Abstract
To define the features of glycemic variations in drug naïve type 2 diabetic (T2D) patients with different HbA1c values using continuous glucose monitoring (CGM), a total of 195 drug naïve T2D patients were admitted. The subjects were divided into the following groups: lower HbA1c values (≤8%), moderate HbA1c values (>8% and ≤10%), and higher HbA1c values (>10%). The patients underwent oral glucose tolerance tests and were then subjected to 3-day CGM. The primary endpoint was the differences in the 24-hr mean amplitude of glycemic excursions (MAGE) in patients with different HbA1c values. Patients with higher HbA1c values had larger MAGEs than those in the moderate and lower groups (7.44 ± 3.00 vs. 6.30 ± 2.38, P < 0.05, 7.44 ± 3.00 vs. 5.20 ± 2.35, P < 0.01, respectively). The 24-hr mean glucose concentrations increased incrementally in the patients with lower, moderate and higher HbA1c values. Moreover, the patients with higher HbA1c values exhibited higher peak glucose concentrations and prolongation in the time to peak glucose. Patients with higher HbA1c values had larger MAGE compared with those with lower and moderate HbA1c values. Our data indicated patients with higher HbA1c values should receive special therapy aimed at reducing the larger glycemic variations.
Introduction
Large glucose fluctuations in patients with may have implications for the risk for long-term diabetic complications1, 2. The underlying mechanisms might be the acute glucose fluctuations, or more specifically, the triggering the oxidative stress by acutely increased postprandial blood glucose levels3.
Postprandial glucose is an independent risk factor for cardiovascular disease4. Acute glucose fluctuations during postprandial periods other than chronic hyperglycemia have been shown to play an important role in oxidative stress in patients with type 2 diabetes (T2D)3. Microvascular and macrovascular complications are mainly5, 6, or at least partially6, 7, dependent on hyperglycemia. The rapid rise in postprandial blood glucose concentrations induce an over-production of peroxynitrite and nitrotyrosine3, 8, 9. Continued efforts have been made to suppress postprandial hyperglycemia in patients with T2D10. Research has indicated that improved postprandial excursions could smooth the oxidative and nitrosative stress11.
HbA1c is very useful as evidence of long term improvement in mean glucose in the large scale clinical studies of the treatment of T2D12–14. Reductions in HbA1c value in patients with diabetes leads to a reduction in the risk of death, myocardial infarction, and microvascular complications7. HbA1c does not necessarily reflect daily plasma glucose fluctuations; however, as different glucose profiles can confer similar HbA1c values, patients with similar HbA1c values do not necessarily bear the same glycemic variations1, 2, 15. Therefore, glycemic fluctuations should be considered when constructing strategies aimed to reduce the burden of diabetic complications as well as HbA1c values2. Continuous Glucose Monitoring (CGM) provides a unique opportunity to examine the 24-hrs glucose excursions in patients with T2D.
We therefore performed a single-center, open and retrospective study. In this study, we determined 24-hr glycemic variations using CGM in drug naïve T2D patients with different or even similar HbA1c values.
Results
A total of 195 drug naïve T2D patients who met the inclusion criteria (129 men and 66 women, aged 51.06 ± 9.87 years, BMI 25.08 ± 2.98 kg/m², and HbA1c values 9.32 ± 1.62%) were recruited into the current study.
The patients were then divided into three groups according to HbA1c values (L group with HbA1c values ≤8%, M group with HbA1c values >8% and ≤10%, and H group with HbA1c values >10%). A total of 55, 90 and 50 subjects were allocated into the L, M and H groups with mean HbA1c values of 7.60 ± 0.32%, 9.13 ± 0.53% and 11.55 ± 1.36%, respectively. We observed that the BMI in the H group was significantly lower than that in the L group (24.19 ± 3.11 vs. 25.47 ± 2.91, P < 0.05). Moreover, patients in the H and M groups were younger than those in the L group (48.40 ± 9.08 vs. 54.09 ± 9.73, P < 0.01, 50.68 ± 9.98 vs. 54.09 ± 9.73, P < 0.05, respectively) (Table 1).
Table 1.
Baseline for the study subjects.
| Items | L Group | M Group | H Group |
|---|---|---|---|
| N (Male/Female) | 55 (37/18) | 90 (56/34) | 50 (36/14) |
| Age (Yrs) | 54.09 ± 9.73 | 50.68 ± 9.98a* | 48.40 ± 9.08a** |
| BMI | 25.47 ± 2.91 | 25.34 ± 2.88 | 24.19 ± 3.11a* |
| HbA1c (%) | 7.60 ± 0.32 | 9.13 ± 0.53 | 11.55 ± 1.36 |
| Bg 0 (mmol/L) | 8.53 ± 1.66 | 10.09 ± 1.78a** | 12.40 ± 2.24ab** |
| Bg 30 (mmol/L) | 14.69 ± 2.46 | 15.59 ± 2.43 | 18.16 ± 3.41ab** |
| Bg 120 (mmol/L) | 18.47 ± 3.43 | 21.50 ± 3.75a* | 24.51 ± 4.58ab* |
| Cp 0 (pmol/L) | 2.58 ± 0.90 | 2.42 ± 0.82 | 1.81 ± 0.58ab* |
| Cp 30 (pmol/L) | 4.40 ± 2.15 | 3.54 ± 1.59a* | 2.50 ± 1.11ab* |
| Cp 120 (pmol/L) | 8.10 ± 2.51 | 5.74 ± 2.02a* | 3.72 ± 1.52ab* |
| Ins 0 (mU/L) | 8.97 ± 4.85 | 8.06 ± 4.75a* | 4.84 ± 2.26ab* |
| Ins 30 (mU/L) | 24.07 ± 17.25 | 18.04 ± 15.69 | 8.44 ± 4.87ab* |
| Ins 120 (mU/L) | 48.50 ± 26.40 | 28.03 ± 17.63a* | 14.72 ± 10.08ab* |
| HOMA-IR | 3.42 ± 1.87 | 3.69 ± 2.35 | 2.58 ± 1.15ab* |
| Matsuda Index | 85.04 ± 75.47 | 94.63 ± 71.63 | 125.88 ± 59.67ab* |
| HOMA-B | 37.81 ± 23.88 | 25.46 ± 15.90a** | 12.01 ± 6.81ab** |
Data are presented as the means ± SD. L group: Lower HbA1c values group, M group: Moderate HbA1c values group, H group: higher HbA1c values group, a*compared with the L Group (P < 0.05), a**compared with M Group (P < 0.01), b*compared with M Group (P < 0.05), b**compared with M Group (P < 0.01), Bg 0: blood glucose concentrations before glucose loading, Bg 30: blood glucose concentrations at 30 min after glucose loading, Bg 120: blood glucose concentrations at 120 min after glucose loading, Cp 0: serum C-Peptide concentrations before glucose loading, Cp 30: serum C-Peptide concentrations 30 min after glucose loading, Cp 120: serum C-Peptide concentrations 120 min after glucose loading, Ins 0: serum insulin concentrations before glucose loading, Ins 30: serum insulin concentrations 30 min after glucose loading, Ins 120: serum insulin concentrations 120 min after glucose loading.
Oral glucose tolerance test (OGTT) data showed that patients with higher HbA1c values exhibited higher blood glucose concentrations and lower C-peptide and insulin levels at 0, 30, and 120 min after glucose load (Table 2).
Table 2.
CGM monitored blood profiles in study subjects.
| Items | L Group | M Group | H Group |
|---|---|---|---|
| 24-hrs MG (mmol/L) | 9.46 ± 1.85 | 11.47 ± 2.19a** | 13.52 ± 2.20ab** |
| SDMG (mmol/L) | 2.00 ± 0.79 | 2.55 ± 0.90a** | 2.88 ± 0.99a**b* |
| MAGE (mmol/L) | 5.20 ± 2.35 | 6.30 ± 2.38a* | 7.44 ± 3.00a**b* |
| AUC (<3.9 mmol/L*Day) | 0.01 ± 0.07 | 0.00 ± 0.01 | 0.00 ± 0.03 |
| AUC (>10.0 mmol/L*Day) | 0.80 ± 0.93 | 2.18 ± 1.57a** | 3.76 ± 1.90ab** |
| PG (mmol/L) | 13.23 ± 3.49 | 16.27 ± 3.62a** | 18.63 ± 2.98 a**b** |
| Time to peak (min) | 91.36 ± 31.01 | 82.94 ± 26.54 | 108.00 ± 30.19ab* |
Data are presented as the means ± SD. L Group: Lower HbA1c values group, M Group: Moderate HbA1c values group, H Group: higher HbA1c values group, a*compared with the L Group (P < 0.05), a**compared with M Group (P < 0.01), b*compared with M Group (P < 0.05), b**compared with M Group (P < < 0.01), ab**compared with the L and M Groups (P < 0.01), ab*compared with the L and M Groups (P < 0.05), 24-hr MG: 24-hr mean glucose concentrations, SDMG: 24-hr standard deviation of MG, AUC: the incremental area under the curve, PG: peak glucose concentrations.
CGM data showed that 24-hr mean glucose concentrations (MG) (11.47 ± 2.19 vs. 9.46 ± 1.85 mmol/L, P < 0.01; 13.52 ± 2.20 vs. 9.46 ± 1.85 mmol/L, P < 0.01; 13.52 ± 2.20 vs. 11.47 ± 2.19 mmol/L, P < 0.01, respectively); the 24-hr standard deviation of the MG (SDMG) (2.55 ± 0.90 vs. 2.00 ± 0.79 mmol/L, P < 0.01; 2.88 ± 0.99 vs. 2.00 ± 0.79 mmol/L, P < 0.01; 2.88 ± 0.99 vs. 2.55 ± 0.90 mmol/L, P < 0.05, respectively); the 24-hr mean amplitude of glycemic excursions (MAGE) (6.30 ± 2.38 vs. 5.20 ± 2.35 mmol/L, P < 0.05; 7.44 ± 3.00 vs. 5.20 ± 2.35 mmol/L, P < 0.01; 7.44 ± 3.00 vs. 6.30 ± 2.38 mmol/L, P < 0.05, respectively), and the incremental area under the curve (AUC) of the glucose above 10 mmol/L (2.18 ± 1.57 vs. 0.80 ± 0.93 mmol/L*Day, P < 0.01; 3.76 ± 1.90 vs. 0.80 ± 0.93 mmol/L*Day, P < 0.01; 3.76 ± 1.90 vs. 2.18 ± 1.57 mmol/L*Day, P < 0.01, respectively) were progressively and significantly increased alongside HbA1c values in drug naïve patients with T2D. There were no differences in the incremental AUC less than 3.9 mmol/L between the L, M and H groups (0.007 ± 0.07 vs. 0.009 ± 0.01 mmol/L*Day, P > 0.05; 0.007 ± 0.07 vs. 0.002 ± 0.003 mmol/L*Day, P > 0.05; 0.009 ± 0.01 vs. 0.002 ± 0.03 mmol/L*Day, P > 0.05, respectively) (Table 2). We also compared glycemic variations in subjects with HbA1c values from 8% to 10%, although there were no differences in the MAGE, the 24-hr MG, the incremental AUC above 10 mmol/L or less than 3.9 mmol/L, or the SDBG between the two subgroups (HbA1c values >8 and <9%, and ≥9 and <10%), with the exception of patients with HbA1c values ≥ 9 and <10%, who had higher peak glucose concentrations than those with HbA1c values ≥ 8 and <9% (Supplementary Table 1). In agreement with the similar glycemic variations, the hourly mean plasma glucose concentrations did not differ between the two groups, with the exception of the 24-hr mean glucose concentrations (Fig. 2).
Figure 2.
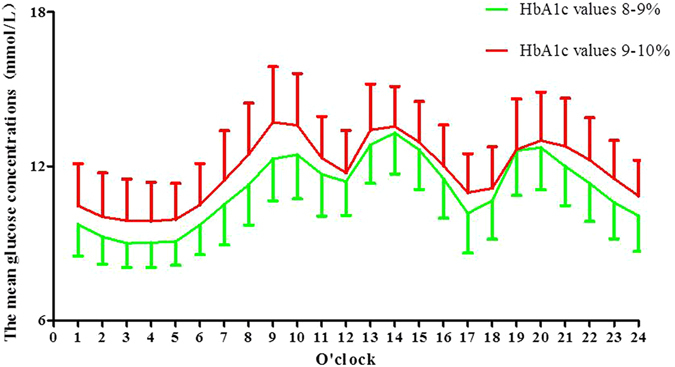
The average glucose concentrations per hour in patients with HbA1c values from 8% to 10%.
The average blood glucose concentrations per hour in the H group were higher than in the M group. As expected, the average blood glucose concentrations per hour in the M group were also significantly higher than those in the L group (Fig. 1). Moreover, higher peak glucose concentrations and prolongation in the time to glucose peak after breakfast were observed in the H group. Subjects in the H group exhibited higher peak glucose concentrations than in the L and M groups (18.63 ± 2.98 vs. 13.23 ± 3.49 mmol/L, P < 0.01, 18.63 ± 2.98 vs. 16.27 ± 3.62 mmol/L, P < 0.01, respectively). In accordance with the increased peak glucose concentrations, the time to peak in patients with higher HbA1c values was significantly prolonged compared with subjects in the L and M groups (108.00 ± 30.19 vs. 91.36 ± 31.01 min, P < 0.05, 108.00 ± 30.19 vs. 82.94 ± 26.54 min, P < 0.01, respectively). However, prolongation of glucose time to peak after lunch and dinner were not observed in the current study.
Figure 1.
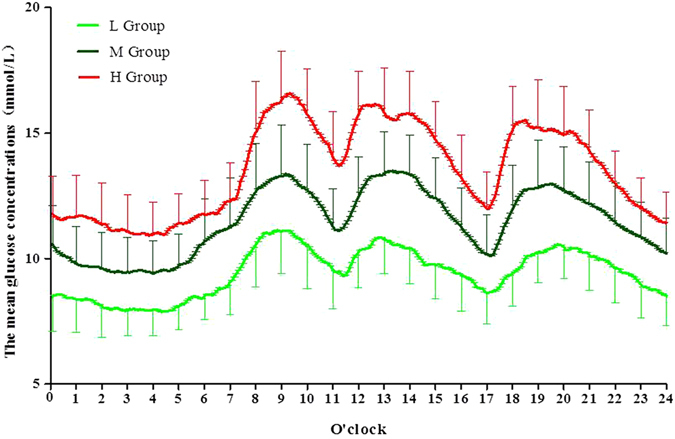
The average glucose concentrations per hour in the L, M, and H groups.
We also used HbA1c as a continuous rather than a discrete variable. Multiple linear regression analyses were performed to assess the independent effects (MBG, SDMG, MAGE, FBG, PBG, HOMA-B, and HOMA-IR) on HbA1c. Our data showed that only FBG, MBG, HOMA-IR and SDBG remained significant in the stepwise regression analysis. The standardized regression coefficients were 0.476 (t = 5.735, P < 0.01), 0.260 (t = 3.016, P < 0.01), −0.197 (t = −3.370, P < 0.05) and 0.150 (t = 2.444, P < 0.05), respectively.
We also observed the contributions of SDMG and MAGE to HbA1c values. The patients were further divided into three equal groups according to the tertiles of SDBG or MAGE. The HbA1c values gradually increased from the lowest to the highest tertile of SDBG or MAGE (8.69 ± 1.35 vs. 9.37 ± 1.49 vs. 9.90 ± 1.77 mmol/L, all P < 0.01, and 8.64 ± 1.27 vs. 9.57 ± 1.60 vs. 9.75 ± 1.73 mmol/L, all P < 0.01). Our data showed that both SDMG and MAGE were strongly correlated with HbA1c values (r = 0.361, P < 0.01, and r = 0.319, P < 0.01, respectively).
We also analyzed β-cell function and insulin sensitivity in patients with different HbA1c values. The multivariate analysis controlled for age and BMI to determine the significance of the differences between groups, particularly with respect to the insulin sensitivity/resistance. Our data showed that, as expected, patients with higher HbA1c values had lower HOMA-B values (H group vs. M group vs. L group: 12.01 ± 6.81 vs. 25.46 ± 15.90 vs. 37.81 ± 23.88, P < 0.01, respectively). Interestingly, patients with higher HbA1c values (>10%) had significant induction of Matsuda index (125.88 ± 59.67 vs. 94.63 ± 71.63, P < 0.05, 125.88 ± 59.67 vs. 85.04 ± 75.47, P < 0.05, respectively) compared to those in the M and L groups. In accordance with the increased Matsuda index, the HOMA-IR in patients with higher HbA1c values was significantly decreased compared to patients in the M group (2.58 ± 1.15 vs. 3.69 ± 2.35, P < 0.05), and was insignificantly reduced compared with those in the L group (2.58 ± 1.15 vs. 3.42 ± 1.87, P > 0.05). We did not observe a difference in HOMA-IR between the M and L groups (Table 1).
Discussion
This relatively large study revealed a novel observation that glycemic variations gradually increased with HbA1c values in drug naïve T2D patients. We also observed that drug naïve patients with HbA1c values above 10% exhibited larger blood glucose fluctuations, higher peak glucose concentrations, and prolongation in the glucose time to peak after breakfast. In addition, patients with moderate HbA1c values (>8% and ≤10%) had similar glycemic variations, with the exception of 24-hrs mean blood glucose. Our data indicated that patients with higher HbA1c values should receive “special therapy” aimed at reducing the larger glycemic variations and the increased peak glucose concentrations.
A achievement of the optimal HbA1c target in patients with T2D is the main consideration for physicians when choosing glucose lowering therapy16. HbA1C is generated by the exposure of overall blood profiles for an extended period, which does not necessarily reflect daily plasma glucose variations throughout the day1, 2. CGM provides a unique opportunity to examine the 24-hr glycemic excursions in patients with T2D, which might be a better tool to determine overall blood glucose profiles. CGM shows the potential effectiveness in subjects with diabetes17 and in patients using intensive insulin therapy18. A set of metrics obtained from CGM could be used to describe the glycemic variations (GV) in type 1 and type 2 diabetes19–21. Furthermore, an observational study reported that prediabetic obese subjects have higher GV, namely SD and MAGE, compared with normal weight individuals22. Studies have demonstrated that there was a high degree of correlation between SD and MAGE17, 23. The percent coefficient of variation (%CV) displays the interpretation of GV17, 23, 24. Using CGM data, clinical researchers and clinicians could efficiently evaluate the quality of the glycemic control, which might be important for decision-making25.
In the current study, our CGM data revealed that patients with different HbA1c values exhibited different glycemic variations. Diabetic patients with higher HbA1c values had larger MAGE, larger SDMG, and increased incremental AUC >10.0 mmol/L, Moreover, patients with higher HbA1c values exhibited increased 24-hr MG. Interestingly, patients with HbA1c values above 10% had higher peak glucose concentrations and a prolongation in the glucose time to peak after breakfast. We could infer that the reason that the patients who had higher HbA1c values exhibited larger glycemic variations might partially depend on the higher peak glucose concentrations and prolongation of glucose time to peak. However, our data could not address the underlying mechanisms of the increase in peak glucose concentrations and the prolongation of the glucose time to peak.
In the current study, the decreased HOMA-IR and increased Matsuda index in the H group compared with those in the L group indicated that the patients with HbA1c values above 10% had lower insulin resistance. Moreover, we also observed that the BMI in the H group was significantly lower than that in the L group. One speculation might be that these patients had very poor glycemic control, and they therefore had suffered weight loss from the urinary loss of calories. The Chinese have lower BMI and smaller waist circumferences when compared to the Western participants who had higher BMI and waist circumferences26. In addition, Chinese patients also have a higher percentage of body fat than Europeans and African Americans at the same level of BMI27, 28. Thus, we could infer that decreased body weight might be the reason for the increased insulin sensitivity, because body fat weight is associated with decreased Matsuda index and increased HOMA-IR29. The increased insulin sensitivity in patients with HbA1c values above 10% compared with patients with HbA1c values less than 10% indicated that HbA1c values might correlate with the Matsuda index and HOMA-IR values. HOMA-IR in patients with higher HbA1c values was significantly reduced compared with the values of those in the M group (2.58 ± 1.15 vs. 3.69 ± 2.35, P < 0.05). The difference in HOMA-IR was not evident between the L and M group. This finding might be attributed to the fact that we used only 138 out of 195 serum samples collected from patients at 0, 30, and 120 min after glucose loading, which were used to measure the glucose, insulin, and C-peptide concentrations. Future studies using HbA1c values are needed to identify changes in insulin resistance. Our data also suggested that, as expected, patients with higher HbA1c values had lower pancreatic β cell function (HOMA-B values).
A stratified analysis comparing the blood glycemic profiles in patients with moderate HbA1c values, from 8% to 10% revealed that each increase of 1% in HbA1c value did not result in any significant differences in hourly blood glucose concentrations, peak glucose concentrations, or blood glycemic variations (Supplementary Table 1).
The current study described a novel observation of gradually increased glycemic variations with HbA1c values in a stepwise manner in drug naïve T2D patients, and patients with higher HbA1c values (>10%) had higher peak glucose concentrations and prolonged glucose time to peak after breakfast. These results differ from those of a previous study that reporting the longer glucose time to peak was observed after breakfast and dinner in drug-naïve, Japanese type 2 diabetic patients with higher HbA1c values30. They observed that the Peak Time and the Increase Range were maximal after dinner30. However, in the current study, the maximal blood glucose increase range was only observed after breakfast, this might be due to the different number of study subjects recruited. Future studies are needed to identify changes in blood variations in drug naïve type 2 diabetic patients with different HbA1c values.
In the current study, we observed that drug naïve diabetic patients with higher HbA1c values (>10%) may require receive more attention in order to address the larger blood glycemic variations, the higher glucose peak concentrations, as well as the prolongation of glucose time to peak. However, the study patient population was limited to the Nanjing area in China; therefore, the situation might not be the same for other geographical regions or populations. Evidence has demonstrated that patients with T2D in China are quite variable when compared to Western countries, such as the thrifty gene, which is prevalent in the Chinese31, the different pattern of intake of nutrients and life-style32, the lower insulin dose requirements, and the higher remission rate following short intensive insulin therapy33. Moreover, Asian T2D populations have the lower BMI and smaller waist circumferences compared to the Western participants26. The “special therapy” recommended in this study might include acarbose34, DPP-4 inhibitor35, GLP-1 receptor agonists36, and SGLT-2 inhibitor therapy37. These therapies have shown potential improvements in glucose fluctuation34–37 and lower insulin dose requirement by patients with T2D to maintain euglycemic control in Chinese populations35, 36. Furthermore, these “special therapies” should be given to patients with high MAGE and HbA1c values over 10%. Our study has other limitations. First, the patient population was very heterogeneous (with A1C value from 6 to 12%). Second, untreated patients were mainly varying in the duration and severity of their diabetes, with differing beta cell reserve, and differing insulin sensitivities.
In conclusion, our data reveal that the glycemic variations gradually increased with the HbA1c values in drug naïve T2D patients. Our data also indicated that patients with higher HbA1c values might need some special therapies aimed at reducing the larger glycemic variations and the prolongation in time-to-peak hyperglycemia, especially after breakfast.
Methods
This was a single-center, open and retrospective trial. Between June 2010 and November 2015, a total of 195 drug naive T2D patients were recruited in Nanjing First Hospital, Nanjing Medical University, China. The inclusion criteria were 1) Patients aged between 18 and 80 years; 2) Newly diagnosed, drug-naive T2D patients; 3) BMI 21 to 35 kg/m2. Patients were excluded if they had ketoacidosis, chronic kidney disease, positive antiglutamic acid decarboxylase (aGAD) antibodies, or if they had maturity onset diabetes in the young (MODY) or mitochondria diabetes mellitus38. Patients with known cancers were excluded38, 39. The study was approved by the ethics committee of Nanjing First Hospital, Nanjing Medical University. All patients gave written informed consent. The methods were conducted in accordance with the Declaration of Helsinki guidelines, including any relevant details.
Recruited patients were admitted as inpatients. Serum samples were obtained at 0, 30, and 120 min after oral administration of 75 g glucose for HbA1c, glucose, insulin and C-peptide concentrations determination. Plasma insulin was determined using an insulin radioimmunoassay kit (Beijing Technology Company, Beijing, China). HbA1c was measured by a DiaSTAT HbA1c analyzer (Bio-Rad, Hercules, CA). C-peptide and glucose concentrations were measured centrally at the central laboratory in Nanjing First Hospital, Nanjing Medical University. After the baseline parameters were assessed, retrospective CGM (Sof-sensor, CGMS-Gold, Medtronic Incorporated, Northridge, USA) was performed for 3 days, as described previously34, 40. Briefly, the CGM sensor was subcutaneously embedded on Day 0 approximately 16:00–17:00 PM. The patients continued with the sensors, if CGM was going well. Subjects were instructed to keep the sensor fixed and waterproof. The study nurse inputted at least 4 calibration readings every day. On Day 4, at approximately 16:00–17:00 PM, subjects had the sensors removed, and the CGM data were saved by the investigator, as described previously34, 40, 41. All subjects were instructed to maintain physical activity according to their doctors’ personalized instructions and received meals consisting of a total daily caloric intake of 25 kcal/kg/day. The percentages of carbohydrate, proteins and fats were 55%, 17% and 28%, respectively. Patients were instructed to have breakfast, lunch and dinner at 0700, 1100 and 1700, respectively. Subjects were then divided into a lower HbA1c values (≤8%) group (L group), a moderate HbA1c values (>8% and ≤10%) group (M group), and a higher HbA1c values (>10%) group (H group).
The 24-hr MG, the SDBG, and the incremental AUC of blood glucose above 10.0 mmol/L or less than 3.9 mmol/L, and the hourly MG were calculated by software provided by Medtronic Incorporated, USA. The MAGE was calculated manually for each patient by measuring the arithmetic mean of the ascending and descending excursions between consecutive peaks and nadirs for the same 24-hr period, and only absolute excursion values >1 SD were considered, as described previously34, 40. The glucose time to peak after breakfast was also calculated among groups. β-cell function was assessed by the homoeostasis model assessment B (HOMA-B), the insulin sensitivity was indicated by HOMA-IR38, 42 and the Matsuda index was calculated as previous described29, 43.
The primary endpoint was the difference in MAGE in patients with different HbA1c values. Secondary endpoints were the differences in 24-hr MG, SDBG, incremental AUC of blood glucose above 10.0 mmol/L or less than 3.9 mmol/L, peak glucose concentrations and glucose time to peak after breakfast, hourly MG, β-cell function and insulin resistance among patients with different HbA1c values.
Statistical analysis
All data are presented as the means ± SD. Statistical significance was determined by one-way analysis of variance (ANOVA), or two-way ANOVA for repeated measurements for the group comparisons, followed by Bonferroni-Dunn post hoc test. P < 0.05 was considered to be statistically significant. We used correlation coefficients and multiple linear regressions analyses to examine the interrelationships among the glycemic variations and HbA1c. All of the statistical analyses were performed using the Statistical Product and Services Solutions (SPSS) package (Version 11.5, SPSS, Science, Chicago, USA).
Electronic supplementary material
Acknowledgements
This research was funded by the Nanjing Public Health Bureau Project (No. YKK11110), the Science and Technology Support Program of Jiangsu Province (No. BL2014010) and a Project funded by the China Postdoctoral Science Foundation (No. 2015M581829).
Author Contributions
J.H.M., and L.Y. contributed to the conception and design of the study. F.F.L., P.H.Z., R.N.Y., D.F.Z., and H.Q.L., contributed to the conduct/data collection. B.L.L., X.F.S., H.H.Z., and J.D.W. contributed to the data analysis. F.F.L. contributed to writing the manuscript and the final approval of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Feng-fei Li, Bing-li Liu, Reng-na Yan, Hong-hong Zhu and Pei-hua Zhou contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01719-y
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nathan DM, et al. Translating the A1C assay into estimated average glucose values. Diabetes care. 2008;31:1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Prato S. In search of normoglycaemia in diabetes: controlling postprandial glucose. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2002;26(Suppl 3):S9–17. doi: 10.1038/sj.ijo.0802172. [DOI] [PubMed] [Google Scholar]
- 3.Monnier L, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. Jama. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 4.Nakagami T. Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia. 2004;47:385–394. doi: 10.1007/s00125-004-1334-6. [DOI] [PubMed] [Google Scholar]
- 5.The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes44, 968–983, doi:10.2337/diab.44.8.968 (1995). [PubMed]
- 6.Klein R. Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes care. 1995;18:258–268. doi: 10.2337/diacare.18.2.258. [DOI] [PubMed] [Google Scholar]
- 7.Stratton IM, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceriello A, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57:1349–1354. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y, Liu W, Huang R, Zhang X. Postchallenge plasma glucose excursions, carotid intima-media thickness, and risk factors for atherosclerosis in Chinese population with type 2 diabetes. Atherosclerosis. 2010;210:302–306. doi: 10.1016/j.atherosclerosis.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Gallwitz B. Implications of postprandial glucose and weight control in people with type 2 diabetes: understanding and implementing the International Diabetes Federation guidelines. Diabetes care. 2009;32(Suppl 2):S322–325. doi: 10.2337/dc09-S331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ceriello A, et al. Role of hyperglycemia in nitrotyrosine postprandial generation. Diabetes care. 2002;25:1439–1443. doi: 10.2337/diacare.25.8.1439. [DOI] [PubMed] [Google Scholar]
- 12.Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. The New England journal of medicine342, 381–389, doi:10.1056/NEJM200002103420603 (2000). [DOI] [PMC free article] [PubMed]
- 13.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet352, 837–853 (1998). [PubMed]
- 14.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. The New England journal of medicine329, 977–986, doi:10.1056/NEJM199309303291401 (1993). [DOI] [PubMed]
- 15.Zhou J, et al. Relationship between HbA1c and continuous glucose monitoring in Chinese population: a multicenter study. PloS one. 2013;8:e83827. doi: 10.1371/journal.pone.0083827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inzucchi SE, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 17.Rodbard D, et al. Improved quality of glycemic control and reduced glycemic variability with use of continuous glucose monitoring. Diabetes technology & therapeutics. 2009;11:717–723. doi: 10.1089/dia.2009.0077. [DOI] [PubMed] [Google Scholar]
- 18.Rodbard D, Jovanovic L, Garg SK. Responses to continuous glucose monitoring in subjects with type 1 diabetes using continuous subcutaneous insulin infusion or multiple daily injections. Diabetes technology & therapeutics. 2009;11:757–765. doi: 10.1089/dia.2009.0078. [DOI] [PubMed] [Google Scholar]
- 19.Fabris C, et al. Glucose variability indices in type 1 diabetes: parsimonious set of indices revealed by sparse principal component analysis. Diabetes technology & therapeutics. 2014;16:644–652. doi: 10.1089/dia.2013.0252. [DOI] [PubMed] [Google Scholar]
- 20.Fabris C, et al. Parsimonious Description of Glucose Variability in Type 2 Diabetes by Sparse Principal Component Analysis. Journal of diabetes science and technology. 2015;10:119–124. doi: 10.1177/1932296815596173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodbard D. New and improved methods to characterize glycemic variability using continuous glucose monitoring. Diabetes technology & therapeutics. 2009;11:551–565. doi: 10.1089/dia.2009.0015. [DOI] [PubMed] [Google Scholar]
- 22.Salkind SJ, Huizenga R, Fonda SJ, Walker MS, Vigersky RA. Glycemic variability in nondiabetic morbidly obese persons: results of an observational study and review of the literature. Journal of diabetes science and technology. 2014;8:1042–1047. doi: 10.1177/1932296814537039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodbard D. Increased glycemic variability at the onset and during progression of type 2 diabetes-commentary. Diabetes technology & therapeutics. 2013;15:445–447. doi: 10.1089/dia.2013.0146. [DOI] [PubMed] [Google Scholar]
- 24.Rodbard D. Clinical interpretation of indices of quality of glycemic control and glycemic variability. Postgraduate medicine. 2011;123:107–118. doi: 10.3810/pgm.2011.07.2310. [DOI] [PubMed] [Google Scholar]
- 25.Rodbard D. Interpretation of continuous glucose monitoring data: glycemic variability and quality of glycemic control. Diabetes technology & therapeutics. 2009;11(Suppl 1):S55–67. doi: 10.1089/dia.2008.0132. [DOI] [PubMed] [Google Scholar]
- 26.Ntuk UE, Gill JM, Mackay DF, Sattar N, Pell JP. Ethnic-specific obesity cutoffs for diabetes risk: cross-sectional study of 490,288 UK biobank participants. Diabetes care. 2014;37:2500–2507. doi: 10.2337/dc13-2966. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, et al. Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. The American journal of clinical nutrition. 1994;60:23–28. doi: 10.1093/ajcn/60.1.23. [DOI] [PubMed] [Google Scholar]
- 28.Mott JW, et al. Relation between body fat and age in 4 ethnic groups. The American journal of clinical nutrition. 1999;69:1007–1013. doi: 10.1093/ajcn/69.5.1007. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki R, et al. Association of Waist Circumference and Body Fat Weight with Insulin Resistance in Male Subjects with Normal Body Mass Index and Normal Glucose Tolerance. Intern Med. 2016;55:1425–1432. doi: 10.2169/internalmedicine.55.4100. [DOI] [PubMed] [Google Scholar]
- 30.Ando K, Nishimura R, Tsujino D, Seo C, Utsunomiya K. 24-hour glycemic variations in drug-naive patients with type 2 diabetes: a continuous glucose monitoring (CGM)-based study. PloS one. 2013;8:e71102. doi: 10.1371/journal.pone.0071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? American journal of human genetics. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- 32.Yabe D, Seino Y, Fukushima M, Seino S. beta cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Current diabetes reports. 2015;15:602. doi: 10.1007/s11892-015-0602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeVries JH. Intensive insulin therapy for type 2 diabetes at diagnosis. The lancet. Diabetes &. endocrinology. 2013;1:3–4. doi: 10.1016/S2213-8587(13)70001-9. [DOI] [PubMed] [Google Scholar]
- 34.Li FF, et al. Influence of Acarbose on Plasma Glucose Fluctuations in Insulin-Treated Patients with Type 2 Diabetes: A Pilot Study. International journal of endocrinology. 2015;2015:903524. doi: 10.1155/2015/903524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li FF, et al. Effects of saxagliptin add-on therapy to insulin on blood glycemic fluctuations in patients with type 2 diabetes: A randomized, control, open-labeled trial. Medicine. 2016;95:e5229. doi: 10.1097/MD.0000000000005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li, F. F. et al. Exenatide Add-on to Continuous Subcutaneous Insulin Infusion Therapy Reduces Bolus Insulin Doses in Patients with Type 2 Diabetes: A Randomized, Controlled, Open-Label Trial. Diabetes therapy: research, treatment and education of diabetes and related disorders, doi:10.1007/s13300-016-0222-7 (2016). [DOI] [PMC free article] [PubMed]
- 37.Li FF, et al. Influence of Dapagliflozin on Glycemic Variations in Patients with Newly Diagnosed Type 2 Diabetes Mellitus. Journal of diabetes research. 2016;2016:5347262. doi: 10.1155/2016/5347262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weng J, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371:1753–1760. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 39.Ziegler R, et al. ProAct study: new features of insulin pumps improve diabetes management and glycemic control in patients after transition of continuous subcutaneous insulin infusion systems. Diabetes technology & therapeutics. 2013;15:738–743. doi: 10.1089/dia.2013.0090. [DOI] [PubMed] [Google Scholar]
- 40.Li FF, et al. Blood Glucose Fluctuations in Type 2 Diabetes Patients Treated with Multiple Daily Injections. Journal of diabetes research. 2016;2016:1028945. doi: 10.1155/2016/1028945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou J, et al. Reference values for continuous glucose monitoring in Chinese subjects. Diabetes care. 2009;32:1188–1193. doi: 10.2337/dc09-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matthews DR, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 43.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


