blotting were used to analyze CMTM3 mRNA and protein expression levels in 40 ESCC tissues and 40 adjacent non-tumor tissues. Immunohistochemistry was performed to investigate the clinical relevance of CMTM3 expression in an additional 110 ESCC tissues and 36 adjacent non-tumor tissues.
Materials and methods
Human samples
A total of 40 paired ESCC and normal esophageal tissues were obtained from ESCC patients who underwent complete esophageal cancer resection at The First Affiliated Hospital of China Medical University (Shenyang, China) between April 2014 and August 2014. Patients who underwent palliative surgery were not included. The mean age of the ESCC patients was 57.71 years (range, 44–71 years). The fresh tissues were immediately frozen in liquid nitrogen and stored at −80°C prior to RNA and protein extraction. To investigate the association between CMTM3 expression and the clinicopathological characteristics and prognosis of ESCC patients, an additional 110 archived paraffin-embedded specimens and 36 adjacent non-tumor paraffin-embedded tissues, obtained from ESCC patients who underwent complete esophageal cancer resection at the First Affiliated Hospital of China Medical University between May 2008 and April 2010, were included in this study for immunohistochemical (IHC) analysis. None of the 110 patients received neoadjuvant therapy prior to surgery. The mean age of the ESCC patients was 60.24 years (range, 43–80 years). The differentiation grade, tumor-node-metastasis (TNM) stage and lymph node status were classified according to the International Union Against Cancer/American Joint Committee on Cancer TNM classification (7th edition) (22). Clinicopathological data, which included age, gender, tumor location, TNM stage, differentiation and lymph node metastasis, was obtained from medical records (Table I). Overall survival was determined from the date of surgery to the time of death, estimated by the Kaplan Meier method. The present study was approved by the Speciality Committee on Ethics of Biomedicine Research of The First Affiliated Hospital of China Medical University. Written informed consent was obtained from all patients.
Table I.
Associations between CMTM3 expression and clinicopathological features of 110 ESCC patients.
| CMTM3 expression | ||||
|---|---|---|---|---|
| Clinicopathological parameters | Patients, n | Low | High | P-value |
| Age, years | 0.280 | |||
| ≤60 | 48 | 37 | 11 | |
| >60 | 62 | 42 | 20 | |
| Gender | 0.275 | |||
| Male | 97 | 68 | 29 | |
| Female | 13 | 11 | 2 | |
| Location | 0.357 | |||
| Ut | 26 | 19 | 7 | |
| Mt | 32 | 20 | 12 | |
| Lt | 52 | 40 | 12 | |
| Pathological grade | 0.730 | |||
| Moderately/poorly-differentiated | 61 | 43 | 18 | |
| Well-differentiated | 49 | 36 | 13 | |
| Lymph node metastasis | 0.002 | |||
| Negative | 56 | 33 | 23 | |
| Positive | 54 | 46 | 8 | |
| Clinical stage | 0.001 | |||
| I+II | 58 | 33 | 25 | |
| III+IV | 52 | 46 | 6 | |
CMTM3, human chemokine-like factor-like MARVEL transmembrane domain-containing 3; ESCC, esophageal squamous cell carcinoma; Ut, upper thoracic; Mt, middle thoracic; Lt, lower thoracic.
qRT-PCR
Total RNA (2 µg) was extracted from tissues using TaKaRa RNAiso Reagent (Takara Bio, Inc., Otsu, Japan) according to manufacturer's instructions. The quantity and quality of the Extracted RNA were analyzed by spectrophotometer at 260 nm (ND1000; Nanodrop; Thermo Fisher Scientific, Inc., Wilmington, DE, USA). RNA (2 µg) was reverse-transcribed using PrimeScript RT Reagent Kit with gDNA Eraser (Takara Bio, Inc.) to synthesize cDNA. qRT-PCR was performed using SYBR® Premix Ex Taq™ II (Takara Bio, Inc.) and the Corbet Rotor-Gene 3000 thermocycler (Rotor-Gene 3000; Corbett Research, Mortlake, Australia). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as an internal control. The following primers were used: Sense, 5′-GCTTGTGCTGGCCCATGATG-3′ and antisense, 5′-TGTGGGCTGTGGTCTCATCT-3′ for CMTM3; sense, 5′-CTCCTCCTGTTCGACAGTCAGC-3′ and antisense, 5′-CCCAATACGACCAAATCCGTT-3′ for GAPDH. PCR amplification was performed under the following conditions: 95°C for 1 min, followed by 35 cycles at 95°C for 15 sec and 60°C for 1 min. Experiments were performed in triplicate. CMTM3 mRNA expression was quantified relative to that of GAPDH according to the comparative threshold cycle (2−ΔΔCq) method (23).
Western blot analysis
A total of 40 paired ESCC and non-tumor tissue samples were sectioned, homogenized and lysed in RIPA lysis buffer supplemented with 1% (v/v) protease inhibitor cocktail and phenylmethylsulfonyl fluoride (all Beyotime Institute of Biology, Haimen, China). Protein concentrations were determined using the BCA method with an Enhanced BCA Protein Assay kit (P0009; Beyotime Institute of Biotechnology) and a microplate reader (BioTek, Winooski, VT, USA) according to the manufacturer's instructions. Proteins (50 µg/lane) were separated by 12% SDS-PAGE and transferred to polyvinylidine difluoride filter membranes (EMD Millipore, Billerica, MA, USA) in a wet transfer system (Bio-Rad, Berkeley, CA, USA) at 70 V. Next, Tris-buffered saline with Tween-20 (TBST) containing 5% nonfat milk was used to block the membrane for 1 h at room temperature, followed by incubation with CMTM3 (cat. no. ab198016; 1:1,000; Abcam, Cambridge, UK) and GAPDH (cat. no. ab181602; 1:5,000; Abcam) primary antibodies at 4°C overnight. After washing with TBST, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies (cat. no. sc-2004; 1:10,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at room temperature for 1 h. Immunopositive bands were visualized using enhanced chemiluminescence buffer (Beyotime Institute of Biotechnology) and band intensities were quantified using MF-ChemiBIS 2.0 (DNR Bio-Imaging Systems Ltd., Jerusalem, Israel) and Quantity One software (version 4.62; Bio-Rad).
IHC analysis
A total of 110 paraffin-embedded biopsy samples were cut into 4-µm sections, dewaxed, rehydrated through decreasing concentrations of ethanol citrate buffer and heated in 10 mmol/l sodium citrate buffer (pH 6.0) for antigen retrieval (high-pressure heat method for 5 min). After washing in phosphate-buffered saline (Beyotime Institute of Biotechnology), all tissue slides were blocked with 10% normal goat serum for 30 min and incubated with CMTM3 primary antibody (cat. no. ab198016; 1:100; Abcam) in a humidified chamber at 4°C over night. Membranes were then incubated with horseradish peroxidase-conjugated IgG secondary antibodies (1:1; PV-9000; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) at 37°C for 10 min. Next, the sections were incubated with DAB solution (Beyotime Institute of Biotechnology) for 3 min and counterstained with hematoxylin (Beyotime Institute of Biotechnology). The expression of CMTM3 was determined by two independent pathologists blinded to the clinical data. Briefly, staining intensity was scored on a 4-tiered scale (0, no immunostaining; 1, light-brown color; 2, medium-brown color; 3, brown color). The percentage of positively stained cells per field was defined as: 0, ≤5%; 1, 5–25%; 2, 26–50%; 3, >50%. The score for each field was the product of the percentage and intensity scores and the final score for CMTM3 expression in each case was the mean score of five fields: -, 0 points; +, 1–2 points; ++, 3–5 points; +++, 6–9 points. For analysis, the patients were divided into CMTM3 ‘high expression’ (++ and +++) and ‘low expression’ (+ and-) groups. Discrepancies were resolved by discussion between the pathologists.
Statistical analysis
Data are presented as the mean ± standard deviation. All statistical analysis was performed using SPSS 21.0 statistical software (SPSS, Inc., Chicago, IL, USA). Comparison of CMTM3 expression between ESCC tissues and the corresponding adjacent non-tumor tissues was performed using the paired Student's t-test. χ2 test was used to analyze the correlation between CMTM3 expression and patient clinicopathological features. The Kaplan Meier method was used to plot survival curves and results were analyzed using the log-rank test. Cox proportional hazards regression model was used for univariate and multivariate survival analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
CMTM3 mRNA expression levels are decreased in ESCC tissues compared with adjacent non-tumor tissues
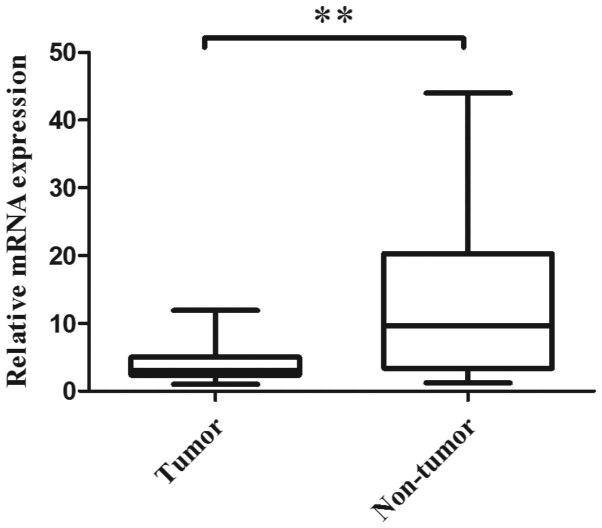
qRT-PCR was performed to evaluate the CMTM3 mRNA expression levels in 40 paired normal and ESCC tumor tissues. The results revealed that 82.5% (33/40) of tumor tissues expressed lower CMTM3 mRNA levels than their adjacent non-tumor tissues (P<0.001; Fig. 1). The mean relative expression levels of CMTM3 mRNA in the tumor and non-tumor tissues were 4.01 and 13.09, respectively. The results indicated that CMTM3 mRNA expression is significantly decreased in tumor tissues when compared with the adjacent non-tumor tissues.
Figure 1.
CMTM3 mRNA expression levels were analyzed in 40 ESCC specimens and 40 adjacent non-tumor tissues. The expression level of CMTM3 mRNA expression was significantly decreased in tumor tissue compared with the paired non-tumor tissue. **P<0.001. Experiments were performed in triplicate. Data are presented as the mean ± standard deviation. CMTM3, human chemokine-like factor-like MARVEL transmembrane domain-containing 3; ESCC, esophageal squamous cell carcinoma.
CMTM3 protein expression levels are decreased in ESCC tissues compared with adjacent non-tumor tissues
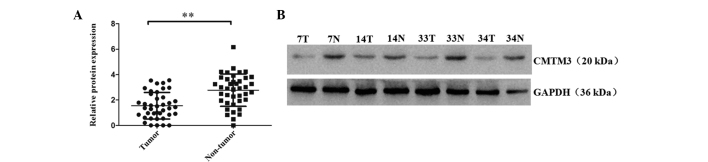
CMTM3 protein levels in 40 paired of ESCC tumor and adjacent non-tumor tissues were analyzed by western blot analysis. The results revealed that 75% (30/40) of tumor tissues expressed lower CMTM3 protein expression levels than their adjacent non-tumor tissues (P<0.001; Fig. 2). The mean relative expression levels of CMTM3 protein in the tumor and non-tumor tissues were 1.55 and 2.77, respectively. These results indicated that CMTM3 protein expression is significantly decreased in ESCC tumor tissue when compared with the paired adjacent non-tumor tissues. Furthermore, low CMTM3 expression may be important in the tumorigenesis of ESCC.
Figure 2.
Western blot analysis of CMTM3 protein expression in ESCC and non-tumor tissues. (A) CMTM3 protein expression levels were analyzed in 40 ESCC and 40 adjacent non-tumor tissues. CMTM3 protein expression was significantly decreased in tumor tissues compared with non-tumor tissues. **P<0.001. Experiments were performed in triplicate. (B) CMTM3 protein expression in four representative pairs of ESCC tumor and adjacent non-tumor tissues. CMTM3 protein expression was decreased in tumor tissue compared with the paired non-tumor tissue. CMTM3, human chemokine-like factor-like MARVEL transmembrane domain-containing 3; ESCC, esophageal squamous cell carcinoma; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
CMTM3 protein expression is associated with lymph node metastasis and clinical stage in ESCC tissues
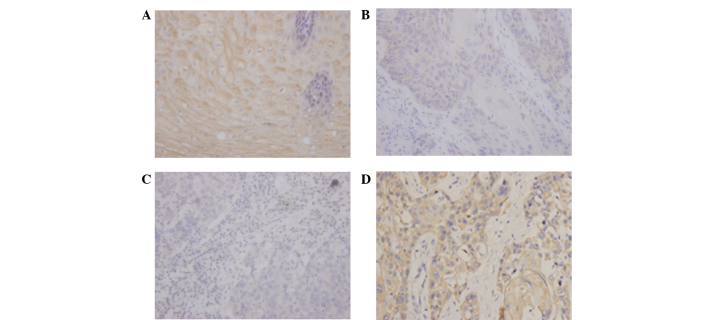
To investigate the association between CMTM3 protein expression and clinicopathological parameters, a total of 110 tissues were obtained from 110 ESCC patients. According to the level of CMTM3 expression in tumor tissues as determined by immunohistochemistry analysis (Fig. 3), the ESCC patients were divided into low and high expression groups. No significant associations were identified between CMTM3 expression and age (P=0.280), gender (P=0.275), tumor location (P=0.357) or pathological grade (P=0.730), however, CMTM3 expression was associated with lymph node metastasis (P=0.002) and clinical stage (P<0.001) in ESCC tissues (Table I).
Figure 3.
Immunohistochemical analysis of CMTM3 expression in ESCC tissue and paired non-tumor tissue. (A) High CMTM3 expression in non-tumor tissue at ×400 magnification. (B) Low CMTM3 expression in well-differentiated ESCC tissue at ×400 magnification. (C) Low CMTM3 expression in poorly-differentiated ESCC at ×400 magnification. (D) High CMTM3 expression in ESCC tissue at ×400 magnification CMTM3, human chemokine-like factor-like MARVEL transmembrane domain-containing 3; ESCC, esophageal squamous cell carcinoma.
Survival time of patients exhibiting low CMTM3 expression is significantly shorter than patients exhibiting high CMTM3 expression in ESCC
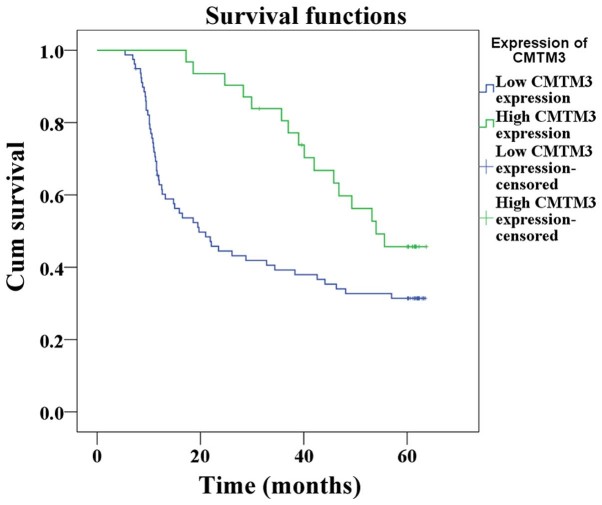
The estimated survival time was calculated according to the Kaplan-Meier method. The median duration of follow up was 35.05 months (range, 5–64 months). Patients that succumbed as a result of postoperative complications were not included in the study. The results revealed that the survival time of the low CMTM3 expression group was significantly shorter than that of the high CMTM3 expression group (32.9 vs. 48.4 %P=0.010; Fig. 4).
Figure 4.
Overall survival curves for ESCC patients exhibiting low and high CMTM3 expression. The survival time of low CMTM3 expression patients (n=79) was significantly shorter than that of high CMTM3 expression patients (n=31) (P=0.010). CMTM3, human chemokine-like factor-like MARVEL transmembrane domain-containing 3; ESCC, esophageal squamous cell carcinoma.
Cox regression analysis was carried out to assess whether CMTM3 is an independent prognostic factor for survival in ESCC. Univariate analysis revealed that patient overall survival was significantly associated with pathological grade (P=0.012), lymph node metastasis (P<0.001), clinical stage (P<0.001) and CMTM3 expression (P=0.012). Multivariate analysis revealed that CMTM3 expression (P=0.036), pathological grade (P=0.001), clinical stage (P<0.001) and lymph node metastasis (P=0.001) were independent prognostic factors for overall survival in ESCC patients (Table II).
Table II.
Univariate and multivariate analysis of overall survival in 110 ESCC patients.
| Univariate analysis | Multivariate analysis | ||
|---|---|---|---|
| Variables | P-value | Hazard ratio (95% CI) | P-value |
| Age, years | 0.726 | 0.897 | |
| ≤60 | 1 | ||
| >60 | 0.967 (0.580–1.580) | ||
| Gender | 0.413 | 0.287 | |
| Male | 1 | ||
| Female | 0.727 (0.353–1.353) | ||
| Location | 0.903 | ||
| Ut | 0.901 | 1 | |
| Mt | 0.918 | 1.065 (0.583–1.583) | 0.838 |
| Lt | 0.704 | 1.059 (0.579–1.579) | 0.853 |
| Pathological grade | 0.012 | 0.001 | |
| Well-differeniated | 1 | ||
| Moderately/poorly-differentiated | 2.594 (1.516–4.516) | ||
| Lymph node metastasis | <0.001 | 0.001 | |
| Negative | 1 | ||
| Positive Clinical | 2.863 (1.574–5.574) | ||
| Clinical stage | <0.001 | <0.001 | |
| IA-IIB | 1 | ||
| IIIA-IV | 3.710 (1.994–6.994) | ||
| CMTM3 expression | 0.012 | 0.036 | |
| High | 1 | ||
| Low | 1.961 (1.046–3.046) | ||
CMTM3, human chemokine-like factor-like MARVEL transmembrane domain-containing 3; ESCC, esophageal squamous cell carcinoma; Ut, upper thoracic; Mt, middle thoracic; Lt, lower thoracic; CI, confidence interval.
Discussion
CMTM3, which is a member of the CMTM family that was first identified by Han et al (7) in 2003. CMTM3 is located at 16q22.1, an important tumor suppressor locus that is associated with the pathogenesis of multiple carcinomas (24–26). Previous studies have demonstrated that CMTM3 is a candidate tumor suppressor gene in a number of tumors, such as renal cell carcinoma (10), testicular cancer (11), gastric cancer (13) and oral squamous cell carcinoma (19). Previously CMTM3 was demonstrated to be silenced or downregulated in 7/18 esophageal cell lines (21), however, CMTM3 expression in ESCC and its association with prognosis remains unknown.
In the present study, qRT-PCR revealed that 82.5% (33/40) of ESCC tissues expressed lower levels of CMTM3 mRNA expression compared with adjacent non-tumor tissues. Consistent with these results, western blot analysis revealed that 75% (30/40) of ESCC tissues expressed lower levels of CMTM3 protein compared with adjacent non-tumor tissues. Furthermore, IHC analysis was performed to analyze associations between CMTM3 expression and clinicopathological features in 110 ESCC patients. No significant association was identified between CMTM3 expression and patient age (P=0.280), gender (P=0.275), tumor location (P=0.357) or pathological grade (P=0.730), however, CMTM3 expression was significantly associated with lymph node metastasis (P=0.002) and clinical stage (P<0.001) in ESCC tissues. IHC analysis also revealed that of the 110 ESCC samples, 79 cases (71.82%) exhibited low CMTM3 expression and 31 cases (28.18%) exhibited high CMTM3 expression. Of the 36 adjacent non-tumor tissues, 27 cases (75%) exhibited high CMTM3 expression and 9 cases (25%) exhibited low CMTM3 expression. These results indicate that CMTM3 may be involved in the progression of ESCC and may act as a tumor suppressor in ESCC. In the present study, the correlation between CMTM3 expression and patient prognosis was evaluated in ESCC patients. Kaplan-Meier survival analysis revealed that the overall survival time of patients with low CMTM3 expression was significantly shorter than patients with high CMTM3 expression (P=0.010). Cox multivariate analysis indicated that CMTM3 protein expression was an independent prognostic predictor for ESCC after resection.
CpG methylation resulting in the loss of TSG functions is a major epigenetic alteration that leads to tumor development and progression (27). The CMTM3 promoter contains a typical CpG island consisting of 53 CpG sites, which is methylated by the addition of a methyl group via DNA methyltransferase enzymes (21). However, a previous study reported that CMTM3 was methylated in 3% of esophageal carcinomas (21). Consistent with these results, methylation of the promoter region of CMTM3 is not observed in renal cell carcinoma (10). Thus, it may be hypothesized that unlike the aberrant methylation observed in tumors such as oral squamous cell carcinoma (20), hepatocellular carcinoma (28) and gastric cancer (21), in ESCC low expression of CMTM3 may be associated with other genetic or epigenetic mechanisms. The results of the present indicated that CMTM3 is expressed in a number of ESCC tissues, however, its function in ESCC cell lines remains unclear. Thus, further studies are required to increase understanding with regard to the function of CMTM3 in ESCC cells.
In conclusion, in the present study CMTM3 expression was significantly decreased in ESCC tissue compared with adjacent non-tumor tissue. Furthermore, this study is the first to indicate that CMTM3 protein expression in resected tumors is an effective prognostic biomarker for ESCC.
Acknowledgements
This study was supported by the National Nature Science Foundation of China (grant no. 81201890) and the Natural Science Foundation of Liaoning Province (grant no. 2013021002).
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol. 2009;24:729–735. doi: 10.1111/j.1440-1746.2009.05824.x. [DOI] [PubMed] [Google Scholar]
- 3.Sun ZG, Wang Z, Liu XY, Liu FY. Mucin 1 and vascular endothelial growth factor C expression correlates with lymph node metastatic recurrence in patients with N0 esophageal cancer after Ivor-Lewis esophagectomy. World J Surg. 2011;35:70–77. doi: 10.1007/s00268-010-0829-1. [DOI] [PubMed] [Google Scholar]
- 4.Thallinger CM, Kiesewetter B, Raderer M, Hejna M. Pre- and postoperative treatment modalities for esophageal squamous cell carcinoma. Anticancer Res. 2012;32:4609–4627. [PubMed] [Google Scholar]
- 5.Kranzfelder M, Büchler P, Friess H. Surgery within multimodal therapy concepts for esophageal squamous cell carcinoma (ESCC): The MRI approach and review of the literature. Adv Med Sci. 2009;54:158–169. doi: 10.2478/v10039-009-0044-1. [DOI] [PubMed] [Google Scholar]
- 6.Han W, Lou Y, Tang J, Zhang Y, Chen Y, Li Y, Gu W, Huang J, Gui L, Tang Y, et al. Molecular cloning and characterization of chemokine-like factor 1 (CKLF1), a novel human cytokine with unique structure and potential chemotactic activity. Biochem J. 2001;357:127–135. doi: 10.1042/0264-6021:3570127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han W, Ding P, Xu M, Wang L, Rui M, Shi S, Liu Y, Zheng Y, Chen Y, Yang T, Ma D. Identification of eight genes encoding chemokine-like factor superfamily members 1–8 (CKLFSF1-8) by in silico cloning and experimental validation. Genomics. 2003;81:609–617. doi: 10.1016/S0888-7543(03)00095-8. [DOI] [PubMed] [Google Scholar]
- 8.Imamura Y, Katahira T, Kitamura D. Identification and characterization of a novel BASH N terminus-associated protein, BNAS2. J Biol Chem. 2004;279:26425–26432. doi: 10.1074/jbc.M403685200. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Li T, Qiu X, Mo X, Zhang Y, Song Q, Ma D, Han W. CMTM3 can affect the transcription activity of androgen receptor and inhibit the expression level of PSA in LNCaP cells. Biochem Biophys Res Commun. 2008;371:54–58. doi: 10.1016/j.bbrc.2008.03.143. [DOI] [PubMed] [Google Scholar]
- 10.Xie J, Yuan Y, Liu Z, Xiao Y, Zhang X, Qin C, Sheng Z, Xu T, Wang X. CMTM3 is frequently reduced in clear cell renal cell carcinoma and exhibits tumor suppressor activities. Clin Transl Oncol. 2014;16:402–409. doi: 10.1007/s12094-013-1092-3. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Xie J, Wu J, Li W, Nie L, Sun X, Tang A, Li X, Liu R, Mei H, et al. CMTM3 inhibits human testicular cancer cell growth through inducing cell-cycle arrest and apoptosis. PLoS One. 2014;9:e88965. doi: 10.1371/journal.pone.0088965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dworakowska D, Wlodek E, Leontiou CA, Igreja S, Cakir M, Teng M, Prodromou N, Góth MI, Grozinsky-Glasberg S, Gueorguiev M, et al. Activation of RAF/MEK/ERK and PI3K/AKT/mTOR pathways in pituitary adenomas and their effects on downstream effectors. Endocr Relat Cancer. 2009;16:1329–1338. doi: 10.1677/ERC-09-0101. [DOI] [PubMed] [Google Scholar]
- 13.Su Y, Lin Y, Zhang L, Liu B, Yuan W, Mo X, Wang X, Li H, Xing X, Cheng X, et al. CMTM3 inhibits cell migration and invasion and correlates with favorable prognosis in gastric cancer. Cancer Sci. 2014;105:26–34. doi: 10.1111/cas.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao L, Cui Y, Li H, Liu Y, Zhao H, Wang Y, Zhang Y, Ng KM, Han W, Ma D, Tao Q. CMTM5 exhibits tumor suppressor activities and is frequently silenced by methylation in carcinoma cell lines. Clin Cancer Res. 2007;13:5756–5762. doi: 10.1158/1078-0432.CCR-06-3082. [DOI] [PubMed] [Google Scholar]
- 15.Chowdhury MH, Nagai A, Terashima M, Sheikh A, Murakawa Y, Kobayashi S, Yamaguchi S. Chemokine-like factor expression in the idiopathic inflammatory myopathies. Acta Neurol Scand. 2008;118:106–114. doi: 10.1111/j.1600-0404.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- 16.Li D, Jin C, Yin C, Zhang Y, Pang B, Tian L, Han W, Ma D, Wang Y. An alternative splice form of CMTM8 induces apoptosis. Int J Biochem Cell Biol. 2007;39:2107–2119. doi: 10.1016/j.biocel.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Mimori K, Shiraishi T, Mashino K, Sonoda H, Yamashita K, Yoshinaga K, Masuda T, Utsunomiya T, Alonso MA, Inoue H, Mori M. MAL gene expression in esophageal cancer suppresses motility, invasion and tumorigenicity and enhances apoptosis through the Fas pathway. Oncogene. 2003;22:3463–3471. doi: 10.1038/sj.onc.1206378. [DOI] [PubMed] [Google Scholar]
- 18.Zhang XA, He B, Zhou B, Liu L. Requirement of the p130CAS-Crk coupling for metastasis suppressor KAI1/CD82-mediated inhibition of cell migration. J Biol Chem. 2003;278:27319–27328. doi: 10.1074/jbc.M303039200. [DOI] [PubMed] [Google Scholar]
- 19.Hu F, Yuan W, Wang X, Sheng Z, Yuan Y, Qin C, He C, Xu T. CMTM3 is reduced in prostate cancer and inhibits migration, invasion and growth of LNCaP cells. Clin Transl Oncol. 2015;17:632–639. doi: 10.1007/s12094-015-1288-9. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Zhang J, Nan X, Li X, Qu J, Hong Y, Sun L, Chen Y, Li T. CMTM3 inhibits cell growth and migration and predicts favorable survival in oral squamous cell carcinoma. Tumour Biol. 2015;36:7849–7858. doi: 10.1007/s13277-015-3504-1. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Li J, Cui Y, Li T, Ng KM, Geng H, Li H, Shu XS, Li H, Liu W, et al. CMTM3, located at the critical tumor suppressor locus 16q22.1, is silenced by CpG methylation in carcinomas and inhibits tumor cell growth through inducing apoptosis. Cancer Res. 2009;69:5194–5201. doi: 10.1158/0008-5472.CAN-08-3694. [DOI] [PubMed] [Google Scholar]
- 22.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 23.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 24.Caldeira JR, Prando EC, Quevedo FC, Neto FA, Rainho CA, Rogatto SR. CDH1 promoter hypermethylation and E-cadherin protein expression in infiltrating breast cancer. BMC Cancer. 2006;6:48. doi: 10.1186/1471-2407-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan KY, Lai PB, Squire JA, Beheshti B, Wong NL, Sy SM, Wong N. Positional expression profiling indicates candidate genes in deletion hotspots of hepatocellular carcinoma. Mod Pathol. 2006;19:1546–1554. doi: 10.1038/modpathol.3800674. [DOI] [PubMed] [Google Scholar]
- 26.Li C, Berx G, Larsson C, Auer G, Aspenblad U, Pan Y, Sundelin B, Ekman P, Nordenskjöld M, van Roy F, Bergerheim US. Distinct deleted regions on chromosome segment 16q23-24 associated with metastases in prostate cancer. Genes Chromosomes Cancer. 1999;24:175–182. doi: 10.1002/(SICI)1098-2264(199903)24:3<175::AID-GCC1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 27.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 28.Zhao W, Xu Y, Xu J, Wu D, Zhao B, Yin Z, Wang X. Subsets of myeloid-derived suppressor cells in hepatocellular carcinoma express chemokines and chemokine receptors differentially. Int Immunopharmacol. 2015;26:314–321. doi: 10.1016/j.intimp.2015.04.010. [DOI] [PubMed] [Google Scholar]