Abstract
Cyclophilins (CYPs) are a group of ubiquitous proteins characterized by their ability to bind to the immunosuppressive drug cyclosporin A. The CYP family occurs in a wide range of organisms and contains a conserved peptidyl-prolyl cis/trans isomerase domain. In addition to fulfilling a basic role in protein folding, CYPs may also play diverse important roles, e.g. in protein degradation, mRNA processing, development, and stress responses. We performed a genome-wide database survey and identified a total of 94 CYP genes encoding 91 distinct proteins. Sequence alignment analysis of the putative BnCYP cyclophilin-like domains revealed highly conserved motifs. By using RNA-Seq, we could verify the presence of 77 BnCYP genes under control conditions. To identify phloem-specific BnCYP proteins in a complementary approach, we used LC-MS/MS to determine protein abundances in leaf and phloem extracts. We detected 26 BnCYPs in total with 12 being unique to phloem sap. Our analysis provides the basis for future studies concentrating on the functional characterization of individual members of this gene family in a plant of dual importance: as a crop and a model system for polyploidization and long-distance signalling.
Introduction
As one of the most important crops for nutritional oil, fodder, biodiesel, chemical and pharmaceutical products, Brassica napus (oilseed rape or canola) is widespread in agriculture, especially in the European Union, China, and Canada. Beside its essential agricultural significance, B. napus has also become a model plant for studying long-distance signalling1–5 and evolutionary consequences of polyploidization6, 7. Brassica napus is used as a model plant for studying long-distance signalling, because methods for the collection of sufficient quantities of pure xylem and phloem sap are well established2, 4. Compared to crops like wheat, soybean or rice, the domestication of B. napus was more recent. It is assumed that chromosome doubling occurred after spontaneous hybridization between Brassica rapa (Asian cabbage or turnip rape, 2n = 2 × 10 = 20, genome AA) and Brassica oleracea (Mediterranean cabbage, 2n = 2 × 9 = 18, genome CC)8. The profitable cultivation of B. napus (genome AACC, 2n = 38) has high importance for the economy9.
A group of proteins involved in diverse fundamental cellular functions in many different organisms is called immunophilins, originally discovered as receptors for immunosuppressive drugs in mammals. The family of immunophilins consists of two major groups, FK506-binding proteins (FKBPs)10, 11 and cyclophilins (CYPs)12, 13. Despite the lack of sequence and structure similarity, FKBPs and CYPs each possess a conserved domain responsible for their common peptidyl-prolyl cis/trans isomerase (PPIase) activity, catalyzing the rate-limiting rotation of X-proline peptide bonds from a cis to a trans conformation14. These domains are called the FKBP and cyclophilin-like domain (CLD), respectively. An additional group of proteins exhibiting PPIase activity, the parvulins, cannot be classified as immunophilins in a strict sense, since they do not bind to any known immunosuppressant molecule15. The drugs of CYPs and FKBPs are cyclosporin A (CsA) and FK506/rapamycin, respectively, that bind to the catalytic pocket of the PPIase domain16, thereby inhibiting its activity and forming a high-affinity binding site for the interaction with calcineurin17. However, these drugs do not occur naturally in cells, therefore the consequences of drug treatment have clinical but no physiological relevance18.
Human CYPs were first characterized by their ability to bind the drug cyclosporin A in 198412. The first plant CYPs were described in 1990, where CYP cDNA sequences were identified in tomato (Lycopersicon esculentum), maize (Zea mays), and oilseed rape (B. napus)19. The ubiquitous CYPs show highly conserved structural features20 and are involved in several fundamental cellular functions, including protein folding21–23, protein trafficking24, signalling25–29, pathogen response30, apoptosis31, RNA-binding, regulation of gene expression or transcription32–36, and plant stress responses37–40.
Interestingly, CYPs were found to be a prominent and abundant class of proteins in the phloem long-distance system of higher plants including castor bean and oilseed rape2, 41. Phloem sieve elements lose their ability for transcription and translation during their maturation into transport tubes42. However, phloem exudate contains a complex set of proteins, some of which have been implicated with long-distance signalling43. Such signalling proteins are imported from the adjacent companion cells through plasmodesmata. It was proposed that phloem CYPs might act as molecular chaperones in this process, potentially involved in refolding of non-cell-autonomous proteins after entry into the translocation stream2, 41.
The growing number of sequenced genomes allowed the identification of whole sets of CYPs in various organisms by sequence comparisons. 19 CYPs were detected in the human genome, whereas Saccharomyces cerevisiae possesses 8, Schizosaccharomyces pombe 9, Caenorhabditis elegans 17, Drosophila melanogaster 1444, 45, and the fungus Leptosphaeria maculans 1246 CYPs. Compared to other organisms, plants have a higher number of CYPs with 29 encoded in A. thaliana 47–49, and 27 in rice37. To date, soybean (Glycine max) is reported to have the largest set of CYPs with 62 members50.
Based on the recently sequenced B. napus genome51, the major aim of the present study was the identification and classification of CYP-like proteins in this economically important species. A total number of 94 genes belonging to the CYP gene family (resulting in 91 different CYP proteins) could be identified in the sequenced B. napus cultivar ‘Darmor-bzh’. By applying transcriptome analysis of the B. napus cultivar ‘Drakkar’ we could confirm the expression of 77 BnCYP genes in leaves. To identify CYP proteins specifically occurring in phloem sap, we performed complementary protein analyses by LC-MS/MS on leaf and phloem extracts and found 26 different BnCYP proteins in total, 12 only present in phloem sap.
Results and Discussion
Identification of cyclophilins in the Brassica napus genome
Putative CYPs of B. napus containing full length or partial CYP-like domains were identified by BLASTp of A. thaliana CYPs47, 48. The 94 B. napus (cultivar ‘Darmor-bzh’) gene sequences determined by this approach resulted in 91 distinct proteins which were subjected to additional analysis like sequence alignments with the respective Arabidopsis homologs and the verification of CLDs. The analysis showed that B. napus contains the largest CYP gene family known to date with 94 genes followed by soybean (Glycine max) with 62 members50.
As proposed by Romano et al.47 and He et al.48 we used the nomenclature BnCYP (Brassica napus cyclophilin) followed by the molecular weight and a consecutive number for genes encoding proteins with similar molecular weight. We used the molecular weight of the immature proteins as the basis, because the prediction of potential cleavable signal peptides by different prediction tools was not unambiguous. Table 1 summarizes the information about all identified BnCYPs.
Table 1.
Nomenclature, gene name, accessions, molecular weight, protein sequence length, information about aligned amino acids, theoretical isoelectric point, and predicted exons of the B. napus CYP family.
| Name | Gene Name | GenBank Accession | kDa | aa | CLD | pI (theoretical) | Exons |
|---|---|---|---|---|---|---|---|
| BnCYP5 | BnaA01g25420D | CDY37322.1 | 5.7 | 49 | 2–42 | 8.7 | 1 |
| BnCYP7-1 | BnaA09g37110D | CDY06069.1 | 7.2 | 66 | 1–66 | 10.7 | 3 |
| BnCYP7-2 | BnaA01g36790D | CDY60385.1 | 7.3 | 64 | 1–61 | 4.5 | 1 |
| BnCYP7-3 | BnaA01g36800D | CDY60386.1 | 7.3 | 65 | 1–64 | 4.9 | 1 |
| BnCYP8 | BnaCnng42430D | CDY63671.1 | 8.2 | 71 | 9–65 | 9.1 | 2 |
| BnCYP10-1 | BnaC02g14160D | CDX96235.1 | 10.1 | 92 | 6–55 | 4.7 | 2 |
| BnCYP10-2 | BnaC06g10280D | CDY30664.1 | 10.5 | 94 | 1–68 | 6.7 | 2 |
| BnCYP12-1 | BnaC02g10590D | CDY02830.1 | 12.5 | 109 | 52–96 | 9.8 | 5 |
| BnCYP12-2 | BnaAnng41240D | CDY72459.1 | 12.8 | 117 | 43–116 | 9.0 | 3 |
| BnCYP13 | BnaA02g07550D | CDY32693.1 | 13.1 | 116 | 8–64 | 8.3 | 4 |
| BnCYP14-1 | BnaC04g41450D | CDX97794.1 | 14.0 | 127 | 1–123 | 5.6 | 1 |
| BnCYP14-2 | BnaC04g41430D | CDX97796.1 | 14.2 | 127 | 1–124 | 5.8 | 1 |
| BnCYP14-3 | BnaC04g41440D | CDX97795.1 | 14.2 | 127 | 1–123 | 6.0 | 1 |
| BnCYP14-4 | BnaC08g28870D | CDX71987.1 | 14.3 | 131 | 1–109 | 6.1 | 1 |
| BnCYP14-5 | BnaC09g34640D | CDX80278.1 | 14.4 | 133 | 84–132 | 9.4 | 3 |
| BnCYP14-6 | BnaA01g25470D | CDY37327.1 | 14.9 | 138 | 2–137 | 4.6 | 3 |
| BnCYP16 | BnaA02g10200D | CDY28828.1 | 16.1 | 148 | 3–124 | 4.4 | 3 |
| BnCYP17-1 | BnaA01g25460D | CDY37326.1 | 17.3 | 161 | 68–134 | 7.7 | 3 |
| BnCYP18-1 | BnaC04g09170D/BnaA05g08140D | CDX75068.1/CDX84155.1 | 18.2 | 164 | 7–163 | 8.9 | 5 |
| BnCYP18-2 | BnaC01g03590D | CDX69094.1 | 18.2 | 172 | 1–171 | 8.9 | 1 |
| BnCYP18-3 | BnaA01g02340D | CDX75468.1 | 18.2 | 172 | 1–172 | 8.9 | 1 |
| BnCYP18-4 | BnaA08g16920D/BnaC03g60160D | CDX90292.1/CDY12100.1 | 18.3 | 172 | 2–172 | 8.3 | 1 |
| BnCYP18-5 | BnaA09g08780D | CDY47469.1 | 18.4 | 171 | 1–171 | 8.3 | 1 |
| BnCYP18-6 | BnaA06g37360D | CDY22414.1 | 18.4 | 172 | 2–171 | 8.3 | 1 |
| BnCYP18-7 | BnaC07g47630D | CDX72741.1 | 18.4 | 172 | 2–171 | 8.3 | 1 |
| BnCYP18-8 | BnaC09g09060D | CDY26779.1 | 18.4 | 171 | 1–171 | 8.3 | 1 |
| BnCYP19-1 | BnaA09g35540D | CDY27248.1 | 19.6 | 182 | 1–174 | 7.7 | 1 |
| BnCYP19-2 | BnaC08g26990D | CDX72175.1 | 19.7 | 183 | 1–174 | 7.0 | 1 |
| BnCYP20 | BnaA01g36700D | CDY70394.1 | 20.9 | 192 | 15–191 | 8.2 | 1 |
| BnCYP21-1 | BnaCnng08980D | CDY38037.1 | 21.0 | 193 | 22–191 | 9.2 | 5 |
| BnCYP21-2 | BnaC04g06640D | CDX91486.1 | 21.6 | 200 | 23–200 | 8.2 | 7 |
| BnCYP21-3 | BnaAnng15590D | CDY58941.1 | 21.7 | 201 | 28–199 | 8.9 | 6 |
| BnCYP21-4 | BnaC03g12390D | CDX71155.1 | 21.7 | 201 | 26–199 | 9.3 | 7 |
| BnCYP21-5 | BnaC04g54560D | CDY55458.1 | 21.7 | 201 | 28–199 | 8.9 | 6 |
| BnCYP21-6 | BnaAnng17350D | CDY61026.1 | 21.8 | 201 | 26–199 | 9.3 | 7 |
| BnCYP21-7 | BnaA05g06380D | CDX74892.1 | 21.8 | 204 | 27–204 | 8.2 | 7 |
| BnCYP21-8 | BnaC04g45890D | CDX93307.1 | 21.9 | 205 | 28–205 | 8.2 | 6 |
| BnCYP21-9 | BnaC09g34020D/BnaA10g29520D | CDX80340.1/CDY52123.1 | 22.0 | 204 | 31–202 | 8.9 | 7 |
| BnCYP22-1 | BnaCnng32070D | CDY57430.1 | 22.0 | 191 | 133–176 | 6.5 | 4 |
| BnCYP22-2 | BnaA04g22160D | CDY18371.1 | 22.3 | 207 | 26–207 | 8.1 | 7 |
| BnCYP22-3 | BnaC04g41460D | CDX97793.1 | 23.0 | 215 | 55–212 | 5.7 | 2 |
| BnCYP24-1 | BnaC08g26840D | CDX72190.1 | 24.0 | 224 | 53–222 | 6.5 | 8 |
| BnCYP24-2 | BnaA09g35470D | CDY27255.1 | 24.0 | 224 | 53–222 | 6.5 | 8 |
| BnCYP24-3 | BnaA10g12350D | CDY33029.1 | 24.3 | 222 | 86–140 | 9.2 | 4 |
| BnCYP24-4 | BnaA08g10930D | CDX76307.1 | 24.4 | 223 | 42–215 | 6.5 | 7 |
| BnCYP24-5 | BnaC01g03530D | CDX69100.1 | 24.6 | 224 | 44–215 | 7.1 | 7 |
| BnCYP24-6 | BnaA01g02260D | CDX75476.1 | 24.6 | 224 | 43–215 | 7.1 | 7 |
| BnCYP24-7 | BnaC03g65620D | CDY17501.1 | 24.6 | 225 | 44–217 | 6.2 | 7 |
| BnCYP25-1 | BnaA08g20010D | CDY46429.1 | 25.4 | 226 | 26–198 | 8.4 | 8 |
| BnCYP25-2 | BnaC08g48540D | CDY52765.1 | 25.5 | 226 | 23–198 | 8.4 | 8 |
| BnCYP25-3 | BnaA09g29400D | CDY16206.1 | 25.6 | 226 | 20–200 | 6.5 | 8 |
| BnCYP25-4 | BnaA03g21710D | CDX83335.1 | 25.9 | 231 | 79–230 | 8.9 | 6 |
| BnCYP25-5 | BnaC03g25990D | CDX95718.1 | 25.9 | 231 | 79–230 | 8.9 | 6 |
| BnCYP26-1 | BnaA05g30740D | CDY24675.1 | 26.3 | 234 | 74–230 | 8.7 | 7 |
| BnCYP26-2 | BnaA08g08200D | CDX76580.1 | 26.3 | 239 | 151–213 | 6.5 | 4 |
| BnCYP26-3 | BnaC05g45190D | CDY05284.1 | 26.3 | 234 | 74–230 | 9.0 | 7 |
| BnCYP26-4 | BnaA04g17840D | CDY29643.1 | 26.3 | 248 | 77–245 | 6.1 | 1 |
| BnCYP26-5 | BnaCnng32180D | CDY57615.1 | 26.5 | 236 | 76–232 | 8.7 | 7 |
| BnCYP27-1 | BnaC05g44950D | CDY05308.1 | 27.1 | 247 | 6–200 | 4.9 | 3 |
| BnCYP27-2 | BnaC08g31970D | CDX76611.1 | 27.4 | 253 | 81–249 | 8.7 | 7 |
| BnCYP27-3 | BnaC03g05790D | CDX70495.1 | 27.8 | 257 | 80–252 | 9.6 | 7 |
| BnCYP27-4 | BnaA09g39610D | CDY11395.1 | 27.9 | 258 | 86–254 | 9.0 | 7 |
| BnCYP27-5 | BnaA03g04200D | CDX78508.1 | 27.9 | 256 | 79–251 | 9.7 | 7 |
| BnCYP28-1 | BnaA04g27460D | CDY58761.1 | 28.1 | 258 | 86–254 | 8.8 | 7 |
| BnCYP28-2 | BnaC04g21530D | CDX93994.1 | 28.6 | 261 | 89–257 | 8.9 | 7 |
| BnCYP30-1 | BnaC08g08050D | CDY12400.1 | 30.4 | 280 | 68–267 | 6.3 | 2 |
| BnCYP30-2 | BnaA08g07170D | CDY41762.1 | 30.5 | 281 | 75–270 | 5.9 | 2 |
| BnCYP31 | BnaC07g00280D | CDY05775.1 | 32.0 | 277 | 59-173 | 4.8 | 7 |
| BnCYP34-1 | BnaC02g22620D | CDY45858.1 | 34.5 | 320 | 92–314 | 9.1 | 3 |
| BnCYP34-2 | BnaA02g16680D | CDY49138.1 | 34.5 | 320 | 91–314 | 9.1 | 3 |
| BnCYP37-1 | BnaC01g21230D | CDY35601.1 | 37.4 | 340 | 161–319 | 6.1 | 5 |
| BnCYP37-2 | BnaA01g17950D | CDY42959.1 | 37.5 | 340 | 161–316 | 6.6 | 5 |
| BnCYP40-1 | BnaC07g05260D | CDX71247.1 | 40.3 | 361 | 3–175 | 6.3 | 8 |
| BnCYP40-2 | BnaA07g04040D | CDY36653.1 | 40.3 | 361 | 3–175 | 6.0 | 8 |
| BnCYP47-1 | BnaC08g10870D | CDY43466.1 | 47.1 | 418 | 157–312 | 8.2 | 7 |
| BnCYP47-2 | BnaA05g33830D | CDY37518.1 | 47.2 | 433 | 254–433 | 5.1 | 7 |
| BnCYP47-3 | BnaC05g48850D | CDY49057.1 | 47.4 | 436 | 257–436 | 5.0 | 6 |
| BnCYP49 | BnaA05g24020D | CDX97677.1 | 49.8 | 459 | 273–438 | 6.6 | 12 |
| BnCYP50 | BnaC05g38110D | CDX98564.1 | 51.0 | 470 | 284–445 | 5.7 | 12 |
| BnCYP52 | BnaC01g05170D | CDX68936.1 | 52.4 | 467 | 6–186 | 8.6 | 11 |
| BnCYP55 | BnaA01g03810D | CDX75321.1 | 55.3 | 492 | 6–185 | 7.7 | 10 |
| BnCYP62 | BnaAnng12550D | CDY53714.1 | 62.0 | 556 | 4–177 | 10.7 | 13 |
| BnCYP65-1 | BnaA06g24990D | CDY08725.1 | 65.0 | 597 | 335–508 | 8.0 | 11 |
| BnCYP65-2 | BnaC03g48580D | CDY36499.1 | 65.1 | 597 | 335–508 | 7.3 | 11 |
| BnCYP67 | BnaC04g20680D | CDX93909.1 | 67.3 | 612 | 77–251 | 10.6 | 15 |
| BnCYP70-1 | BnaC03g54740D | CDY08708.1 | 70.1 | 622 | 465–619 | 6.0 | 13 |
| BnCYP70-2 | BnaA06g18830D | CDX99192.1 | 70.2 | 622 | 465–619 | 6.0 | 13 |
| BnCYP86-1 | BnaA01g04590D | CDX75243.1 | 86.9 | 765 | 3–174 | 11.8 | 13 |
| BnCYP86-2 | BnaC01g06080D | CDX68845.1 | 87.0 | 765 | 3–174 | 11.7 | 13 |
| BnCYP112 | BnaC03g71020D | CDY10512.1 | 112.6 | 992 | 1–176 | 5.9 | 18 |
| BnCYP146 | BnaA03g29520D | CDX74095.1 | 146.1 | 1268 | 1108–1264 | 6.0 | 13 |
The cyclophilin-like domain
Since CYPs are characterized by the highly conserved CLD, its occurrence in the identified potential CYP sequences was verified. Most of the BnCYPs contained full length CLDs, whereas CYPs with a molecular weight below 17 kDa have partial CLDs missing essential residues or whole secondary structure parts and thereby might be lacking PPIase activity.
As a consequence of the CLD analysis, two proteins annotated as CYPs in the first place, BnaC05g10020D (30 kDa) and BnaC04g45810D (54 kDa), were excluded from subsequent studies. In addition, for one of the putative low molecular weight CYPs, BnCYP7-1, no CLD was predicted. Nevertheless, it was retained, because a BLASTp search and sequence alignment suggested a partial CLD.
Figure 1 depicts the conserved sequence of all identified B. napus CLDs aligned with the secondary structure of human cyclophilin A (hCYPA) as a reference (for a detailed view of the multiple alignment see Supplementary Fig. S1). The crystal structure of hCYPA shows eight β-strands forming a β-barrel structure, plus two additional α-helices located at the top and bottom52. Human CYPA is often referred to as the “archetypal” CYP. Residues important for CYP function as well as residues promoting the secondary structure are conserved, whereas gaps in the conserved sequence represent insertions in individual members of this protein family. In previous studies it has been demonstrated that the highly conserved amino acid W121 of hCYPA (Fig. 1, W225) is required for CsA binding and interacts directly with the phosphatase calcineurin, but it is not essential for PPIase activity53, 54. This amino acid was not present in 57 out of the 91 BnCYP proteins. Further highly conserved amino acids fundamental for the PPIase activity of hCYPA are R55 (Fig. 1, R87), F60 (Fig. 1, F102;), and H126 (Fig. 1, H232)53. Three single mutations of these amino acids reduced the original activity of the human wild-type isomerase to less than 1%. However, the mutant proteins were still able to bind CsA. Since some of the BnCYPs did not possess all of these three amino acids, their PPIase activity needs to be experimentally confirmed. The motif VXGXV of the hydrophobic region around β-sheet-VII is reported to be conserved among all AtCYP proteins47. This motif was also present in 62 BnCYP protein sequences (Fig. 1, V234, G236, V238).
Figure 1.
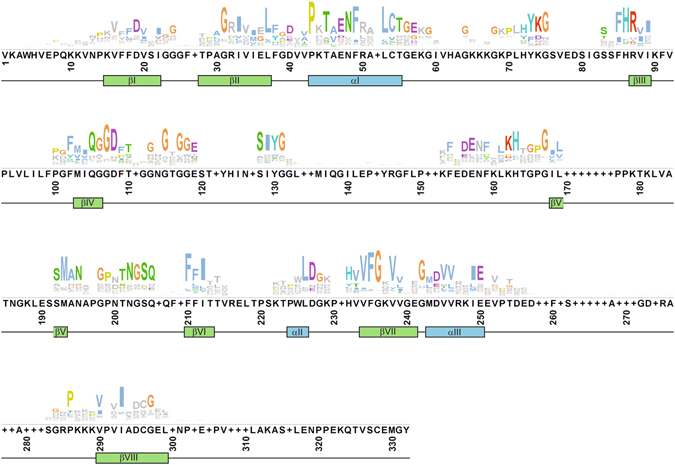
Conserved sequence of the B. napus cyclophilin-like domain. CYP protein sequences were cropped to the predicted sequence for the CLD and aligned with the secondary structure of hCypA. The conserved sequence of 91 predicted CLDs highlights structural features and functional residues. Gaps represent insertions in very few members of this protein family.
Exceptional insertions of amino acids in plant CYPs between β-sheet-I and β-sheet-II (β-I/β-II), β-sheet-IV and β-sheet-V (β-IV/β-V), and β-sheet-VI and α-helix-II (β-VI/α-II)20 have also been observed in this study. In more detail, 3 amino acids are inserted between β-I/β-II (Fig. 1, residues 25–27) in 16 BnCYPs. Yet, 45 BnCYPs do not show this insertion. Interestingly, we also observed deletions in 30 BnCYPs in this region. Only a minority of BnCYPs contains an insertion between β-IV/β-V (Fig. 1, residues 133–152) with 1 to 19 amino acids in length, but also here deletions occurred in some cases. Insertions between β-VI/α-II (Fig. 1, residues 216–221) are only present in 5 BnCYPs with medium and higher molecular masses. An additional insertion between α-helix-I and β-sheet-III (α-I/β-III junction) has been described by Romano et al.47. Here, 8 to 11 amino acids are inserted in several AtCYPs and 3 to 4 amino acids in chloroplast variants. For B. napus a 7 amino acid insertion occurs in several CYPs. For example, except for BnCYP18-1, all 18 and 19 kDa BnCYPs show this insertion.
Domain architecture of cyclophilins and homology
Depending on the domains present, CYPs are classified as single- (SD) and multi-domain (MD) forms48. SD CYPs contain only a CLD which was the case for most of the BnCYPs with a rather low molecular weight (18–30 kDa). While there are mostly two BnCYP homologs with high sequence identity (>80%) corresponding to each AtCYP, caused by polyploidization, there are only a few exceptions with only one corresponding BnCYP homolog. One example is BnCYP18-1 which is the only B. napus homolog of AtCYP18-2 with a full length CLD (with 95% sequence identity, Table 2). It has been shown that the Arabidopsis CYP AtCYP18-2 is recruited by AtSKIP to the nucleus to regulate pre-mRNA splicing55. Because of the high homology, the oilseed rape variant might fulfil a similar role in the nucleus of B. napus cells.
Table 2.
The Arabidopsis cyclophilin family, localization, functions and corresponding Brassica homologs.
| AtCYP | Gene | Subcellular Localization* | Comments | BnCYP homolog (Identity) |
|---|---|---|---|---|
| AtCYP18-1 | At1g01940 | Cytosol (p47, 48) | up-regulated during heat stress81 | BnCYP21-1 (98%) |
| AtCYP18-2 | At2g36130 | Cytosol (p47, 48) | pre-mRNA splicing, down-regulated after pathogen treatment48, 55 | BnCYP18-1 (95%), BnCYP13 (79%), BnCYP8 (56%) |
| AtCYP18-3 | At4g38740 | Cytosol (p47, 48) | plant growth (stem elongation, shoot branching), hormone signalling (links light signal receptors to brassinosteroids), pathogen defence/ETI (by binding of pathogenic proteins/RNA (inhibition of replication (TBSV), interacts with A. tumefaciens VirD2 protein, P. syringae AvRpt2 protease and the plant RIN4 protein)), up-regulated after salt treatment48, 82–89 | BnCYP18-4 (93%), BnCYP18-6 (92%), BnCYP18-7 (92%) |
| AtCYP18-4 | At4g34870 | Cytosol (p47, 48) | pathogen infection (interaction with A. tumefaciens VirD2 protein), down-regulated after salt and cytokinin treatment48, 85 | BnCYP18-2 (83%), BnCYP18-3 (83%), BnCYP17-1 (74%), BnCYP10-1 (35%) |
| AtCYP19-1 | At2g16600 | Cytosol (p47, 48) | seed development, pathogen defence (ROS production, inhibiting pathogen growth), up-regulated after P. syringae infection, down-regulated after ABA treatment30, 48, 90 | BnCYP18-5 (92%), BnCYP18-8 (92%), BnCYP26-4 (66%), BnCYP22-3 (65%), BnCYP20 (62%), BnCYP14-1 (62%), BnCYP14-2 (62%), BnCYP14-3 (60%), BnCYP14-6 (52%), BnCYP16 (50%), BnCYP7-1 (36%) |
| AtCYP19-2 | At2g21130 | Cytosol (p47, 48) | — | BnCYP7-3 (59%), BnCYP27-1 (46%) |
| AtCYP19-3 | At3g56070 | Cytosol (p47, 48) | pathogen defence (inhibition of replication by binding to viral replication proteins (TBSV)), Ca2+ signalling, interaction with calmodulin (35–70 aa), sensitive to Cu2+ (implying redox regulation)84, 91 | BnCYP19-1 (91%), BnCYP19-2 (90%), BnCYP5 (48%) |
| AtCYP19-4 | At2g29960 | Cytosol+SP (e92, 93) | cell polarity (regulates GNOM activity during embryogenesis), up-regulated after salt and cytokinin treatment48, 92, 93 | BnCYP21-5 (93%), BnCYP21-3 (92%), BnCYP12-1 (56%), BnCYP14-4 (52%) |
| AtCYP20-1 | At5g58710 | SP (p47, 48) | UPR (unfolded protein response) in the ER, up-regulated upon ER stress, interaction with PP2A a component of multiple signalling pathways (e.g. auxin transport, growth response)94, 95 | BnCYP21-9 (97%), BnCYP21-4 (92%), BnCYP21-6 (92%) |
| AtCYP20-2 | At5g13120 | TL (e96, 97) | down-regulated after pathogen treatment and up-regulated after light treatment, NAD(P)H dehydrogenase complex subunit, PPIase activity induced by oxidative stress, plant development (interacting with BZR1, a transcription factor responding to brassinosteroids (hormone signalling)), general protein folding catalyst in the TL48, 62, 63, 97–100 | BnCYP27-3 (86%), BnCYP27-5 (86%), BnCYP7-2 (51%) |
| AtCYP20-3 | At3g62030 | Stroma (e96, 97, 101) | light and oxidative stress, redox regulation suggested (interacts with peroxiredoxins PrxA and PrxB), cysteine biosynthesis (interaction with SAT1 (serine acetyltransferase)), JA signalling, binds to JA and OPDA, interacts with A. tumefaciens VirD2 protein38, 40, 85, 102–104 | BnCYP27-2 (89%), BnCYP27-4 (89%), BnCYP28-1 (85%), BnCYP28-2 (85%), BnCYP14-5 (81%), BnCYP24-3 (79%) |
| AtCYP21-1 | At4g34960 | SP (p47, 48) | — | BnCYP24-5 (95%), BnCYP24-6 (95%), BnCYP24-4 (85%), BnCYP24-7 (83%) |
| AtCYP21-2 | At3g55920 | SP (p47, 48) | water stress105 | BnCYP24-1 (92%), BnCYP24-2 (92%) |
| AtCYP21-3 | At2g47320 | Mitochondria (p47, 48) | — | BnCYP25-4 (83%), BnCYP25-5 (83%) |
| AtCYP21-4 | At3g66654 | Mitochondria (p47, 48) | down-regulated after dark treatment48 | BnCYP26-3 (89%), BnCYP26-1 (88%), BnCYP146 (87%), BnCYP26-5 (86%) |
| AtCYP22 | At2g38730 | Cytosol (p47, 48) | — | BnCYP21-2 (93%), BnCYP21-7 (93%), BnCYP21-8 (92%), BnCYP22-2 (92%) |
| AtCYP23 | At1g26940 | SP (p47, 48) | — | BnCYP25-3 (92%), BnCYP25-2 (87%), BnCYP25-1 (86%) |
| AtCYP26-1 | At3g22920 | Cytosol (p47, 48) | — | — |
| AtCYP26-2 | At1g74070 | TL (p47, 48, 106) | down-regulated after sucrose treatment48 | BnCYP34-1 (84%), BnCYP34-2 (83%) |
| AtCYP28 | At5g35100 | TL (e96, 106) | down-regulated after dark and high CO2 treatment48 | BnCYP30-1 (86%), BnCYP30-2 (85%) |
| AtCYP37 | At3g15520 | TL (e96, 106) | down-regulated after dark treatment48 | BnCYP49 (87%), BnCYP50 (87%) |
| AtCYP38 | At3g01480 | TL (e96, 97) | no PPIase activity in the TL, PsbQ-like domain, photo system II: folding of subunits and assembly, down-regulated after dark treatment and up-regulated after light treatment48, 61–65 | BnCYP47-2 (86%), BnCYP47-3 (84%), BnCYP22-1 (69%), BnCYP31 (51%) |
| AtCYP40 | At2g15790 | Cytosol (p47, 48) | interaction with HSP90, AGO1, miRNA156, regulating RISC complex, regulation of vegetative phase change56, 57, 107 | BnCYP40-2 (93%), BnCYP40-1 (92%) |
| AtCYP57 | At4g33060 | Cytosol/Nucleus (p47, 48) | pathogen defence (callose accumulation), up-regulated after P. syringae infection, RNA-interacting region30, 47, 48 | BnCYP55 (86%), BnCYP52 (80%) |
| AtCYP59 | At1g53720 | Nucleus (e67) | cyclophilin-RNA interacting protein (CRIP), Zinc finger motif, pre-mRNA processing, transcription (modulates RNA polymerase II activity)32, 36, 67 | BnCYP12-2 (97%), BnCYP112 (85%), BnCYP10-2 (69%) |
| AtCYP63 | At3g63400 | Nucleus (p47, 48) | may be involved in RNA metabolism47 | BnCYP62 (65%), BnCYP67 (65%) |
| AtCYP65 | At5g67530 | Cytosol (p47, 48) | suggested to be involved in the ubiquitin degradation pathway47 | BnCYP65-1 (92%), BnCYP65-2 (92%), BnCYP26-2 (33%), BnCYP47-1 (27%), BnCYP37-1 (26%), BnCYP37-2 (26%) |
| AtCYP71 | At3g44600 | Nucleus (e35) | plant development, gene expression (histone modification, chromatin assembly), down-regulated after auxin treatment and after knox (transcription factors) overexpression35, 48, 60 | BnCYP70-1 (91%), BnCYP70-2 (91%) |
| AtCYP95 | At4g32420 | Nucleus (p47, 48) | may be involved in RNA metabolism47 | BnCYP86-1 (69%), BnCYP86-2 (67%) |
*Localization was either p = predicted (SP, secretory pathway; TL, thylakoid lumen) or e = experimentally proven as described in the references.
For 12 B. napus CYPs additional domains are predicted which promote diverse capabilities like interaction (protein-protein, protein-DNA, protein-RNA) and modification (ubiquitination) (Fig. 2). These seem to be conserved among Brassicaceae, since similar domain structures exist in the corresponding A. thaliana homologs.
Figure 2.
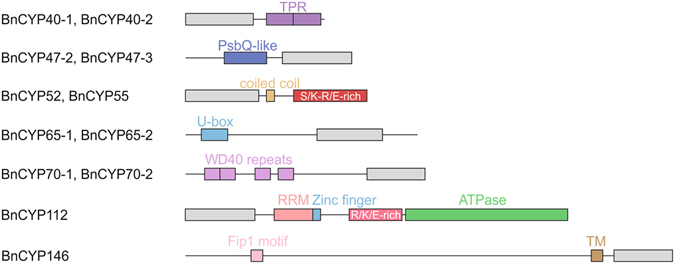
Domain structure of multi-domain B. napus cyclophilins. While most of the CYPs are single-domain proteins, 12 BnCYPs possess additional domains for specific tasks. CLDs are represented by a grey box. RRM = RNA recognition motif, TPR = tetratricopeptide repeat, TM = transmembrane domain.
The MD CYPs BnCYP40-1 and BnCYP40-2 are characterized by a tetratricopeptide repeat (TPR) motif with two TPRs, each 34 amino acids long. Such motifs mediate protein-protein interactions and can thereby assist in the assembly of multi-protein complexes. BnCYP40-1 and BnCYP40-2 show a high degree of sequence identity (92 and 93%, respectively) to AtCYP40 (Table 2). A cytoplasmic interaction partner of AtCYP40 is HSP90, which mediates its recruitment to an intermediate RISC complex by its TPR motifs56, 57. Thereby, AtCYP40 forms, together with HSP90, a complex with AGO1 and a small RNA duplex before the mature RISC complex consisting of AGO1 and a siRNA or miRNA strand is formed56. The mechanism of CYP40-HSP90 binding is conserved between different species. Besides plants, human HSP90 is also known to bind to hCYP40, the human AtCYP40 homolog58. Thus, a similar function for the highly identical CYPs BnCYP40-1 and BnCYP40-2 can be assumed.
Other CYPs showing classical protein-protein interaction domains are BnCYP70-1 and BnCYP70-2, both containing four tryptophan-aspartic acid (WD40) repeats. These are short structural motifs typically forming a four stranded anti-parallel β-sheet. Multiple copies build a circular β-propeller structure promoting protein-protein interactions. Both show 91% sequence identity to their homolog in Arabidopsis, AtCYP71 (Table 2), which is located in the nucleus and functions in the regulation of gene expression and organogenesis. The WD40 domain enables AtCYP71 to associate with histone H3 affecting its methylation35. Data from yeast PPIases provide evidence that they are responsible for histone modification by utilizing the peptidyl-prolyl bond isomerisation as a molecular switch for transcription regulation59. Furthermore, AtCYP71 was shown to interact with FAS1 (a subunit of Chromatin Assembly Factor-1) and LHP1 (a heterochromatin protein)60, suggesting that AtCYP71 is involved in histone modification and chromatin assembly.
BnCYP47-2 and BnCYP47-3 both contain a putative PsbQ-like domain and have only small differences in their N-terminal amino acid sequences. Their closest homolog is AtCYP38 with 86 and 84% sequence identity (Table 2), which also contains a PsbQ-like domain. This domain is typical for proteins localized in the chloroplast, but its function is mostly unknown. AtCYP38 is the only Arabidopsis CYP for which a crystal structure is available. This revealed the additional PsbQ-like domain61. AtCYP38 is experimentally assigned to the chloroplast thylakoid lumen, does not show any PPIase activity62, 63, and plays a critical role in the assembly and maintenance of photosystem II61, 64, 65. It is suggested to interact with the E-loop of chlorophyll protein47 (CP47), a component of the photosystem II (PSII) complex61, via its CLD. Furthermore it is proposed to be responsible for proper folding and insertion of D1 and CP43, both components of PSII65.
There are also two BnCYPs showing classical domains for protein modification. BnCYP65-1 and BnCYP65-2 both contain a Zinc finger U-box motif. Therefore, they might be involved in the ubiquitin degradation pathway.
Four BnCYPs contain sequences characteristic for RNA-interacting proteins. BnCYP52 and BnCYP55 both possess a coiled coil domain. This domain represents a structural motif with coiled α-helices. Proteins containing such a domain can, for example, be transcription factors involved in the regulation of gene expression. The respective A. thaliana homolog is AtCYP57, which shows, besides a predicted coiled coil domain, also an S/K-R/E-rich region47. Sequence comparisons of BnCYP52, BnCYP55 and AtCYP57 revealed a high sequence identity in the AtCYP57 S/K-R/E-rich region (data not shown). This region is supposed to mediate the interaction with ribonucleoproteins66.
BnCYP112 contains an RNA recognition motif (RRM), a Zinc finger motif (CCHC-type), a positively charged region (arginine, lysine, glutamate) and an actin-like ATPase domain. Besides AtCYP59 in A. thaliana, homologs to BnCYP112 can be found in different species like P. tetraurelia, S. pombe, C. elegans, D. melanogaster, and H. sapiens 32. This group of proteins is called CRIPs, for cyclophilin-RNA interacting proteins32. Characteristic for this group of proteins is an RRM in addition to the CLD. Nevertheless, CRIPs differ in their C-terminal region. AtCYP59 and BnCYP112 are the only members containing a Zinc finger motif. AtCYP59 interacts with the C-terminal domain of the largest subunit of RNA polymerase II and thereby influences its phosphorylation state. Furthermore, binding of an RNA transcript decreases PPIase activity of AtCYP59. This might modulate RNA polymerase II activity36. Therefore, it is suggested that AtCYP59 connects pre-mRNA processing and transcription36, 67. Moreover, BnCYP112 is the only homolog of all CRIPs showing an additional actin-like ATPase domain.
BnCYP146 is the largest CYP found in oilseed rape. It contains a transmembrane domain and a Fip1 motif. In yeast, the Fip1 protein ensures the polyadenylation of mRNAs by interacting with poly(A) polymerase. There is no A. thaliana homolog showing the same domain structure as BnCYP146, but its CLD is closely related to AtCYP21-4 (Table 2).
Subcellular localization and phylogenetic relationships
The phylogenetic analysis of the full length amino acid sequences revealed clusters of proteins with high sequence similarity (Fig. 3). Often, these groups possess the same additional domains, consistent subcellular localization, and the same A. thaliana CYP homolog.
Figure 3.
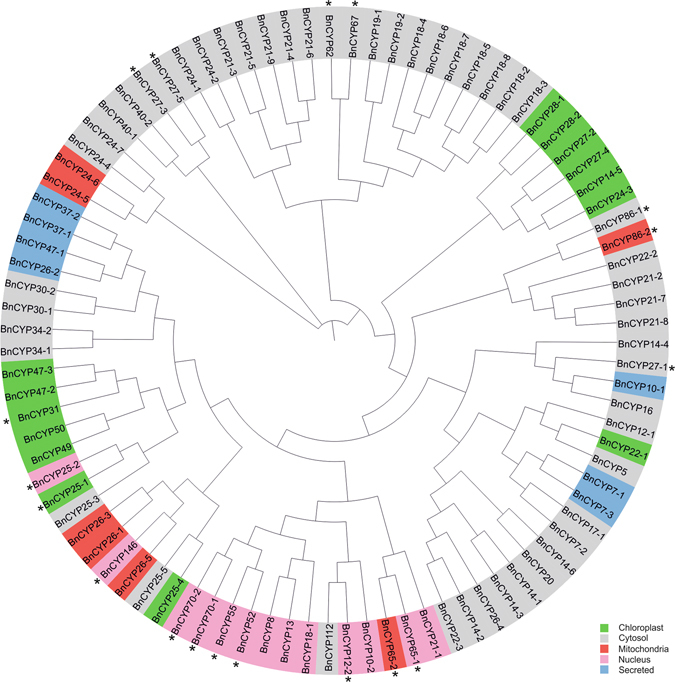
Phylogenetic tree of B. napus cyclophilins. The phylogenetic analysis was based on a sequence alignment by ClustalOmega and constructed as described in Materials and Methods with ignored branch lengths. As indicated by colour code, this protein family is distributed over all intercellular organelles. In addition, some BnCYPs possess putative nuclear localization signals (marked by *).
As previously described for other organisms (e.g. A. thaliana homologs, see Table 2), B. napus CYPs are targeted to all intercellular organelles. At least 50 BnCYPs are predicted to be located in the cytosol. This result is comparable to the subcellular distribution of the A. thaliana CYPs where 14 of 29 are either predicted or experimentally proven to be located in the cytosol (Table 2). Furthermore, 14 BnCYPs are predicted to be located in chloroplasts, 7 in mitochondria, 13 in the nucleus, and 7 are secreted (summarized in Fig. 3, for a detailed prediction see Supplementary Table S1). In addition, some BnCYPs contain nuclear localization signals (NLS). Proteins predicted to be cytosolic or nuclear which possess NLS might relocate between both compartments.
Genomic distribution of cyclophilins
CYP genes are distributed on all 19 chromosomes of the B. napus genome (Fig. 4a). There is no chromosome that is not encoding any CYP. Chromosomes A07 and C06 contain only one CYP each. Chromosome A01 has the largest number with 11 CYP genes. All other chromosomes contain between 2 and 10 CYP genes (Fig. 4b). The 4 CYP genes BnaAnng41240D (BnCYP12-2), BnaAnng15590D (BnCYP21-3), BnaAnng17350D (BnCYP21-6), and BnaAnng12550D (BnCYP62) were mapped to the A chromosomes, but without a detailed information regarding the exact chromosomal location. Likewise, the 4 CYP genes BnaCnng42430D (BnCYP8), BnaCnng08980D (BnCYP21-1), BnaCnng32070D (BnCYP22-1), and BnaCnng32180D (BnCYP26-5) were mapped to the C chromosomes, but again without a detailed information regarding the exact chromosomal location. The A chromosomes contain 42 CYP genes and 4 not fully assigned genes, and the C chromosomes contain 44 CYP genes and 4 not fully assigned genes. Thus, both chromosome sets (A and C), which originate from the B. rapa and B. oleracea genome, contain a similar number of CYP genes.
Figure 4.
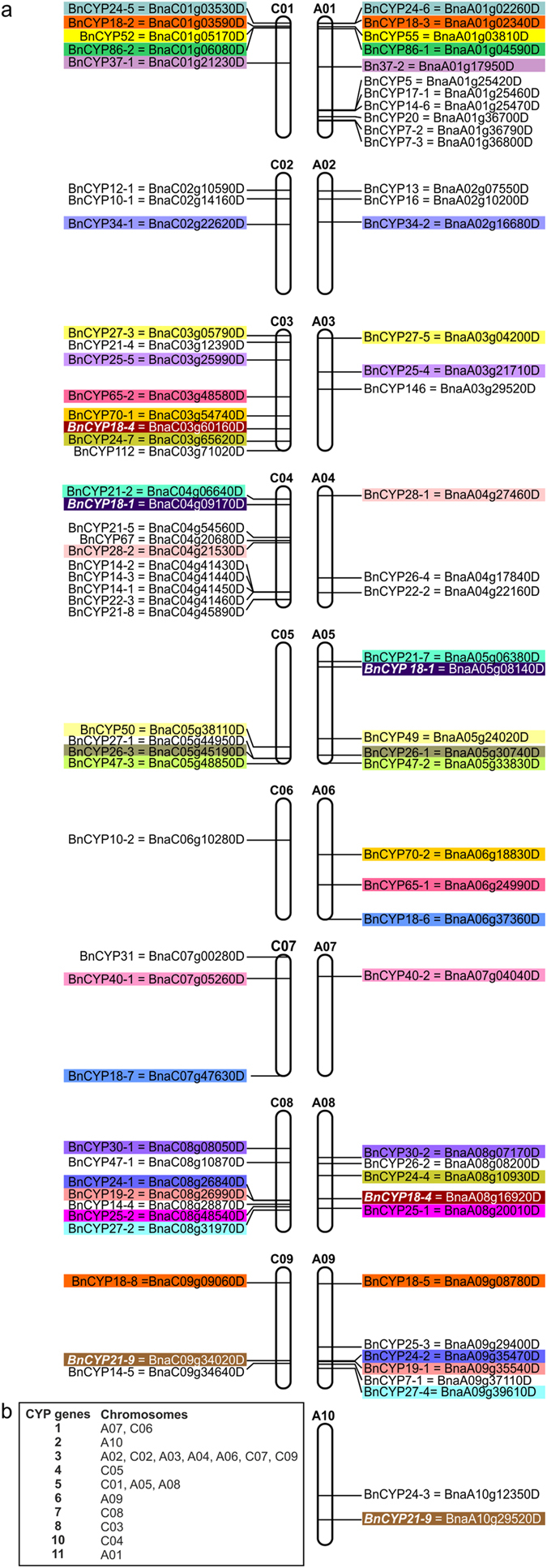
Genomic distribution of cyclophilin genes on B. napus chromosomes. (a) Chromosomal locations of BnCYP genes are indicated based on the information provided by the Brassica napus Genome Browser. Highlighted by identical colours are BnCYP genes which encode protein isoforms. Genes encoding identical proteins are written in italics and white colour. (b) Summary of the number of BnCYP genes encoded on each chromosome.
Interestingly, there are three CYP proteins that are each encoded by two of the six following genes BnaA05g08140D and BnaC04g09170D (BnCYP18-1), BnaA08g16920D and BnaC03g60160D (BnCYP18-4), or BnaA10g29520D and BnaC09g34020D (BnCYP21-9). Thus, one copy of these genes occurs on each of the chromosomal sets. Alignments of these CYP genes from the A and respective C chromosome showed slightly different nucleotide sequences. Nevertheless, these encode identical amino acid sequences (data not shown).
Due to the two progenitor chromosomal sets, several CYP genes encode proteins with high sequence homologies. Most often these occur as pairs, each gene originating from one progenitor chromosomal set. They are highlighted with identical colours on the A and C chromosomes in Fig. 4 and are from now on called isoforms. Some of them are located on the respective A and C chromosome, others are spread on different chromosomes, probably due to chromosomal rearrangements of the B. napus genome (as recently described by Cheng et al.68).
In summary, the allopolyploidy of oilseed rape results in two isoforms of many proteins. These show high sequence identities on the nucleotide and amino acid levels and originate from either the A or C genome. Due to their similarity, they cluster in the phylogenetic tree and often the same localization is predicted (Fig. 3). They might either possess the ability to replace each other or might be specialized for certain tasks and interaction partners.
One of the biggest groups of isoforms among the BnCYPs is the group of 18 kDa proteins. These contain a full-length CLD with a high degree of conservation, a cytosolic localization, and can be further subdivided by their homology. The phylogenetic tree reveals isoform pairs: BnCYP18-2 and BnCYP18-3, BnCYP18-5 and BnCYP18-8, and BnCYP18-6 and BnCYP18-7 (Fig. 3). Each pair has a representative gene on chromosome A and C, respectively (Fig. 4a). Interestingly, BnCYP18-4 which is closely related to the pair BnCYP18-6/18-7 is encoded by two genes located on C03 and A08 that might derive from a gene duplication event. The A. thaliana homologs of the described 18 kDa isoforms are AtCYP18-3, AtCYP18-4, and AtCYP19-1 which are shown to be important for various processes from plant growth to pathogen defence (summarized in Table 2). In contrast to the other 18 kDa BnCYPs, BnCYP18-1 is the only 18 kDa CYP with introns and without the prominent insertion between α-helix-I and β-sheet-III. Moreover, its localization is predicted to be nuclear instead of cytosolic (Fig. 3).
Expression analysis of the predicted BnCYPs on transcript and protein levels
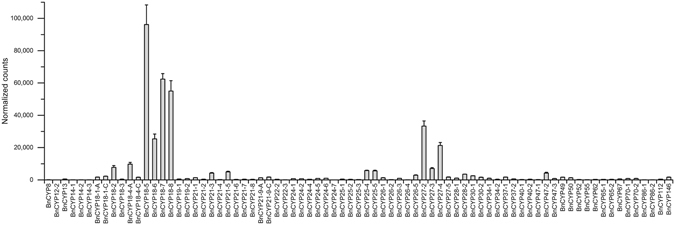
To investigate the mRNA expression of the bioinformatically predicted CYP genes, RNA-Seq of RNA isolated from leaves was performed. This approach revealed 77 expressed BnCYPs under the applied conditions (summarized in Table 3). The expression pattern indicates a large variance in the abundance of BnCYP transcripts (Fig. 5, for raw data see Supplementary Table S2). The results thus show that most of the predicted CYP genes are indeed transcribed. Transcripts for CYP genes not detected in this study could be expressed in other tissues, specific cell types, or under different experimental conditions.
Table 3.
Identified B. napus cyclophilins by RNA-Seq and LC-MS/MS.
| Name | Gene Name | BnCYPs identified by RNA-Seq | BnCYPs identified by LC-MS/MS | |
|---|---|---|---|---|
| Leaf | Phloem | |||
| BnCYP5 | BnaA01g25420D | |||
| BnCYP7-1 | BnaA09g37110D | |||
| BnCYP7-2 | BnaA01g36790D | |||
| BnCYP7-3 | BnaA01g36800D | |||
| BnCYP8 | BnaCnng42430D | x | ||
| BnCYP10-1 | BnaC02g14160D | |||
| BnCYP10-2 | BnaC06g10280D | |||
| BnCYP12-1 | BnaC02g10590D | |||
| BnCYP12-2 | BnaAnng41240D | x | ||
| BnCYP13 | BnaA02g07550D | x | x | |
| BnCYP14-1 | BnaC04g41450D | x | ||
| BnCYP14-2 | BnaC04g41430D | x | ||
| BnCYP14-3 | BnaC04g41440D | x | ||
| BnCYP14-4 | BnaC08g28870D | |||
| BnCYP14-5 | BnaC09g34640D | |||
| BnCYP14-6 | BnaA01g25470D | |||
| BnCYP16 | BnaA02g10200D | |||
| BnCYP17-1 | BnaA01g25460D | |||
| BnCYP18-1 | BnaA05g08140D | x | x | |
| BnaC04g09170D | x | |||
| BnCYP18-2 | BnaC01g03590D | x | x | |
| BnCYP18-3 | BnaA01g02340D | x | ||
| BnCYP18-4 | BnaC03g60160D | x | x | x |
| BnaA08g16920D | x | |||
| BnCYP18-5 | BnaA09g08780D | x | x | x |
| BnCYP18-6 | BnaA06g37360D | x | x | |
| BnCYP18-7 | BnaC07g47630D | x | x | |
| BnCYP18-8 | BnaC09g09060D | x | ||
| BnCYP19-1 | BnaA09g35540D | x | x | |
| BnCYP19-2 | BnaC08g26990D | x | x | x |
| BnCYP20 | BnaA01g36700D | |||
| BnCYP21-1 | BnaCnng08980D | x | x | |
| BnCYP21-2 | BnaC04g06640D | x | ||
| BnCYP21-3 | BnaAnng15590D | x | x | x |
| BnCYP21-4 | BnaC03g12390D | x | x | |
| BnCYP21-5 | BnaC04g54560D | x | ||
| BnCYP21-6 | BnaAnng17350D | x | ||
| BnCYP21-7 | BnaA05g06380D | x | ||
| BnCYP21-8 | BnaC04g45890D | x | ||
| BnCYP21-9 | BnaA10g29520D | x | x | |
| BnaC09g34020D | x | |||
| BnCYP22-1 | BnaCnng32070D | |||
| BnCYP22-2 | BnaA04g22160D | x | x | |
| BnCYP22-3 | BnaC04g41460D | x | ||
| BnCYP24-1 | BnaC08g26840D | x | x | |
| BnCYP24-2 | BnaA09g35470D | x | ||
| BnCYP24-3 | BnaA10g12350D | |||
| BnCYP24-4 | BnaA08g10930D | x | ||
| BnCYP24-5 | BnaC01g03530D | x | ||
| BnCYP24-6 | BnaA01g02260D | x | ||
| BnCYP24-7 | BnaC03g65620D | x | ||
| BnCYP25-1 | BnaA08g20010D | x | ||
| BnCYP25-2 | BnaC08g48540D | x | ||
| BnCYP25-3 | BnaA09g29400D | x | ||
| BnCYP25-4 | BnaA03g21710D | x | ||
| BnCYP25-5 | BnaC03g25990D | x | ||
| BnCYP26-1 | BnaA05g30740D | x | ||
| BnCYP26-2 | BnaA08g08200D | x | ||
| BnCYP26-3 | BnaC05g45190D | x | ||
| BnCYP26-4 | BnaA04g17840D | x | ||
| BnCYP26-5 | BnaCnng32180D | x | ||
| BnCYP27-1 | BnaC05g44950D | |||
| BnCYP27-2 | BnaC08g31970D | x | x | x |
| BnCYP27-3 | BnaC03g05790D | x | x | x |
| BnCYP27-4 | BnaA09g39610D | x | x | x |
| BnCYP27-5 | BnaA03g04200D | x | ||
| BnCYP28-1 | BnaA04g27460D | x | x | x |
| BnCYP28-2 | BnaC04g21530D | x | x | |
| BnCYP30-1 | BnaC08g08050D | x | x | |
| BnCYP30-2 | BnaA08g07170D | x | ||
| BnCYP31 | BnaC07g00280D | |||
| BnCYP34-1 | BnaC02g22620D | x | x | |
| BnCYP34-2 | BnaA02g16680D | x | ||
| BnCYP37-1 | BnaC01g21230D | x | ||
| BnCYP37-2 | BnaA01g17950D | x | ||
| BnCYP40-1 | BnaC07g05260D | x | ||
| BnCYP40-2 | BnaA07g04040D | x | ||
| BnCYP47-1 | BnaC08g10870D | x | ||
| BnCYP47-2 | BnaA05g33830D | x | x | |
| BnCYP47-3 | BnaC05g48850D | x | ||
| BnCYP49 | BnaA05g24020D | x | x | |
| BnCYP50 | BnaC05g38110D | x | ||
| BnCYP52 | BnaC01g05170D | x | ||
| BnCYP55 | BnaA01g03810D | x | ||
| BnCYP62 | BnaAnng12550D | x | x | |
| BnCYP65-1 | BnaA06g24990D | x | ||
| BnCYP65-2 | BnaC03g48580D | x | ||
| BnCYP67 | BnaC04g20680D | x | ||
| BnCYP70-1 | BnaC03g54740D | x | ||
| BnCYP70-2 | BnaA06g18830D | x | ||
| BnCYP86-1 | BnaA01g04590D | x | ||
| BnCYP86-2 | BnaC01g06080D | x | x | |
| BnCYP112 | BnaC03g71020D | x | ||
| BnCYP146 | BnaA03g29520D | x | ||
Figure 5.
Expression pattern of BnCYP genes from leaf material. In case of two genes encoding the same BnCYP protein, the transcript is abbreviated by its BnCYP name with -A or -C depending on the chromosomal location of the gene, respectively. Raw data are provided in Supplementary Table S2.
In the analysed leaf sample, the 18 kDa and 27 kDa family members belong to the strongest expressed ones, with BnCYP18-5 showing the highest read count. Interestingly, some isoform pairs had differing read counts, e.g. BnCYP18-2/BnCYP18-3, BnCYP18-4/BnCYP18-6/BnCYP18-7, BnCYP27-3/BnCYP27-5.
Since CYPs are a prominent protein class in the phloem long-distance transport system of higher plants including oilseed rape with a potential function as a molecular chaperone important for protein long-distance transport2, 41, we performed LC-MS/MS analysis of phloem protein extract and compared the CYP protein profile to that of leaf extract. Table 3 summarizes the BnCYPs identified from leaf and phloem protein extracts. In total, 26 BnCYPs could be detected at the protein level under the applied conditions with six being unique to leaves and 12 to phloem exudate. Since sieve elements do not have a functional transcription machinery, RNA-Seq was not performed with phloem samples. However, phloem samples contain a specific set of mobile RNAs that have in part been implicated with long-distance signalling43, but this was not the subject of the present study.
Most of the identified BnCYP proteins are predicted to be localized in the cytosol, but some potentially chloroplastic, mitochondrial and nuclear BnCYPs were found as well. Besides several SD CYPs, also one MD CYP, the putative chloroplastic protein BnCYP47-2, could be identified in leaf extract. The analysis of phloem sap revealed a low molecular weight CYP, BnCYP13, which is suggested to contain only a partial CLD. Figure 6 shows the abundance of the identified CYP proteins in the two examined organs (more details in Supplementary Tables S3, S4, S5, S6). Whereas BnCYP18-5, BnCYP24-1 and BnCYP27-4 were the most abundant CYP proteins in leaf extract, only BnCYP18-5 was the most abundant one in phloem sap with a considerably higher relative abundance observed than for the other identified BnCYP proteins from this compartment. Interestingly, the most abundant CYP protein in both compartments is BnCYP18-5. The finding of so many CYPs in the phloem transport system suggests an essential function of this protein family in this compartment, probably in protein transport or long-distance signalling.
Figure 6.
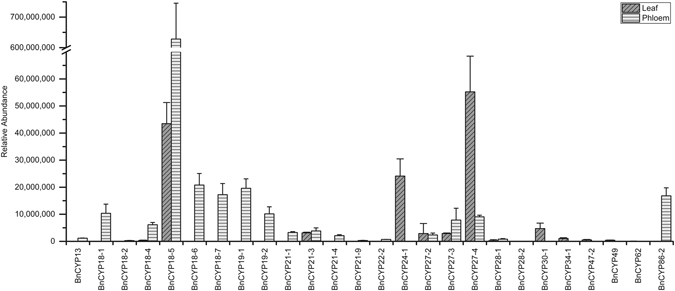
Relative abundance of leaf and phloem BnCYP proteins. The relative abundance of each BnCYP refers to the abundance of its unique peptides either from leaf extract (n = 3) or phloem sap (n = 4) samples.
Conclusions
Cyclophilins are ubiquitous proteins that constitute a multigene family in higher organisms. Exceptionally high numbers of CYPs have been found in plants, underlining their essential importance in many essential physiological processes. However, the physiological roles of most CYPs in plants are not well understood.
The present study applied bioinformatic tools for a genome-wide identification of CYPs in the important oil crop B. napus. Sequence similarity searches with known Arabidopsis CYPs, sequence alignments and CLD prediction identified a surprisingly high number of 94 CYP-coding genes. Therefore, B. napus contains the highest number of CYPs known so far. As in other plants, CYPs are predicted to be localized in all compartments, most of them being probably cytosolic. Most BnCYPs are single-domain proteins.
Transcriptome analysis confirmed the expression of 77 distinct CYPs in the B. napus cultivar ‘Drakkar’ from leaf material under normal growth conditions. The occurrence of 26 BnCYP proteins was confirmed by LC-MS/MS analysis. It is likely that additional CYPs are expressed on transcript and protein levels in different cell types, plant parts, or under different environmental conditions. Interestingly, 12 BnCYPs were exclusively found in phloem samples and not in leaf extract supporting a fundamental and specific role in this specialized compartment.
Future studies must now focus on the functional characterization of the high number of CYPs in B. napus in order to better understand the diverse roles of CYPs in oilseed rape and in plant biology in more general. In this regard, elucidating the role(s) of the phloem CYPs in protein refolding and long-distance signalling will be of special interest.
Materials and Methods
Sequence analysis
Brassica napus CYPs were identified by BLASTp of the Arabidopsis thaliana CYP18-1 (At1g01940) and CYP19-1 (At2g16600) against the B. napus genome sequence database51 deposited at NCBI (http://www.ncbi.nlm.nih.gov/)69. BLASTp searches with the remaining AtCYPs revealed the same BnCYPs as already identified. Amino acid and cDNA sequences were obtained by the European Nucleotide Archive (http://www.ebi.ac.uk/ena/data/view/PRJEB5043). To identify A. thaliana homologs for the individual BnCYPs, the amino acid sequences of BnCYPs were used as queries for a BLAST search on UniProtKB (http://www.uniprot.org/)70.
All identified proteins were analyzed for the presence of a CLD and potential additional domains with InterPro (http://www.ebi.ac.uk/interpro/)71 and drawn by CorelDRAW. The theoretical isoelectric point was determined by the ProteinProspector Tool MS-Digest (http://prospector.ucsf.edu/prospector/cgi-bin/msform.cgi?form=msdigest), subcellular localization was predicted by LocTree3 (https://rostlab.org/services/loctree3/)72 and nuclear localization signals (NLS) by using the NLS mapper (http://nls-mapper.iab.keio.ac.jp/cgi-bin/NLS_Mapper_form.cgi)73.
Chromosome mapping of the CYPs was performed using the Brassica napus Genome Browser (http://www.genoscope.cns.fr/brassicanapus/cgi-bin/gbrowse/colza/) available by Genoscope - Centre National de Séquençage and redrawn by CorelDRAW.
Protein sequence alignment and phylogenetic analysis
Sequences were aligned using ClustalOmega (http://www.ebi.ac.uk/Tools/msa/clustalo/)74 and displayed with Jalview 2.9.0b275. The secondary structure annotation is based on the structure of the crystallized human CYPA (4N1M.pdb).
The phylogenetic tree was calculated with ClustalW2 Phylogeny (http://www.ebi.ac.uk/Tools/phylogeny/clustalw2_phylogeny/) by using the multiple alignment from ClustalOmega, and subsequently processed with iTOL (http://itol.embl.de/)76, 77.
Plant material and growth conditions
Brassica napus cultivar ‘Drakkar’ plants were grown in 19 cm pots on soil (LAT-Terra Standard P, Industrie-Erdenwerk Archut, Germany) in a glasshouse under controlled conditions with 70% humidity and a 16 h/8 h light/dark (day/night) and 22 °C/18 °C (day/night) cycle. Plants were watered once per day and fertilized with 2 g/l Osmocote Exact Standard High K (Scotts, the Netherlands).
Expression profile of cyclophilins
Transcriptome data were generated by GAMAVIR, a tri-national research activity aiming at characterizing plant:virus interactions in rapeseed (ANR-13-KBBE-0005). PolyA RNA isolated from leaf disks of 6 weeks-old Brassica napus cultivar ‘Drakkar’ plants (n = 3) was sequenced using Illumina technology. Clean RNAseq reads were aligned against the re-sequenced Brassica napus cultivar ‘Drakkar’ genome using Tophat2 v2.0.1378 and counted with Samtools v1.179.
Protein extraction for proteomics
Phloem sap was collected as described previously by Giavalisco et al.2 at the inflorescence of oilseed rape plants before flowering. Phloem sap samples were collected on ice four times (n = 4), frozen in liquid nitrogen and stored at −80 °C until further processing. Proteins were precipitated in 4 volumes of 90% (v/v) acetone, 10% (v/v) methanol, 10 mM DTT over night at −20 °C. The precipitates were pelleted at 14,000 × g at 4 °C for 15 min, washed twice with 100% acetone and air-dried.
Leaf material was harvested from three different plants (n = 3) and for each sample 100 mg material was grinded in liquid nitrogen. Proteins were extracted with 800 µl extraction buffer (50 mM MOPS/pH 7.5, 5% glycerol, 0.55% PVPP, 0.5% Nonidet P-40, 5 mM L-ascorbic acid, 5 mM DTT, 1x protease inhibitor (cOmplete Protease Inhibitor Cocktail, Roche), 1x phosphatase inhibitor (PhosStop, Phosphatase Inhibitor Cocktail, Roche). Centrifuging at 14,000 × g at 4 °C for 15 min allowed the separation of soluble proteins from insoluble material. Proteins were precipitated as described above by acetone/methanol/DTT.
Analysis of phloem sap and leaf proteins by LC-MS/MS
For the analysis of phloem proteins the extracted and precipitated phloem sap proteins were resuspended in extraction buffer (6 M urea, 2 M thiourea, 15 mM DTT, 2% CHAPS). Here, each protein pellet was dissolved in exactly the same volume deployed for the initial phloem sap precipitation. Once the proteins were in solution, samples were sonicated for 10 min in a sonication bath, followed by 30 min incubation on an orbital shaker (100 rpm) at room temperature. Solubilised proteins were centrifuged at 10,000 × g for 5 min and the protein concentration was determined from the collected supernatant.
For the analysis of leaf tissue proteins, the precipitated proteins were resuspended in sufficient protein extraction buffer to provide a final protein concentration of 2 µg/µl.
25 µg of phloem sap and 50 µg of leaf tissue protein extract were then digested in solution using a Trypsin/Lys-C mixture (Mass Spec Grade, Promega, Madison, WI, USA) according to the instruction manual. After the digestion, the samples were desalted using C18-stage tips as described in Rappsilber et al.80.
After the elution of the digested and desalted peptides from C18-stage tips, the samples were concentrated to near dryness in a SpeedVac and the peptide mixtures were reconstituted in 30 µl resuspension buffer (5% acetonitrile, 0.1% formic acid in water). 5 µl of this peptide mix was injected onto an Acclaim PepMap RSLC HPLC column (75 µm × 15 cm, Thermo Scientific) connected to the EASY-nLC 1000 system (Thermo Scientific). The eluting peptides were then analyzed on a Q Exactive Plus (Thermo Scientific, Bremen, Germany) high-resolution mass spectrometer.
The peptides were separated using a binary buffer system of 0.1% formic acid in water (Buffer A) and 60% acetonitrile containing 0.1% formic (Buffer B). The flow rate was adjusted to 300 nl/min. Peptides were eluted with on a linear gradient of 0–40% buffer B for 50 min followed by a linear gradient between 40–80% buffer B for additional 30 min. Peptides were analyzed in the mass spectrometer using one full scan (300–1600 m/z, R = 70,000 at 200 m/z), followed by up to fifteen data-dependent MS/MS scans (Top 15 approach) with higher-energy collisional dissociation (HCD) at a resolution of 17,500 at 200 m/z. Dynamic exclusion was set to 15 s.
Raw data were processed using the Progenesis QI for proteomics (Progenesis QI for Proteomics Version 3.0, Nonlinear Dynamics, Newcastle, UK) software and the protein sequences of all identified CYPs from Brassica napus. Protein identifications were filtered with a false discovery rate better than 1%, at least two peptides, one unique peptide and a score of 50.
Electronic supplementary material
Acknowledgements
We are grateful to S. Graindorge and D. Pflieger at the IBMP BioImage and Bioinformatics platform for providing access to the bioinformatic resources and the analysis of RNAseq data. We would like to acknowledge the financial contribution to the research activities by a Career Integration Grant (CIG; PCIG14-GA-2013-63 0734) by the European Commission within the 7th framework program, the grant LFF-GK06 ‘DELIGRAH’ (Landesforschungsförderung Hamburg), and a DFG grant (DFG KE 856_6-1) awarded to J.K., and funding by the Agence National de la Recherche (ANR-13-KBBE-0005-01) awarded to M.H.
Author Contributions
P.H. and M.T. performed the original database searches and contributed to the design of the project. P.H. performed bioinformatic predictions, phloem sap sampling and protein extraction. K.A. performed RNA extraction, K.A. and M.H. contributed to RNA-Seq data analysis. K.B. and P.G. performed LC-MS/MS. P.H. and P.G. analyzed the protein data. J.K. and M.H. participated in data analysis and discussion. P.H. and M.T. drafted the initial manuscript, J.K. conceived of and supervised the study and complemented the writing. All authors reviewed the manuscript and approved the final article.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01596-5
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Buhtz A, Pieritz J, Springer F, Kehr J. Phloem small RNAs, nutrient stress responses, and systemic mobility. BMC Plant Biology. 2010;10:64. doi: 10.1186/1471-2229-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giavalisco P, Kapitza K, Kolasa A, Buhtz A, Kehr J. Towards the proteome of Brassica napus phloem sap. Proteomics. 2006;6:896–909. doi: 10.1002/pmic.200500155. [DOI] [PubMed] [Google Scholar]
- 3.Pant BD, Buhtz A, Kehr J, Scheible WR. MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. The Plant Journal. 2008;53:731–738. doi: 10.1111/j.1365-313X.2007.03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kehr J, Buhtz A, Giavalisco P. Analysis of xylem sap proteins from Brassica napus. BMC Plant Biology. 2005;5:11. doi: 10.1186/1471-2229-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostendorp A, Pahlow S, Deke J, Thieß M, Kehr J. Protocol: optimisation of a grafting protocol for oilseed rape (Brassica napus) for studying long-distance signalling. Plant Methods. 2016;12:1–8. doi: 10.1186/s13007-016-0122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kagale S, et al. Polyploid evolution of the Brassicaceae during the Cenozoic era. Plant Cell. 2014;26:2777–2791. doi: 10.1105/tpc.114.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmutzer T, et al. Species-wide genome sequence and nucleotide polymorphisms from the model allopolyploid plant Brassica napus. Scientific data. 2015;2:150072. doi: 10.1038/sdata.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iñiguez-Luy, F. L. F., M. L. In Genetics and Genomics of the Brassicaceae (ed I.; Schmidt Bancroft, R.) 291–322 (New York, NY: Springer, 2011).
- 9.Friedt, W. & Snowdon, R. In Oil Crops Vol. 4 Handbook of Plant Breeding (eds Johann Vollmann & Istvan Rajcan) Ch. 4, 91–126 (Springer New York, 2010).
- 10.Harding MW, Galat A, Uehling DE, Schreiber SL. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989;341:758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- 11.Bierer BE, et al. Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proc Natl Acad Sci USA. 1990;87:9231–9235. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984;226:544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 13.Harding MW, Handschumacher RE, Speicher DW. Isolation and amino acid sequence of cyclophilin. J Biol Chem. 1986;261:8547–8555. [PubMed] [Google Scholar]
- 14.Kiefhaber T, Quaas R, Hahn U, Schmid FX. Folding of ribonuclease T1. 2. Kinetic models for the folding and unfolding reactions. Biochemistry. 1990;29:3061–3070. doi: 10.1021/bi00464a024. [DOI] [PubMed] [Google Scholar]
- 15.Rahfeld JU, et al. Confirmation of the existence of a third family among peptidyl-prolyl cis/trans isomerases. Amino acid sequence and recombinant production of parvulin. FEBS Lett. 1994;352:180–184. doi: 10.1016/0014-5793(94)00932-5. [DOI] [PubMed] [Google Scholar]
- 16.Galat A. Peptidylprolyl cis/trans isomerases (immunophilins): biological diversity–targets–functions. Current topics in medicinal chemistry. 2003;3:1315–1347. doi: 10.2174/1568026033451862. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-H. [DOI] [PubMed] [Google Scholar]
- 18.Barik S. Immunophilins: for the love of proteins. Cell Mol Life Sci. 2006;63:2889–2900. doi: 10.1007/s00018-006-6215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasser CS, Gunning DA, Budelier KA, Brown SM. Structure and expression of cytosolic cyclophilin/peptidyl-prolyl cis-trans isomerase of higher plants and production of active tomato cyclophilin in Escherichia coli. Proc Natl Acad Sci USA. 1990;87:9519–9523. doi: 10.1073/pnas.87.24.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galat A. Variations of sequences and amino acid compositions of proteins that sustain their biological functions: An analysis of the cyclophilin family of proteins. Arch Biochem Biophys. 1999;371:149–162. doi: 10.1006/abbi.1999.1434. [DOI] [PubMed] [Google Scholar]
- 21.Klappa P, Freedman RB, Zimmermann R. Protein disulphide isomerase and a lumenal cyclophilin-type peptidyl prolyl cis-trans isomerase are in transient contact with secretory proteins during late stages of translocation. Eur J Biochem. 1995;232:755–764. doi: 10.1111/j.1432-1033.1995.tb20870.x. [DOI] [PubMed] [Google Scholar]
- 22.Kern G, Kern D, Schmid FX, Fischer G. A kinetic analysis of the folding of human carbonic anhydrase II and its catalysis by cyclophilin. J Biol Chem. 1995;270:740–745. doi: 10.1074/jbc.270.2.740. [DOI] [PubMed] [Google Scholar]
- 23.Zander K, et al. Cyclophilin A interacts with HIV-1 Vpr and is required for its functional expression. J Biol Chem. 2003;278:43202–43213. doi: 10.1074/jbc.M305414200. [DOI] [PubMed] [Google Scholar]
- 24.Romano P, Gray J, Horton P, Luan S. Plant immunophilins: functional versatility beyond protein maturation. New Phytol. 2005;166:753–769. doi: 10.1111/j.1469-8137.2005.01373.x. [DOI] [PubMed] [Google Scholar]
- 25.Allain F, Denys A, Spik G. Characterization of surface binding sites for cyclophilin B on a human tumor T-cell line. J Biol Chem. 1994;269:16537–16540. [PubMed] [Google Scholar]
- 26.Schiene-Fischer C, Yu C. Receptor accessory folding helper enzymes: the functional role of peptidyl prolyl cis/trans isomerases. FEBS Lett. 2001;495:1–6. doi: 10.1016/S0014-5793(01)02326-2. [DOI] [PubMed] [Google Scholar]
- 27.Brazin KN, Mallis RJ, Fulton DB, Andreotti AH. Regulation of the tyrosine kinase Itk by the peptidyl-prolyl isomerase cyclophilin A. Proc Natl Acad Sci USA. 2002;99:1899–1904. doi: 10.1073/pnas.042529199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bukrinsky MI. Cyclophilins: unexpected messengers in intercellular communications. Trends Immunol. 2002;23:323–325. doi: 10.1016/S1471-4906(02)02237-8. [DOI] [PubMed] [Google Scholar]
- 29.Jing H, et al. Peptidyl-prolyl isomerization targets rice Aux/IAAs for proteasomal degradation during auxin signalling. Nat Commun. 2015;6:7395. doi: 10.1038/ncomms8395. [DOI] [PubMed] [Google Scholar]
- 30.Pogorelko GV, et al. Characterization of three Arabidopsis thaliana immunophilin genes involved in the plant defense response against Pseudomonas syringae. Gene. 2014;538:12–22. doi: 10.1016/j.gene.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 31.Lin DT, Lechleiter JD. Mitochondrial targeted cyclophilin D protects cells from cell death by peptidyl prolyl isomerization. J Biol Chem. 2002;277:31134–31141. doi: 10.1074/jbc.M112035200. [DOI] [PubMed] [Google Scholar]
- 32.Krzywicka A, et al. KIN241: a gene involved in cell morphogenesis in Paramecium tetraurelia reveals a novel protein family of cyclophilin-RNA interacting proteins (CRIPs) conserved from fission yeast to man. Mol Microbiol. 2001;42:257–267. doi: 10.1046/j.1365-2958.2001.02634.x. [DOI] [PubMed] [Google Scholar]
- 33.Anderson M, et al. A new family of cyclophilins with an RNA recognition motif that interact with members of the trx/MLL protein family in Drosophila and human cells. Dev Genes Evol. 2002;212:107–113. doi: 10.1007/s00427-002-0213-8. [DOI] [PubMed] [Google Scholar]
- 34.Dubourg B, et al. The human nuclear SRcyp is a cell cycle-regulated cyclophilin. J Biol Chem. 2004;279:22322–22330. doi: 10.1074/jbc.M400736200. [DOI] [PubMed] [Google Scholar]
- 35.Li H, et al. A WD40 domain cyclophilin interacts with histone H3 and functions in gene repression and organogenesis in Arabidopsis. Plant Cell. 2007;19:2403–2416. doi: 10.1105/tpc.107.053579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bannikova O, et al. Identification of RNA targets for the nuclear multidomain cyclophilin atCyp59 and their effect on PPIase activity. Nucleic Acids Res. 2013;41:1783–1796. doi: 10.1093/nar/gks1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahn JC, et al. Classification of rice (Oryza sativa L. Japonica nipponbare) immunophilins (FKBPs, CYPs) and expression patterns under water stress. BMC Plant Biol. 2010;10:253. doi: 10.1186/1471-2229-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dominguez-Solis JR, et al. A cyclophilin links redox and light signals to cysteine biosynthesis and stress responses in chloroplasts. Proc Natl Acad Sci USA. 2008;105:16386–16391. doi: 10.1073/pnas.0808204105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruan SL, et al. Proteomic identification of OsCYP2, a rice cyclophilin that confers salt tolerance in rice (Oryza sativa L.) seedlings when overexpressed. BMC Plant Biol. 2011;11:34. doi: 10.1186/1471-2229-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park SW, et al. Cyclophilin 20-3 relays a 12-oxo-phytodienoic acid signal during stress responsive regulation of cellular redox homeostasis. Proc Natl Acad Sci USA. 2013;110:9559–9564. doi: 10.1073/pnas.1218872110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gottschalk M, et al. Ricinus communis cyclophilin: functional characterisation of a sieve tube protein involved in protein folding. Planta. 2008;228:687–700. doi: 10.1007/s00425-008-0771-8. [DOI] [PubMed] [Google Scholar]
- 42.Esau, K. The phloem. Vol. 5 (Gebr. Borntraeger, 1969).
- 43.Kehr J. Long-distance transport of macromolecules through the phloem. F1000 Biol Rep. 2009;1:31. doi: 10.3410/B1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galat A. Function-dependent clustering of orthologues and paralogues of cyclophilins. Proteins. 2004;56:808–820. doi: 10.1002/prot.20156. [DOI] [PubMed] [Google Scholar]
- 45.Pemberton TJ, Kay JE. The cyclophilin repertoire of the fission yeast Schizosaccharomyces pombe. Yeast. 2005;22:927–945. doi: 10.1002/yea.1288. [DOI] [PubMed] [Google Scholar]
- 46.Singh K, Zouhar M, Mazakova J, Rysanek P. Genome wide identification of the immunophilin gene family in Leptosphaeria maculans: a causal agent of Blackleg disease in Oilseed Rape (Brassica napus) OMICS. 2014;18:645–657. doi: 10.1089/omi.2014.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romano PG, Horton P, Gray JE. The Arabidopsis cyclophilin gene family. Plant Physiol. 2004;134:1268–1282. doi: 10.1104/pp.103.022160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He Z, Li L, Luan S. Immunophilins and parvulins. Superfamily of peptidyl prolyl isomerases in Arabidopsis. Plant Physiol. 2004;134:1248–1267. doi: 10.1104/pp.103.031005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vasudevan D, et al. Plant immunophilins: a review of their structure-function relationship. Biochim Biophys Acta. 2014;1850:2145–58. doi: 10.1016/j.bbagen.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 50.Mainali HR, Chapman P, Dhaubhadel S. Genome-wide analysis of Cyclophilin gene family in soybean (Glycine max) BMC Plant Biol. 2014;14:282. doi: 10.1186/s12870-014-0282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chalhoub B, et al. Plant genetics. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science. 2014;345:950–953. doi: 10.1126/science.1253435. [DOI] [PubMed] [Google Scholar]
- 52.Ke HM, Zydowsky LD, Liu J, Walsh CT. Crystal structure of recombinant human T-cell cyclophilin A at 2.5 A resolution. Proc Natl Acad Sci USA. 1991;88:9483–9487. doi: 10.1073/pnas.88.21.9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zydowsky LD, et al. Active site mutants of human cyclophilin A separate peptidyl-prolyl isomerase activity from cyclosporin A binding and calcineurin inhibition. Protein Sci. 1992;1:1092–1099. doi: 10.1002/pro.5560010903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J, Chen CM, Walsh CT. Human and Escherichia coli cyclophilins: sensitivity to inhibition by the immunosuppressant cyclosporin A correlates with a specific tryptophan residue. Biochemistry. 1991;30:2306–2310. doi: 10.1021/bi00223a003. [DOI] [PubMed] [Google Scholar]
- 55.Lee SS, et al. Rice cyclophilin OsCYP18-2 is translocated to the nucleus by an interaction with SKIP and enhances drought tolerance in rice and Arabidopsis. Plant Cell Environ. 2015;38:2071–87. doi: 10.1111/pce.12531. [DOI] [PubMed] [Google Scholar]
- 56.Iki T, Yoshikawa M, Meshi T, Ishikawa M. Cyclophilin 40 facilitates HSP90-mediated RISC assembly in plants. EMBO J. 2012;31:267–278. doi: 10.1038/emboj.2011.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Earley KW, Poethig RS. Binding of the cyclophilin 40 ortholog SQUINT to Hsp90 protein is required for SQUINT function in Arabidopsis. J Biol Chem. 2011;286:38184–38189. doi: 10.1074/jbc.M111.290130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ratajczak T, Carrello A. Cyclophilin 40 (CyP-40), mapping of its hsp90 binding domain and evidence that FKBP52 competes with CyP-40 for hsp90 binding. J Biol Chem. 1996;271:2961–2965. doi: 10.1074/jbc.271.6.2961. [DOI] [PubMed] [Google Scholar]
- 59.Nelson CJ, Santos-Rosa H, Kouzarides T. Proline isomerization of histone H3 regulates lysine methylation and gene expression. Cell. 2006;126:905–916. doi: 10.1016/j.cell.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 60.Li H, Luan S. The cyclophilin AtCYP71 interacts with CAF-1 and LHP1 and functions in multiple chromatin remodeling processes. Mol Plant. 2011;4:748–758. doi: 10.1093/mp/ssr036. [DOI] [PubMed] [Google Scholar]
- 61.Vasudevan D, Fu A, Luan S, Swaminathan K. Crystal structure of Arabidopsis cyclophilin38 reveals a previously uncharacterized immunophilin fold and a possible autoinhibitory mechanism. Plant Cell. 2012;24:2666–2674. doi: 10.1105/tpc.111.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shapiguzov A, Edvardsson A, Vener AV. Profound redox sensitivity of peptidyl-prolyl isomerase activity in Arabidopsis thylakoid lumen. FEBS Lett. 2006;580:3671–3676. doi: 10.1016/j.febslet.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 63.Edvardsson A, Eshaghi S, Vener AV, Andersson B. The major peptidyl-prolyl isomerase activity in thylakoid lumen of plant chloroplasts belongs to a novel cyclophilin TLP20. FEBS Lett. 2003;542:137–141. doi: 10.1016/S0014-5793(03)00366-1. [DOI] [PubMed] [Google Scholar]
- 64.Fu A, et al. A chloroplast cyclophilin functions in the assembly and maintenance of photosystem II in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2007;104:15947–15952. doi: 10.1073/pnas.0707851104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sirpio S, et al. AtCYP38 ensures early biogenesis, correct assembly and sustenance of photosystem II. Plant J. 2008;55:639–651. doi: 10.1111/j.1365-313X.2008.03532.x. [DOI] [PubMed] [Google Scholar]
- 66.Weighardt F, et al. A novel hnRNP protein (HAP/SAF-B) enters a subset of hnRNP complexes and relocates in nuclear granules in response to heat shock. J Cell Sci. 1999;112(Pt 10):1465–1476. doi: 10.1242/jcs.112.10.1465. [DOI] [PubMed] [Google Scholar]
- 67.Gullerova M, Barta A, Lorkovic ZJ. AtCyp59 is a multidomain cyclophilin from Arabidopsis thaliana that interacts with SR proteins and the C-terminal domain of the RNA polymerase II. RNA. 2006;12:631–643. doi: 10.1261/rna.2226106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng F, Wu J, Wang X. Genome triplication drove the diversification of Brassica plants. Horticulture research. 2014;1:14024. doi: 10.1038/hortres.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coordinators NR. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2016;44:D7–19. doi: 10.1093/nar/gkv1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.UniProt C. UniProt: a hub for protein information. Nucleic Acids Res. 2015;43:D204–212. doi: 10.1093/nar/gku989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mitchell A, et al. The InterPro protein families database: the classification resource after 15 years. Nucleic Acids Res. 2015;43:D213–221. doi: 10.1093/nar/gku1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goldberg T, et al. LocTree3 prediction of localization. Nucleic Acids Res. 2014;42:W350–355. doi: 10.1093/nar/gku396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kosugi S, Hasebe M, Tomita M, Yanagawa H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc Natl Acad Sci USA. 2009;106:10171–10176. doi: 10.1073/pnas.0900604106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sievers F, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539–539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2–a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Letunic I, Bork P. Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics. 2007;23:127–128. doi: 10.1093/bioinformatics/btl529. [DOI] [PubMed] [Google Scholar]
- 77.Letunic I, Bork P. Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res. 2011;39:W475–478. doi: 10.1093/nar/gkr201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim D, et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biology. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rappsilber J, Mann M, Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat Protoc. 2007;2:1896–1906. doi: 10.1038/nprot.2007.261. [DOI] [PubMed] [Google Scholar]
- 81.Larkindale J, Vierling E. Core genome responses involved in acclimation to high temperature. Plant Physiol. 2008;146:748–761. doi: 10.1104/pp.107.112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trupkin SA, Mora-Garcia S, Casal JJ. The cyclophilin ROC1 links phytochrome and cryptochrome to brassinosteroid sensitivity. Plant J. 2012;71:712–723. doi: 10.1111/j.1365-313X.2012.05013.x. [DOI] [PubMed] [Google Scholar]
- 83.Li M, et al. Proline isomerization of the immune receptor-interacting protein RIN4 by a cyclophilin inhibits effector-triggered immunity in Arabidopsis. Cell Host Microbe. 2014;16:473–483. doi: 10.1016/j.chom.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kovalev N, Nagy PD. Cyclophilin A binds to the viral RNA and replication proteins, resulting in inhibition of tombusviral replicase assembly. J Virol. 2013;87:13330–13342. doi: 10.1128/JVI.02101-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deng W, et al. Agrobacterium VirD2 protein interacts with plant host cyclophilins. Proc Natl Acad Sci USA. 1998;95:7040–7045. doi: 10.1073/pnas.95.12.7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma X, Song L, Yang Y, Liu D. A gain-of-function mutation in the ROC1 gene alters plant architecture in Arabidopsis. New Phytol. 2013;197:751–762. doi: 10.1111/nph.12056. [DOI] [PubMed] [Google Scholar]
- 87.Coaker G, Falick A, Staskawicz B. Activation of a phytopathogenic bacterial effector protein by a eukaryotic cyclophilin. Science. 2005;308:548–550. doi: 10.1126/science.1108633. [DOI] [PubMed] [Google Scholar]
- 88.Coaker G, Zhu G, Ding Z, Van Doren SR, Staskawicz B. Eukaryotic cyclophilin as a molecular switch for effector activation. Mol Microbiol. 2006;61:1485–1496. doi: 10.1111/j.1365-2958.2006.05335.x. [DOI] [PubMed] [Google Scholar]
- 89.Aumuller T, Jahreis G, Fischer G, Schiene-Fischer C. Role of prolyl cis/trans isomers in cyclophilin-assisted Pseudomonas syringae AvrRpt2 protease activation. Biochemistry. 2010;49:1042–1052. doi: 10.1021/bi901813e. [DOI] [PubMed] [Google Scholar]
- 90.Stangeland B, et al. Molecular analysis of Arabidopsis endosperm and embryo promoter trap lines: reporter-gene expression can result from T-DNA insertions in antisense orientation, in introns and in intergenic regions, in addition to sense insertion at the 5′ end of genes. J Exp Bot. 2005;56:2495–2505. doi: 10.1093/jxb/eri242. [DOI] [PubMed] [Google Scholar]
- 91.Kaur G, et al. Characterization of Peptidyl-Prolyl Cis-Trans Isomerase- and Calmodulin-Binding Activity of a Cytosolic Arabidopsis thaliana Cyclophilin AtCyp19-3. PloS one. 2015;10:e0136692. doi: 10.1371/journal.pone.0136692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saito T, et al. Expression of a gene for cyclophilin which contains an amino-terminal endoplasmic reticulum-targeting signal. Plant Cell Physiol. 1999;40:77–87. doi: 10.1093/oxfordjournals.pcp.a029477. [DOI] [PubMed] [Google Scholar]
- 93.Grebe M, et al. A conserved domain of the arabidopsis GNOM protein mediates subunit interaction and cyclophilin 5 binding. Plant Cell. 2000;12:343–356. doi: 10.1105/tpc.12.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kamauchi S, Nakatani H, Nakano C, Urade R. Gene expression in response to endoplasmic reticulum stress in Arabidopsis thaliana. FEBS J. 2005;272:3461–3476. doi: 10.1111/j.1742-4658.2005.04770.x. [DOI] [PubMed] [Google Scholar]
- 95.Jackson K, Soll D. Mutations in a new Arabidopsis cyclophilin disrupt its interaction with protein phosphatase 2A. Mol Gen Genet. 1999;262:830–838. doi: 10.1007/s004380051147. [DOI] [PubMed] [Google Scholar]
- 96.Schubert M, et al. Proteome map of the chloroplast lumen of Arabidopsis thaliana. J Biol Chem. 2002;277:8354–8365. doi: 10.1074/jbc.M108575200. [DOI] [PubMed] [Google Scholar]
- 97.Ingelsson B, Shapiguzov A, Kieselbach T, Vener AV. Peptidyl-prolyl isomerase activity in chloroplast thylakoid lumen is a dispensable function of immunophilins in Arabidopsis thaliana. Plant Cell Physiol. 2009;50:1801–1814. doi: 10.1093/pcp/pcp122. [DOI] [PubMed] [Google Scholar]
- 98.Zhang Y, et al. The cyclophilin CYP20-2 modulates the conformation of BRASSINAZOLE-RESISTANT1, which binds the promoter of FLOWERING LOCUS D to regulate flowering in Arabidopsis. Plant Cell. 2013;25:2504–2521. doi: 10.1105/tpc.113.110296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sirpio S, Holmstrom M, Battchikova N, Aro EM. AtCYP20-2 is an auxiliary protein of the chloroplast NAD(P)H dehydrogenase complex. FEBS Lett. 2009;583:2355–2358. doi: 10.1016/j.febslet.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 100.Edvardsson A, Shapiguzov A, Petersson UA, Schroder WP, Vener AV. Immunophilin AtFKBP13 sustains all peptidyl-prolyl isomerase activity in the thylakoid lumen from Arabidopsis thaliana deficient in AtCYP20-2. Biochemistry. 2007;46:9432–9442. doi: 10.1021/bi700426q. [DOI] [PubMed] [Google Scholar]
- 101.Lippuner V, et al. Cloning and characterization of chloroplast and cytosolic forms of cyclophilin from Arabidopsis thaliana. J Biol Chem. 1994;269:7863–7868. [PubMed] [Google Scholar]
- 102.Motohashi K, Koyama F, Nakanishi Y, Ueoka-Nakanishi H, Hisabori T. Chloroplast cyclophilin is a target protein of thioredoxin. J Biol Chem. 2003;278:31848–31852. doi: 10.1074/jbc.M304258200. [DOI] [PubMed] [Google Scholar]
- 103.Laxa M, Konig J, Dietz KJ, Kandlbinder A. Role of the cysteine residues in Arabidopsis thaliana cyclophilin CYP20-3 in peptidyl-prolyl cis-trans isomerase and redox-related functions. Biochem J. 2007;401:287–297. doi: 10.1042/BJ20061092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liebthal M, et al. Redox-Dependent Conformational Dynamics of Decameric 2-Cysteine Peroxiredoxin and its Interaction with Cyclophilin 20-3. Plant and Cell Physiology. 2016;57:1415–1425. doi: 10.1093/pcp/pcw031. [DOI] [PubMed] [Google Scholar]
- 105.Kim JH, Nguyen NH, Nguyen NT, Hong SW, Lee H. Loss of all three calreticulins, CRT1, CRT2 and CRT3, causes enhanced sensitivity to water stress in Arabidopsis. Plant Cell Rep. 2013;32:1843–1853. doi: 10.1007/s00299-013-1497-z. [DOI] [PubMed] [Google Scholar]
- 106.Tomasic Paic A, Fulgosi H. Chloroplast immunophilins. Protoplasma. 2015;253:249–58. doi: 10.1007/s00709-015-0828-z. [DOI] [PubMed] [Google Scholar]
- 107.Smith MR, et al. Cyclophilin 40 is required for microRNA activity in Arabidopsis. Proc Natl Acad Sci USA. 2009;106:5424–5429. doi: 10.1073/pnas.0812729106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


