Abstract
Natural T cells [cluster of differentiation (CD) 3+CD56+] and natural killer (NK) cells (CD3−CD56+) are particularly abundant in the human liver and serve an important role in immune responses in the liver. The aim of the present study was to extensively determine the phenotypic and functional characteristics of natural T and NK cells in human hepatocellular carcinoma (HCC). Tumorous and non-tumorous tissue infiltrating lymphocytes (TILs and NILs, respectively) and peripheral blood mononuclear cells (PBMCs) from patients with hepatocellular carcinoma (HCC) were obtained to determine the frequency and phenotype of natural T/NK cells by a multicolor fluorescence activated cell sorting analysis. The abundance of natural T cells and NK cells was decreased in TILs vs. NILs (natural T cells, 6.315±1.002 vs. 17.16±1.804; NK cells, 6.324±1.559 vs. 14.52±2.336, respectively). However such results were not observed in PBMCs from HCC patients vs. that of healthy donors. Notably, a substantial fraction of the natural T cells (21.96±5.283) in TILs acquired forkhead box P3 (FOXP3) expression, and the FOXP3+ natural T cells lost the expression of interferon-γ and perforin. Conversely, being similar to the conventional FOXP3+ regulatory T cells, the FOXP3+ natural T cells assumed a specific phenotype that was characteristic of CD25+, CD45RO+ and cytotoxic T-lymphocyte-associated protein 4+. Consistent with the phenotypic conversion, the present functional results indicate that FOXP3 expression in natural T cells contributes to the acquisition of a potent immunosuppressive capability. In conclusion, the present study describes a different representation of natural T cells and NK cells in local tumor tissues and in the periphery blood of patients with HCC, and identified a new type of FOXP3-expressing natural T cell spontaneously arising in the TILs of HCC.
Keywords: natural T cells, natural killer cells, human hepatocellular carcinoma, regulatory T cell
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common type of malignancy worldwide and is the third leading cause of cancer-associated mortality worldwide (1). The majority of patients with HCC are diagnosed when the disease is at an advanced stage, and potentially curative therapeutic options for HCC are limited (2). Thus, there is an urgent need to identify and establish alternative approaches for the treatment of HCC. Immunotherapy has gained attention in previous years as a promising adjuvant therapy for HCC patients. A complex role of the immune system in the development and progression of HCC has been highlighted by a growing number of studies (3–5). To improve the clinical efficacy of cancer immunotherapy, a detailed understanding of the immunological mechanism of tumorigenesis and progression is required.
The tumor microenvironment is a systematic concept that defines the behavior of cancer not only by the genetics of the tumor cells alone but also by the surrounding milieu. Tumor infiltrating lymphocytes (TILs), an important part of the tumor surveillance system, have been detected in a number of patients with HCC. In particular, cluster of differentiation (CD) 8+ cytotoxic T lymphocytes (CTLs) were found to be linked to an improved patient survival (6,7). However, the spontaneous clearance of established HCC lesions by endogenous immune mechanisms is rare, despite spontaneous humoral and cellular immune responses having been detected in a significant proportion of patients with HCC against a number of tumor-associated antigens (TAAs) (8–10). As a number of TAAs are derived from normal self-constituents, anti-tumor immunity against these TAAs was not sufficient to control the growth of the tumors. Additionally, in the pressure of a host immune system, HCC has evolved multiple passive and active mechanisms that generate a suppressive network in order to evade the host's anti-tumor immune response (11,12). Such mechanisms include an accumulation of immunosuppression by regulatory T cells (Tregs), tumor-associated macrophages and dysfunctional dendritic cells (DC) (13–15). Tregs are the most extensively studied mechanism, and an increased frequency of Tregs in the peripheral blood and tumor tissues of HCC has been studied previously (16,17). Furthermore, the upregulation of Tregs is notably coupled with a significant decrease and impaired effector function of cytotoxic CD8+ T cells, and a worse predicted survival rate for patients with HCC (18,19). However, cells with a potential suppressive function against anti-tumor immunity are not confined to this cell subset. Regulatory γδ T cells and natural killer (NK) T cells had been gradually identified as having a potential suppressive function by several studies (20–22). The distribution of forkhead box P3 (FOXP3)+ cells in various cell subsets of HCC has been presented in our previous study (23).
The range of lymphocytes present in the liver differs markedly from that in the peripheral blood and other organs (24–26). Lymphocytes in the liver are characterized by a high proportion of NK cells and significant numbers of several unconventional T cell subsets, such as T cells that co-express CD3 and CD56, which can also be referred to as natural T cells (27). Considering the high prevalence of NK and natural T cells in the liver, it is suggested that they perform a critical role in immune homeostasis and pathogenesis of the liver. The present study was interested in the implication of NK and natural T cells in the pathology of HCC. As reported by Kawarabayashi et al (28) a decreased abundance of NK cells and natural T cells in liver diseases may be involved in their susceptibility to HCC. Therefore, the principal objective of the present study was to determine the representation of NK cells and natural T cells in local tumor tissues and in peripheral blood of patients with HCC. The present study observed a marked reduction in NK cells and natural T cells in TILs vs. non-tumor infiltrating lymphocytes (NILs). However, this pattern was not observed in peripheral blood mononuclear cells (PBMCs) of patients with HCC vs. healthy donors. In addition, a new type of FOXP3-expressing natural T cell was identified in the tumorous tissues of HCC, and several phenotypic and functional tests were then performed on this special cell subset. It is considered that this discovery may provide a new mechanistic explanation for HCC induced immunosuppression, and outline a previously unrecognized potential target for the immunotherapy of HCC.
Materials and methods
Patients and healthy donors
This study was approved by the hospital ethics review committee at the Peking University People's Hospital (Beijing, China), and written informed consent was obtained from all patients prior to the start of the study. A total of 16 paired tumorous and adjacent non-tumorous tissue samples were collected from patients with HCC at the time of surgery. Surgery was performed at the Center of Hepatobiliary Surgery, Tianjin Medical University Cancer Institute and Hospital (Tianjin, China). Blood samples from 11 of these patients were also collected, and blood samples from 11 healthy volunteers were obtained from the blood bank of Beijing Red Cross to use as controls. HCC was diagnosed according to the diagnostic guidelines of the European Association for the Study of the Liver (Geneva, Switzerland). Patient characteristics and demographic data are shown in Table I.
Table I.
Clinical characteristics of 16 patients.
| Variables | Results |
|---|---|
| Gender (male/female) | 10/6 |
| Age (year, range of median) | 60 (38–74) |
| α-fetoprotein level (ng/ml, range of median) | 366 (0–1,210) |
| Hepatitis B surface antigen positivity (%) | 68.75 |
| Hepatitis C virus antibody positivity (%) | 6.25 |
| Tumor-node-metastasis stage (I/II/III/IV) | 3/6/5/2 |
| Differentiation (high/medium/low) | 2/12/2 |
Cell isolation and purification
Tumorous and non-tumorous (>5 cm from the tumor margin) tissue infiltrating lymphocytes (TILs and NILs) were obtained using a method based on mechanical dissociation and collagenase treatment as described previously (23). Freshly obtained blood samples were immediately centrifuged (800 × g, 25°C) for 20 min on Ficoll-Paque density gradients (Beijing Solarbio Science & Technology, Beijing, China) to collect PBMCs. All isolated cells were either used immediately in experiments, or cryopreserved for future use. TILs or NILs were stained with the appropriate combinations of fluorescence labeled antibodies for fluorescence activated cell sorting (FACS) analysis or sorted using a BD FACSAria cell sorter (BD Biosciences, San Diego, CA, USA) for purification of CD25+CD3+CD56+, CD4+CD25high and CD4+CD25− cell subsets with a purity of 95–98%.
Phenotypic and functional flow cytometric analysis
Multicolor FACS analysis was performed to determine the frequency and phenotype of natural T/NK cells in TILs, NILs and PBMCs using a series of fluorescence labeled monoclonal antibodies in 1:10 dilutions, including peridinin-chlorophyll-protein complex-Cy5.5-anti-human CD3 (catalog no. 340949), phycoerythrin-anti-human CD56 (catalog no. 555516), fluorescein isothiocyanate (FITC)-anti-human CD4 (catalog no. 555346), allophycocyanin (APC)-anti-human CD8 (catalog no. 555349), FITC-anti-human CD25 (catalog no. 555431), APC-anti-human CD25 (catalog no. 555434), FITC-anti-human CD45RO (catalog no. 11-0457), and FITC-anti-human cytotoxic T-lymphocyte-associated protein 4 (CTLA-4; catalog no. 11-1529), with appropriate isotype-matched controls. Antibodies against CD45RO and CTLA-4 were obtained from eBioscience, Inc. (San Diego, CA, USA), and the rest were from BD Pharmingen (San Diego, CA, USA). For intracellular staining of interferon (IFN)-γ and perforin, FITC-anti-human IFN-γ (1:10; catalog no. 552887) and Alexa 488-anti-human perforin (1:10; catalog no. 563764; BD Pharmingen) antibodies were used. The cells were stimulated with 50 ng/ml phorbol-12-myristate-13-acetate (PMA, Sigma-Aldrich; Merck Millipore, Darmstadt, Germany), and 10 µg/ml ionomycine (Sigma-Aldrich; Merck Millipore) for 4 h at 37°C. For the remaining 2 h of this incubation, 1 µg/ml brefeldin A (Sigma-Aldrich; Merck Millipore) was added into the culture. The culture was subsequently incubated with previously mentioned fluorescent-labeled antibodies at 4°C for 30 min and CytoFix/Cytoperm (BD Pharmingen) as previously mentioned. Staining for the FOXP3 protein was performed using a FOXP3 kit (1:20; APC-anti-human FOXP3; catalog no. 77-5774; eBioscience Inc.) according to the manufacturer's protocol. Cells were analyzed using the FACSCalibur flow cytometer (BD Biosciences) and analyzed using CellQuest software (BD Biosciences).
T cell proliferation and suppression assay
CD25+CD3+CD56+ cells from the tumor tissues of 3 patients with HCC were isolated and purified using FACSAria (BD Biosciences). These cells were used to represent FOXP3+ natural T cells (NTreg), since FOXP3, which is an intracellular molecule, could not be used as a marker in cell sorting to keep cells alive for the functional assay. To detect the anti-proliferative effect of NTreg, the present study performed a co-culture test using purified autologous CD4+CD25− cells as responder cells (5×104 cells/well). These cells were preloaded with carboxyfluorescein diacetate succinimidyl ester (CFSE) with NTreg at a ratio of 1:1 in 96-well round-bottomed plates (Corning Costar Incorporated, NY, USA) in triplicate.
Cellular proliferation was induced by stimulation with the anti-CD3 antibody (1 µg/ml) in the presence of irradiated allogeneic PBMCs as antigen-presenting cells (2×105 cells/well, irradiated at 35 Gy) for 4 days at 37°C. Proliferation was measured by monitoring CFSE dilution and the results were shown in histograms subsequent to gating on CFSE+ cells.
Statistical analysis
Data are presented as the mean ± standard error of the mean for percentages. The significance of the difference between group means was determined using the Student's t-test by using GraphPad Prism (version5.0; GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Reduced natural T and NK cells in TILs of patients with HCC
Considering the abundance of natural T cells and NK cells in the human liver, it was necessary to investigate the representation of natural T cells and NK cells in local tumor tissues of human HCC. TILs and NILs were obtained as described previously (23). Flow cytometry was then performed on a total of 16 paired TILs and NILs using fluorescence labeled monoclonal antibodies specific for CD3 and CD56. The percentages of natural T cells (6.315±1.002 vs. 17.16±1.804, P<0.001) and NK cells (6.324±1.559 vs. 14.52±2.336, P<0.01) were markedly reduced in TILs compared with that in NILs, as determined by flow cytometry analysis (Fig. 1).
Figure 1.
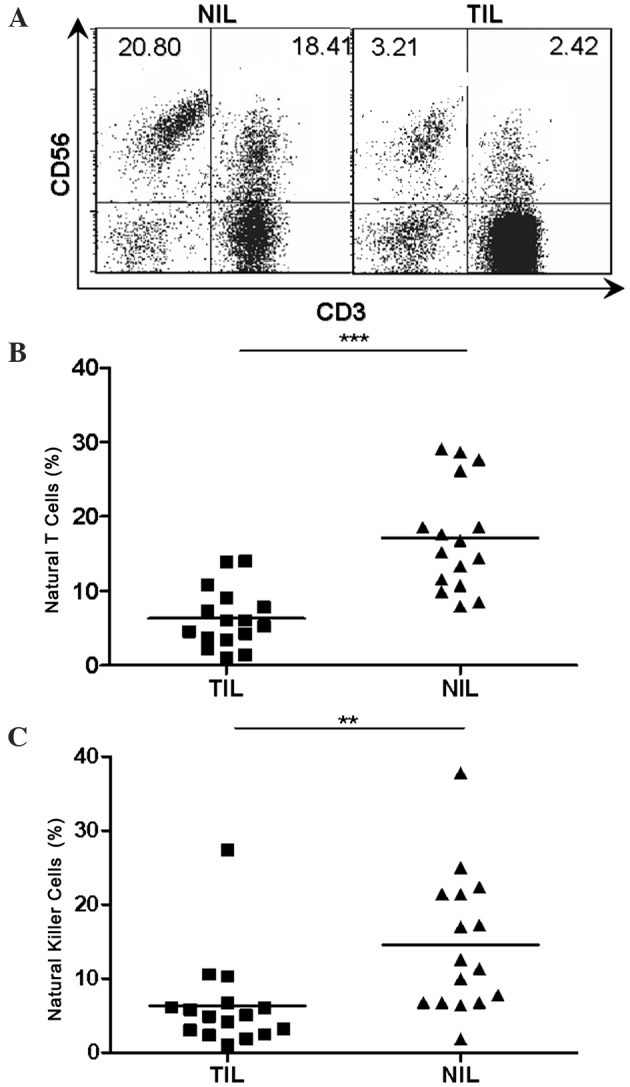
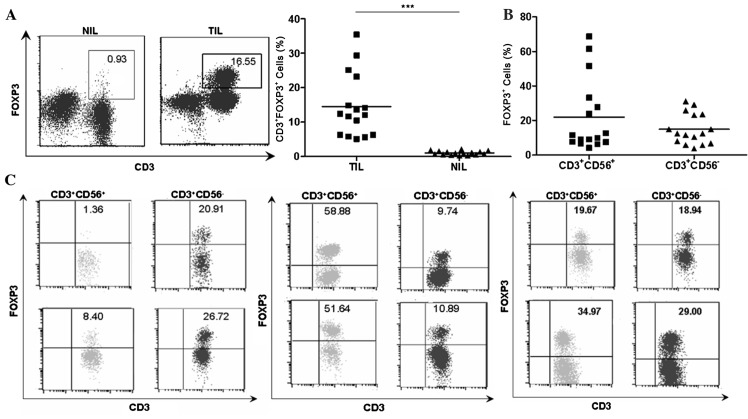
Percentage of natural T cells and natural killer cells in TILs vs. NILs in HCC. (A) CD3/CD56 staining pattern of infiltrating lymphocytes in tumor and adjacent non-tumor tissues from a representative patient. The numbers in each dot plots represented the percentages of natural killer cells (left) and natural T cells (right) in NIL and TIL from a representative patient with HCC. (B) Percentages of natural T cells in individual patients (n=16). (C) Percentages of natural killer cells in individual patients (n=16). **P<0.01, ***P<0.001. CD, cluster of differentiation; HCC, hepatocellular carcinoma; TIL, tumor infiltrating lymphocytes; NIL, non-tumor infiltrating lymphocytes.
Identification of FOXP3+ natural T cells in TILs of patients with HCC
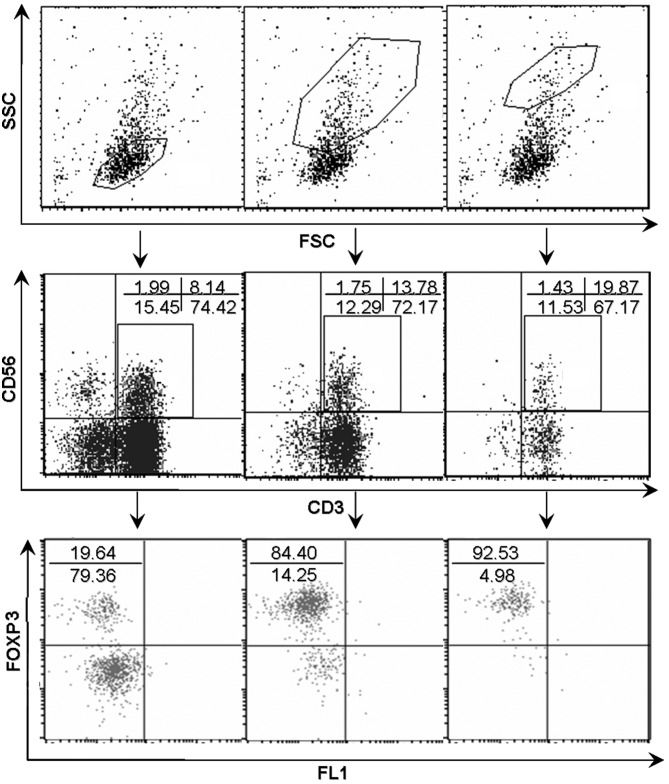
As shown in Fig. 2A, the percentages of FOXP3+ T cells were higher in TILs compared with NILs (14.48±2.297 vs. 1.023±0.1411, P<0.001) despite a large variation among individual patients with HCC. The present study subsequently analyzed the respective FOXP3 expression in different cell subsets. Notably, FOXP3+ cells were identified not in conventional T cells (CD3+CD56−) and in natural T cells (CD3+CD56+; Fig. 2B). The NK cells (CD3−CD56+) in TILs did not acquire FOXP3 expression. As shown in in Fig. 2C, there were 3 different distribution patterns of FOXP3+ cell subset in autologous natural T cell and conventional T cell populations in TILs, which suggested that there was no association between the level of FOXP3 and CD56 expression. By contrast, dot plots in Fig. 3 showed a positive association between the level of FOXP3 expression and forward scatter/side scatter gating in natural T cells.
Figure 2.
Detection of FOXP3 expression in natural T cells and conventional T cells in tumor tissues. (A) FOXP3 staining of infiltrating CD3+ lymphocytes in TIL and NIL from a representative patient with HCC and the percentages of CD3+FOXP3+ cells in TIL and NIL in individual patients (n=16). The numbers represent the percentage of FOXP3-expressing cells in NIL and TIL from a representative patient. (B) The percentage of FOXP3+ cells in natural T cells (CD3+CD56+) and conventional T cells (CD3+CD56−) in tumor (n=16). (C) A total of 3 different distribution patterns of FOXP3+ cell subsets were observed in autologous natural T cell (CD3+CD56+) and corresponding conventional T cells (CD3+CD56−) from 6 representative patients. The percentage of FOXP3+ cells in natural T cells was low, but the percentage of autologous conventional T cells was high (n=2; left panel). The percentage of FOXP3+ cells in natural T cells was high, but the percentage of autologous conventional T cells was low (middle panel; n=2). The percentage of FOXP3+ cells in autologous natural T cells and corresponding conventional T cells was similar (right panel; n=2). The numbers indicate the percentage of corresponding subsets. ***P<0.001. FOXP3, forkhead box P3; TIL, tumor infiltrating lymphocytes; NIL, non-tumor infiltrating lymphocytes.
Figure 3.
FOXP3 expression in natural T cells in tumor infiltrating lymphocytes subsequent to gating on different FSC/SSC subsets. A dot plot analysis revealed a positive trend between the level of FOXP3 expression and FSC/SSC gating. The numbers in the dot plots indicated the percentages of corresponding subsets. FOXP3, forkhead box P3; FSC, forward scatter; SSC, side scatter; CD, cluster of differentiation; FL1, green fluorescence.
Representation of natural T cells and NK cells in PBMCs of patients with HCC
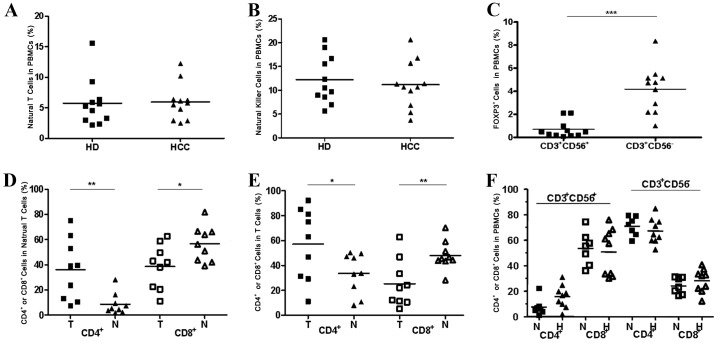
In contrast with what was found in the local tumor tissues, there was no clear change in the percentages of natural T cells (Fig. 4A, P>0.05) and NK cells (Fig. 4B, P>0.05) in the peripheral blood from patients with HCC in comparison with that from healthy donors. In addition, the prevalence of FOXP3+ cells was not observed in natural T cell populations in the peripheral blood of patients with HCC, despite a significant accumulation of FOXP3+ cells in autologous conventional T cell population in TILs having been detected at the same time (Fig. 4C, P<0.001).
Figure 4.
Comparison of natural T cells and NK cells in PBMCs between patients with HCC and healthy donors and an altered CD4/CD8 distribution pattern in natural T cells and conventional T cells in tumorous tissues compared with non-tumorous tissues. (A) The percentages of natural T cells in PBMCs from patients with HCC (n=11) and from healthy donors (n=11). (B) The percentages of NK cells in PBMCs from patients with HCC (n=11) and in healthy donors (n=11). (C) The percentages of FOXP3+ cells in natural T cells (CD3+CD56+) and conventional T cells (CD3+CD56−) in PBMCs from individual patients (n=11). (D) An altered CD4/CD8 distribution pattern in natural T cells in tumor tissues compared with non-tumor tissues. (E) An altered CD4/CD8 distribution pattern in conventional T cells in tumorous tissues compared with non-tumorous tissues was observed. (F) An unaltered CD4/CD8 distribution pattern in natural T cells and T cells in PBMCs from patients with HCC compared with normal donors. *P<0.05, **P<0.01, ***P<0.001. PBMCs, peripheral blood mononuclear cells; HCC, hepatocellular carcinoma; CD, cluster of differentiation; HD, healthy donors; FOXP3, forkhead box P3; T, tumor; H, patients with HCC; N, normal donors.
One unique characteristic of resident human hepatic lymphocytes is a reversed CD4/CD8 ratio (1:3.5) compared with that in PBMCs (2:1) (24). The present study found the percentage of CD4+ cells to be observably higher in the natural T cell subset (Fig. 4D, P<0.01) and the conventional T cell subset (Fig. 4E, P<0.05) in TILs than in NILs. Conversely, the percentage of the CD8+ cell subset was markedly reduced in TILs compared with NILs (Fig. 4D, P<0.05; Fig. 4E, P<0.01). Nevertheless, there were no notable changes in the percentages of the CD4+ cell population and the CD8+ cell population in the natural T cell subset and the conventional T cell subset when referring to the PBMCs from patients with HCC in comparison with that from healthy donors (Fig. 4F, P>0.05).
Phenotypic and functional characterization of FOXP3+ natural T cells in TILs
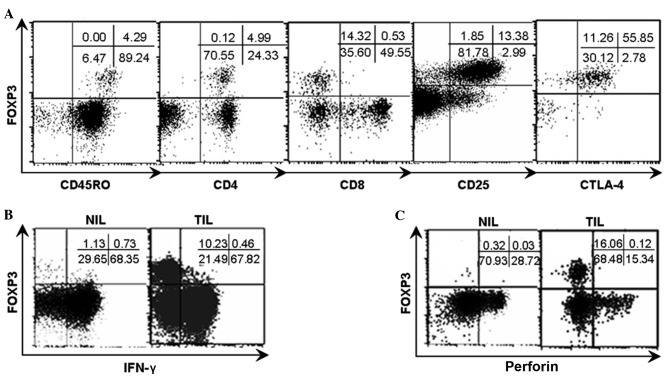
Similar to conventional Tregs, the majority of the FOXP3+ cell population in natural T cells exhibited positive expression of CD25 and CTLA-4 (Fig. 5A).
Figure 5.
Phenotypic characteristics of FOXP3+ natural T cells and the FOXP3/IFN-γ and FOXP3/perforin patterns in TILs and NILs from representative patients. Analyses were repeated with samples from 8–10 HCC patients with similar results. (A) The phenotypic analysis indicated that the FOXP3+ natural T cells were restricted to CD45RO+, CD4+, CD25+, CTLA-4+ and CD8− populations. (B) TILs and NILs were stimulated with PMA and ionomycine for 4 h respectively and subsequently analyzed for FOXP3/IFN-γ pattern subsequent to gating on natural T cells by flow cytometry. (C) TILs and NILs were analyzed for FOXP3/perforin pattern subsequent to gating on natural T cells by flow cytometry. The numbers indicate the percentage of corresponding subsets. FOXP3, forkhead box P3; IFN-γ, interferon-γ; TILs, tumor infiltrating lymphocytes; NILs, non-tumor infiltrating lymphocytes; HCC, hepatocellular carcinoma; CD, cluster of differentiation; CTLA-4+, cytotoxic T-lymphocyte-associated protein 4; PMA, phorbol-12-myristate-13-acetate.
IFN-γ and perforin are important effector molecules for natural T cells
TILs and NILs were stimulated with PMA (50 ng/ml) and ionomycine (1 µg/ml) for 4 h, respectively, and subsequently examined for the expression of IFN-γ and perforin in natural T cells in the context of FOXP3 expression by flow cytometry. The present results demonstrate that the acquisition of FOXP3 appeared to be accompanied with the loss of expression of IFN-γ and perforin, due to the mutually exclusive expression between IFN-γ and perforin and FOXP3 as shown in the dot plots (Fig. 5B and C).
Immunosuppressive function of FOXP3+ natural T cells in TILs of patients with HCC
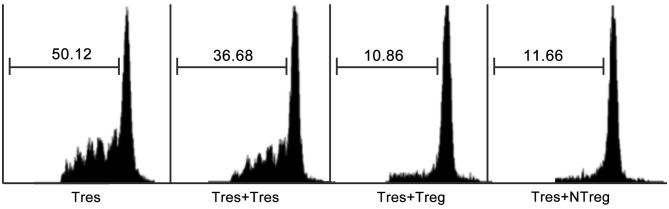
It was important to distinguish whether the expression of FOXP3 in natural T cells represented the immunosuppressive function acquisition. As an intracellular molecule, FOXP3 could not be used as a marker to obtain live cells for functional analysis. CD25+CD3+CD56+ cells were purified from TILs of patients with HCC to represent FOXP3+ natural T cells, since the majority of FOXP3+ natural T cells were CD25+, whereas CD25 expression was hardly detected in FOXP3− natural T cells as indicated in Fig. 5A. In order to test the immunosuppressive function of FOXP3+ natural T cells in TILs, a CFSE assay through co-culture of CD25+CD3+CD56+ cells with CFSE labeled autologous CD4+CD25− responder T cells was performed as previously described. As expected, results from the CFSE dilution assay indicated that FOXP3+ natural T cells exerted a comparable inhibitory effect on the proliferation of responder T cells to that mediated by conventional Tregs (Fig. 6).
Figure 6.
Anti-proliferative effect of tumor infiltrating forkhead box P3+ natural T cells. CD25+CD3+CD56+ cells were purified from the tumorous tissues of patients with HCC as NTreg cells and the CD4+CD25− cells were from autologous non-tumorous tissues as responder T cells. Histograms represent carboxyfluorescein diacetate succinimidyl ester dilution of labeled autologous responder Tres or at a 1:1 ratio with CD4+CD25− (Tres), Treg or CD25+CD3+CD56+ cells (NTreg). Data are representative of 3 independent experiments from 3 individual patients with HCC. HCC, hepatocellular carcinoma; Tres, T cells cultured alone; Treg, regulatory T cells; NTreg, natural Treg.
Discussion
A number of studies have documented HCC progress in the presence of tumor-specific immune responses in a majority of patients with HCC, indicating that HCC has evolved multiple mechanisms to evade host anti-tumor immunity (3–5,11,12). To improve the efficacy of immunotherapy for HCC, it is critical to improve understanding of the mechanisms behind this immune suppression.
Although it is well established that reduced CD8+ CTLs and increased FOXP3+ cells in HCC are coupled with poor prognosis (18,19), it is now also being recognized that the innate arm of the immune system may also perform a potentially important role in immune responses in liver diseases (25,28–32). Suppressive innate immunocytes including regulatory DCs, γδ T cells and NKT cells have gained attention in previous years (15,20–22).
The present study describes a different representation of natural T cells and NK cells between TILs and peripheral lymphocytes of human HCC. Natural T cells and NK cells were markedly decreased in TILs vs. NILs, whilst the proportions were not altered in PBMCs from patients with HCC in comparison with that from healthy donors. By contrast, several studies have indicated a reduction in the proportion of NK cells in vPBMCs from patients with HCC (33,34). Conversely, a previous investigation from 28 patients with HCC in tumor node metastasis stage I demonstrated that the proportion of NK cells in PBMCs did not differ from the proportion of NK cells in PBMCs from healthy controls (35). These conflicting studies may be in part due to the relatively low proportion of NK cells in PBMCs of the included healthy donors in the present study.
The present study identified a novel FOXP3+ natural T cell population (CD3+CD56+FOXP3+) with a similar phenotype and comparable suppressive capacity to conventional Tregs in TILs of HCC. Due to the resemblance between FOXP3+ natural T cells and conventional FOXP3+ Tregs, it is necessary to determine whether or not the FOXP3+ natural T cells are a special subset of Tregs that acquire CD56 expression at the same time. A correlation analysis was firstly performed between the proportion of FOXP3-expressing cell population in the natural T cell subset (CD3+CD56+) and that in the conventional T cell subset (CD3+CD56−) in TILs. These results however suggested that there is no significant correlation between these factors. The mean proportion of FOXP3 expressing cells was higher in the natural T cell subset compared with that in the conventional T cell subset in TILs (Fig. 2B). In individuals, the proportions of FOXP3+ cells in the natural T cell subset and in the conventional T cell subset in TILs were not always equivalent (Fig. 2C). Regarding the PBMCs from patients with HCC, an unaltered representation of natural T cells and NK cells was observed in comparison with that from healthy donors, and a significant proportion of the FOXP3+ cell population only existed in the conventional T cell subset but not in the natural T cell subset. In NK cells, no FOXP3-expressing cells were detected in either TILs or PBMCs. In summary, it was identified that FOXP3+ natural T cells spontaneously existed in TILs of HCC as a new regulatory cell subset, which were mostly derived from natural T cells infiltrating in the local microenvironment of HCC but not recruited from the peripheral blood.
As studied, apart from conventional CD4+FOXP3+ regulatory cells, CD8+ and T cell receptor (TCR) γδ+ regulatory T cells have been identified in prostate and breast cancer, respectively (36,37). Although TCRγδ+ and CD8+ cell populations were dominant in the natural T cell subset in the human liver, the identified FOXP3+ natural T cells in TILs of HCC were confined to TCRγδ− and CD8− cell populations. In addition, the acquisition of FOXP3, appeared to be accompanied with the loss of classical functions of natural T cells, since the expression of FOXP3, IFN-γ and perforin in natural T cells was mutually exclusive on single cell level through the flow cytometric scatter plots graphs (Fig. 5B and C). By contrast, granzyme B and perforin were found to be relevant for Tregs-mediated suppression of tumor clearance in vivo (38).
FOXP3 is essential for the programming, development, differentiation and suppressive functioning of Tregs, and is a definitive marker for Tregs (39). A number of studies have demonstrated that overexpression of FOXP3 in naïve T cells and/or T cell lines through lentiviral infection resulted in acquisition of suppressive activity (40–45). The authors of the present study speculate whether in vitro FOXP3-lentiviral infection is sufficient for the acquisition of immunosuppressive activity in natural T cells, and this will be investigated in further studies. Although there was a high percentage of natural T cells with FOXP3+ expression in TILs, the prevalence of FOXP3+ natural T cells in TILs was not as high as Tregs in TILs, due to a decrease in cell numbers in the overall natural T cell subset in TILs.
In conclusion, the identification of the unique FOXP3+ natural T cells may be helpful to improve understanding of the immunosuppressive local microenvironment in HCC, and it is hypothesized that it may also provide useful clues for the improvement of therapeutic intervention of HCC in the future.
Acknowledgements
The present study was supported in part by grants from the National Science and Technology Major Project (grant no. 2013ZX09303001), the National Natural Science Foundation of China (grant nos. 81501984, 81601377), the Tianjin Municipal Bureau of Health Science & Technology Fund (grant no. 2013KZ088) and the Tianjin Medical University Science Fund (grant no. 2013KYQ07).
References
- 1.Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am. 2015;24:1–17. doi: 10.1016/j.soc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Kulik LM, Chokechanachaisakul A. Evaluation and management of hepatocellular carcinoma. Clin Liver Dis. 2015;19:23–43. doi: 10.1016/j.cld.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Hui D, Qiang L, Jian W, Ti Z, Da-Lu K. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig Liver Dis. 2009;41:36–41. doi: 10.1016/j.dld.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Wirth TC. Spontaneous and therapeutic immune responses in hepatocellular carcinoma: Implications for current and future immunotherapies. Expert Rev Gastroenterol Hepatol. 2014;8:101–110. doi: 10.1586/17474124.2014.862497. [DOI] [PubMed] [Google Scholar]
- 5.Breous E, Thimme R. Potential of immunotherapy for hepatocellular carcinoma. J Hepatol. 2011;54:830–834. doi: 10.1016/j.jhep.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Unitt E, Marshall A, Gelson W, Rushbrook SM, Davies S, Vowler SL, Morris LS, Coleman N, Alexander GJ. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J Hepatol. 2006;45:246–253. doi: 10.1016/j.jhep.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, Schemmer P, Bruns H, Eiermann T, Price DA, et al. Immunodominance and functional alterations of tumor-associated antigen specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59:1415–1426. doi: 10.1002/hep.26731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang HG, Chen HS, Peng JR, Shang XY, Zhang J, Xing Q, Pang XW, Qin LL, Fei R, Mei MH, et al. Specific CD8(+) T cell responses to HLA-A2 restricted MAGE-A3 p271-279 peptide in hepatocellular carcinoma patients without vaccination. Cancer Immunol Immunother. 2007;56:1945–1954. doi: 10.1007/s00262-007-0338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Daley S, Evdokimova VN, Zdobinski DD, Potter DM, Butterfield LH. Hierarchy of alpha fetoprotein (AFP)-specific T cell responses in subjects with AFP-positive hepatocellular cancer. J Immunol. 2006;177:712–721. doi: 10.4049/jimmunol.177.1.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korangy F, Ormandy LA, Bleck JS, Klempnauer J, Wilkens L, Manns MP, Greten TF. Spontaneous tumor-specific humoral and cellular immune responses to NY-ESO-1 in hepatocellular carcinoma. Clin Cancer Res. 2004;10:4332–4341. doi: 10.1158/1078-0432.CCR-04-0181. [DOI] [PubMed] [Google Scholar]
- 11.Zhao F, Korangy F, Greten TF. Cellular immune suppressor mechanisms in patients with hepatocellular carcinoma. Dig Dis. 2012;30:477–482. doi: 10.1159/000341695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unitt E, Rushbrook SM, Marshall A, Davies S, Gibbs P, Morris LS, Coleman N, Alexander GJ. Compromised lymphocytes infiltrate hepatocellular carcinoma: The role of T-regulatory cells. Hepatology. 2005;41:722–730. doi: 10.1002/hep.20644. [DOI] [PubMed] [Google Scholar]
- 13.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Shirabe K, Mano Y, Muto J, Matono R, Motomura T, Toshima T, Takeishi K, Uchiyama H, Yoshizumi T, Taketomi A, et al. Role of tumor-associated macrophages in the progression of hepatocellular carcinoma. Surg Today. 2012;42:1–7. doi: 10.1007/s00595-011-0058-8. [DOI] [PubMed] [Google Scholar]
- 15.Han YM, Chen ZB, Yang Y, Jiang Z, Gu Y, Liu Y, Lin C, Pan Z, Yu Y, Jiang M, et al. Human CD14+CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-Lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology. 2014;59:567–579. doi: 10.1002/hep.26694. [DOI] [PubMed] [Google Scholar]
- 16.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 17.Yang XH, Yamagiwa S, Ichida T, Matsuda Y, Sugahara S, Watanabe H, Sato Y, Abo T, Horwitz DA, Aoyagi Y. Increase of CD4+CD25+ regulatory T-cells in the liver of patients with hepatocellular carcinoma. J Hepatol. 2006;45:254–262. doi: 10.1016/j.jhep.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 18.Fu J, Xu DP, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 19.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 20.Moreira-Teixeira L, Resende M, Devergne O, Herbeuval JP, Hermine O, Schneider E, Dy M, Cordeiro-da-Silva A, Leite-de-Moraes MC. Rapamycin combined with TGF-β converts human invariant NKT cells into suppressive Foxp3+ regulatory cells. J Immunol. 2012;188:624–631. doi: 10.4049/jimmunol.1102281. [DOI] [PubMed] [Google Scholar]
- 21.Monteiro M, Almeida CF, Caridade M, Ribot JC, Duarte J, Agua-Doce A, Wollenberg I, Silva-Santos B, Graca L. Identification of regulatory Foxp3+ invariant NKT cells induced by TGF-beta. J Immunol. 2010;185:2157–2163. doi: 10.4049/jimmunol.1000359. [DOI] [PubMed] [Google Scholar]
- 22.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique Toll-like receptor signaling pathway. Immunity. 2007;27:334–348. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Pang YL, Zhang HG, Peng JR, Pang XW, Yu S, Xing Q, Yu X, Gong L, Yin YH, Zhang Y, Chen WF. The immunosuppressive tumor microenvironment in hepatocellular carcinoma. Cancer Immunol Immunother. 2009;58:877–886. doi: 10.1007/s00262-008-0603-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norris S, Collins C, Doherty DG, Smith F, McEntee G, Traynor O, Nolan N, Hegarty J, O'Farrelly C. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J Hepatol. 1998;28:84–90. doi: 10.1016/S0168-8278(98)80206-7. [DOI] [PubMed] [Google Scholar]
- 25.Doherty DG, O'Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunol Rev. 2000;174:5–20. doi: 10.1034/j.1600-0528.2002.017416.x. [DOI] [PubMed] [Google Scholar]
- 26.Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, O'Farrelly C. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163:2314–2321. [PubMed] [Google Scholar]
- 27.Norris S, Doherty DG, Collins C, McEntee G, Traynor O, Hegarty JE, O'Farrelly C. Natural T cells in the human liver: Cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogenous and include Valpha24-JalphaQ and gammadelta T cell receptor bearing cells. Hum Immunol. 1999;60:20–31. doi: 10.1016/S0198-8859(98)00098-6. [DOI] [PubMed] [Google Scholar]
- 28.Kawarabayashi N, Seki S, Hatsuse K, Ohkawa T, Koike Y, Aihara T, Habu Y, Nakagawa R, Ami K, Hiraide H, Mochizuki H. Decrease of CD56(+) T Cells and natural killer cells in cirrhotic livers with hepatitis C may be involved in their susceptibility to hepatocellular carcinoma. Hepatology. 2000;32:962–969. doi: 10.1053/jhep.2000.19362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golden-Mason L, Castelblanco N, O'Farrelly C, Rosen HR. Phenotypic and functional changes of cytotoxic CD56pos natural T cells determine outcome of acute hepatitis C virus infection. J Virol. 2007;81:9292–9298. doi: 10.1128/JVI.00834-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye L, Wang X, Wang S, Wang Y, Song L, Hou W, Zhou L, Li H, Ho W. CD56+ T cells inhibit hepatitis C virus replication in human hepatocytes. Hepatology. 2009;49:753–762. doi: 10.1002/hep.22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gulubova M, Manolova I, Kyurkchiev D, Julianov A, Altunkova I. Decrease in intrahepatic CD56+ lymphocytes in gastric and colorectal cancer patients with liver metastases. APMIS. 2009;117:870–879. doi: 10.1111/j.1600-0463.2009.02547.x. [DOI] [PubMed] [Google Scholar]
- 32.Doskali M, Tanaka Y, Ohira M, Ishiyama K, Tashiro H, Chayama K, Ohdan H. Possibility of adoptive immunotherapy with peripheral blood-derived CD3-CD56+ and CD3+CD56+ cells for inducing antihepatocellular carcinoma and antihepatitis C virus activity. J Immunother. 2011;34:129–138. doi: 10.1097/CJI.0b013e3182048c4e. [DOI] [PubMed] [Google Scholar]
- 33.Liao Y, Wang B, Huang ZL, Shi M, Yu XJ, Zheng L, Li S, Li L. Increased circulating Th17 cells after transarterial chemoembolization correlate with improved survival in stage III hepatocellular carcinoma: A prospective study. PLoS One. 2013;8:e60444. doi: 10.1371/journal.pone.0060444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, Shi M, Zhang H, Yang Y, Wu H, et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008;129:428–437. doi: 10.1016/j.clim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Nakajima T, Mizushima N, Kanai K. Relationship between natural killer activity and development of hepatocellular carcinoma in patients with cirrhosis of the liver. Jpn J Clin Oncol. 1987;17:327–332. [PubMed] [Google Scholar]
- 36.Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM, Thompson TC, Old LJ, Wang RF. CD8+Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13:6947–6958. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 37.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27:334–348. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 38.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 39.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 40.Fontenot JD, Gavin MA, Rudensky AY. Pillars Article: Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003. 4: 330–336. J Immunol. 2017;198:986–992. [PubMed] [Google Scholar]
- 41.Hori S, Nomura T, Sakaguchi S. Pillars Article: Control of regulatory T cell development by the transcription factor Foxp3. Science 2003. 299: 1057–1061. Science. 2017;198:981–985. [PubMed] [Google Scholar]
- 42.Kim JY, Kim HJ, Hurt EM, Chen X, Howard OM, Farrar WL. Functional and genomic analyses of FOXP3-transduced Jurkat-T cells as regulatory T (Treg)-like cells. Biochem Biophys Res Commun. 2007;362:44–50. doi: 10.1016/j.bbrc.2007.07.187. [DOI] [PubMed] [Google Scholar]
- 43.Ziegler SF. FOXP3: Of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 44.Yaqi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S, Sakaguchi S. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 45.Allan SE, Alstad AN, Merindol N, Crellin NK, Amendola M, Bacchetta R, Naldini L, Roncarolo MG, Soudeyns H, Levings MK. Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol Ther. 2008;16:194–202. doi: 10.1038/sj.mt.6300341. [DOI] [PubMed] [Google Scholar]