Abstract
While the aberrant expression and the controversial results of serum- and glucocorticoid-regulated kinase (SGK1) have been reported in a number of malignancies, the expression of SGK1 and its possible association with the progression of adenocarcinoma in the esophagogastric junction (AEG) remain to be elucidated. To the best of our knowledge, the expression and localization of SGK1 was examined for the first time in the present study in cancerous and adjacent tissue from 60 patients with AEG, and compared with 20 healthy mucosa control tissue samples. Furthermore, the association between SGK1 expression and the clinicopathological characteristics, and prognosis of patients with AEG was statistically analyzed. The expression level of SGK1 was identified to be significantly higher (P<0.0001) in the cancerous AEG tissue samples (65%) compared with that of the adjacent tissue (31.7%) and healthy control (10%) samples. Enhanced SGK1 was primarily localized in the cytoplasm and the expression level of SGK1 was associated with the differentiation (P=0.045) and lymph node metastasis (P=0.006) of AEG. Notably, increased expression of SGK1 was demonstrated to be significantly correlated with poor overall survival (P=0.027). The results of the present study revealed the expression profile of SGK1 in AEG and demonstrated that SGK1 expression in cancerous tissue is an indicator for the progression of AEG. Thus, SGK1 may be a potential molecular marker for the diagnosis, interference therapy and prognosis of AEG.
Keywords: serum- and glucocorticoid-regulated kinase, adenocarcinoma, esophagogastric junction, prognosis
Introduction
Adenocarcinoma of the esophagogastric junction (AEG) is a type of adenocarcinoma at the junction between the esophagus and stomach. As classified by Siewert and Stein (1), the prototypical AEG is located within the region 1 cm above and 2 cm below the esophagogastric junction. Due to its unique epidemiological, genetic and prognostic characteristics, AEG has been regarded as a disease that is distinct from other types of gastric cancer (2). Previous studies have demonstrated that the incidence of AEG has increased in Western countries during last decade (3,4). Due to the lack of diagnostic molecular biomarkers, >80% of AEG cases are diagnosed at the advanced stage, resulting in a poor prognosis with <30% of patients having 5-year overall survival (OS) (5).
SGK1 (serum- and glucocorticoid-induced protein kinase-1) is a ubiquitous serine/threonine kinase belonging to the protein kinase A, G and C family, which is able to be activated by multiple stimuli, such as serum, follicle stimulating hormone, osmotic shock, ischemia, glucocorticoids and other cytokines (6,7). As a downstream molecule in the phosphatidylinositol-3 kinase (PI3K) signaling pathway, SGK1 regulates numerous target genes, influencing many physiological processes, such as proliferation, differentiation and apoptosis (8,9). Previous studies have demonstrated that the expression of SGK1 is significantly increased in many types of cancer, including colorectal, breast and lung cancer (10–12). Inhibition of SGK1 or SGK1 gene knockdown was revealed to inhibit the growth of tumor cells and increase the resistance of cells to chemically-mediated carcinogenesis (10,13). However, several studies reported conflicting results, whereby SGK1 expression was demonstrated to be decreased in cancerous tissue compared with adjacent control groups in prostate cancer, ovarian cancer, and adrenocortical tumor (14–16). Although these results suggest aberrant expression of SGK1 occurs in various cancerous tissue, which may affect the progression and prognosis of different types of cancer, the expression of SGK1 in AEG, particularly the association with the clinicopathological characteristics and prognosis of patients with AEG, remains to be established.
In the present study, the expression of SGK1 was determined in cancerous tissue samples of patients with AEG. Furthermore, the association between SGK1 expression and various clinicopathological characteristics were assessed in order to determine whether SGK1 is a potential molecular marker for the diagnosis, interference therapy and prognosis of AEG.
Materials and methods
Patient tissue samples
A total of 60 cases of postoperative pathologically diagnosed AEG and 20 healthy controls between 2012 and 2014 from The First Affiliated Hospital of the University of Henan University of Science and Technology (HAUST; Luoyang, China) and Anyang Tumor Hospital (Anyang, China were investigated. The tissue samples (cancerous and adjacent healthy) resected from patients were retrieved from the pathology departments of the two hospitals. The specimens were fixed in 10% formalin at 37°C for 24 h and embedded in paraffin wax. The paraffin embedded tissue blocks were freshly cut into 4-µm thick slices for subsequent immunohistochemistry analysis. Adjacent tissue samples were obtained 3 cm away from the cancerous tissue. Healthy control samples were selected from esophagogastric junction biopsy tissues obtained during endoscopic examinations of 20 age- and gender-matched participants. All samples, including healthy controls, were confirmed histologically by two pathologists. Demographics (gender and age) and clinicopathological characteristics (differentiation status, lymphatic invasion, lymph node metastasis and tumor-node-metastasis stage) were obtained from patient medical records. OS rates were determined over 60 months. Up to the date of follow-up, 2 were unavailable, 45 succumbed and 13 were alive by the end of the follow-up period. The present study was approved by the Institutional Review Board of HAUST. Ethical permission was received from the Regional Ethical Board of the First Affiliated Hospital of HAUST and Anyang Tumor Hospital, and the written informed consent was obtained from all patients.
Immunohistochemistry analysis
The slides were dewaxed and rehydrated in a descending series of alcohol, immunohistochemical detection was performed using an indirect immunoperoxidase technique (17) following high-temperature (100°C) antigen retrieval in 10 mM citric acid monohydrate buffer (pH 6.5; Sigma; Merck KGaA, Darmstadt, Germany) for 13 min. Blocking of endogenous peroxidase and non-specific staining was performed. Samples were incubated at 4°C overnight with primary rabbit monoclonal antibody directed against SGK1 (cat. no. ab32374; dilution, 1:200; Abcam, Cambridge, MA, USA) and PBS was used as a negative control for the primary antibody. Signal amplification was achieved using biotin-labeled anti-rabbit/rat and chain mildew avidin-peroxidase conjugated secondary antibody (cat. no. ZS-9001; OriGene Technologies, Inc., Beijing, China) at room temperature for 1 h. The Streptavidin-Biotin DAB kit (cat. no. ZLI-9019; OriGene Technologies, Inc.) was used to stain sections for between 2 and 5 min, nuclei were counterstained with hematoxylin. SGK1 was exclusively expressed in the cytoplasm. Immunostaining scores were calculated using light microscopy (Eclipse 80i; Nikon Corporation, Tokyo, Japan) by two independent experienced pathologists who were unaware of the patients' clinical details. Whenever discrepancies occurred, the results were jointly assessed by the two investigators and the final score was formed by consensus. The staining intensity of SGK1 expression was classified using the following numerical scale: <10% stained cells, negative (grade 0); 10–30%, weak (grade 1); 30–60%, moderate (grade 2); and ≥60%, strong (grade 3). A grade of ≥2 was considered positively-stained for the SGK1 antibody.
Statistical analysis
The continuous data are presented as the mean ± standard deviation. The χ2 test was used to analyze the differences in the distribution of clinicopathological characteristics and to analyze differences between categorical variables. OS was defined as the time between the date of primary diagnosis and mortality. Kaplan-Meier estimator analysis and the log-rank test were used to estimate differences in OS in strata according to high and low SGK1 expression. All tests were two-tailed. The corresponding 95% confidence interval (CI) was calculated, and the univariate and multivariate analysis were performed using SPSS software (version 21.0; IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of SGK1 is higher in the cancerous tissue of patients with AEG compared with non-cancerous tissue and the healthy control group
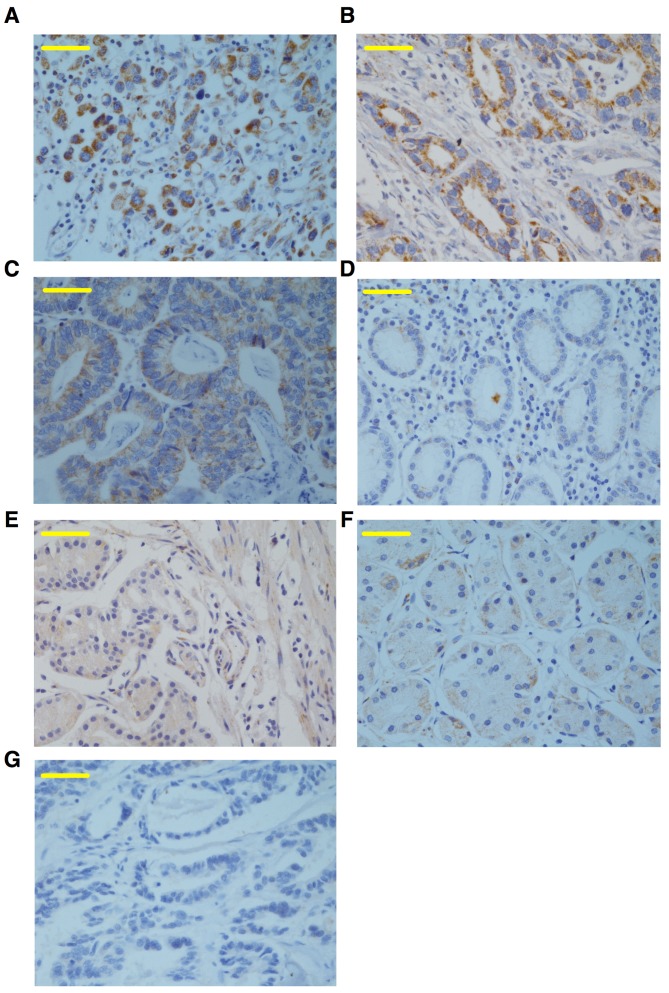
To determine SGK1 expression in cancerous, adjacent and healthy control tissue samples, immunohistochemical staining for SGK1 was performed on samples from 60 cases of paraffin-embedded AEG and healthy control tissues. The results revealed that SGK1 was positively expressed as brown-yellow granules, which were primarily localized to the epithelial cell cytoplasm and weakly expressed in the nuclei (Fig. 1). The expression of SGK1 was significantly higher in cancerous tissue from patients with AEG (65%), compared with the adjacent non-cancerous tissues (31.7%) and healthy controls (10%) (both P<0.0001; Fig. 1; Table I), indicating that the expression of SGK1 may be a biomarker for AEG.
Figure 1.
Immunohistochemical detection of SGK1 in cancerous and adjacent tissues from patients with AEG, and the healthy control group. (A) Prototypical image of SGK1-positive staining in the cancerous tissue of AEG, demonstrating SGK1 was primarily immunolocalized to the cell cytoplasm and barely present in the nuclei. Representative images of the (B) high, (C) medium, and (D) low expression of SGK1 in AEG. Representative images of SGK1 expression in (E) adjacent non-cancerous tissue from the patients with AEG and (F) healthy control tissue. (G) Representative negative image of SGK1 immunostaining using pre-immune rabbit immunoglobulin G in the cancerous AEG tissue. Magnification, ×40; scale bars, 50 µm. SGK1, serum- and glucocorticoid-regulated kinase; AEG, adenocarcinoma of esophagogastric junction.
Table I.
Expression of serum- and glucocorticoid-regulated kinase in the cancerous and adjacent tissues of patients with AEG and the healthy control group.
| Group | No. of patients | Positive (%) | Negative (%) | P-value |
|---|---|---|---|---|
| AEG cancerous | 60 | 39 (65) | 21 (35) | |
| Adjacent control | 60 | 19 (31.7) | 41 (62.3) | <0.0001 |
| Healthy control | 20 | 2 (10) | 18 (90) |
The P-value compared the groups of AEG cancerous tissue and adjacent control tissue. AEG, adenocarcinoma of esophagogastric junction.
Higher expression of SGK1 is positively correlated with poor differentiation and severe lymph node metastasis
As the expression of SGK1 was demonstrated to be higher in the cancerous AEG tissue, the association between the expression of SGK1 and the progression of AEG was examined. Clinicopathological characteristics of the patients with AEG are presented in Table II. Although the expression of SGK1 was not significantly associated with age, gender, pathological type, tumor size and tumor stage, SGK1 expression was positively associated with the differentiation and lymph node metastasis status (both P<0.05; Table II). Positive SGK1 immunostaining was present in 61.5% (24/39) of the poorly differentiated tissue samples, which was significantly higher compared with the well (3/39; 7.7%) or moderately (12/39; 30.8%) differentiated samples (both P<0.05; Table II). Furthermore, the percentage of SGK1-positively stained tissue in the N2-3 lymph node metastasis group was 79.4% (31/39), which was significantly higher compared with that in the N0-1 group (20.6%) (P=0.006; Table II). These results reveal that a higher expression of SGK1 is positively correlated with poor differentiation and severe lymph node metastasis, suggesting that higher SGK1 expression may be a novel etiological agent and potential indicator of poor prognosis for AEG.
Table II.
Association between the expression of SGK1 and clinicopathological characteristics of patients with adenocarcinoma of esophagogastric junction.
| Clinicopathological characteristic | Null/low SGK1 expression | Medium SGK1 expression | Strong SGK1 expression | No. of patients | χ2 | P-value |
|---|---|---|---|---|---|---|
| Gender | 0.277 | 0.87 | ||||
| Male | 11 | 10 | 13 | 34 | ||
| Female | 8 | 6 | 12 | 26 | ||
| Age, years | 0.371 | 0.543 | ||||
| ≤62 | 12 | 8 | 11 | 31 | ||
| >62 | 9 | 8 | 12 | 29 | ||
| Differentiation | 9.746 | 0.045a | ||||
| Well | 6 | 1 | 2 | 9 | ||
| Middle | 9 | 3 | 9 | 21 | ||
| Low | 6 | 12 | 12 | 30 | ||
| Lymph node status | 7.447 | 0.006a | ||||
| N0-N1 | 13 | 3 | 5 | 21 | ||
| N2-N3 | 8 | 13 | 18 | 39 | ||
| Tumor size | 3.313 | 0.191 | ||||
| T2 | 8 | 10 | 8 | 26 | ||
| T3 | 13 | 6 | 15 | 34 | ||
| Tumor-node-metastasis | 3.504 | 0.061 | ||||
| I–II | 9 | 3 | 4 | 16 | ||
| III | 12 | 13 | 19 | 44 | ||
| Pathological type | 0.196 | 0.658 | ||||
| Protruding | 2 | 0 | 6 | 8 | ||
| Ulcerative | 12 | 9 | 9 | 30 | ||
| Ulcer infiltrating | 3 | 2 | 3 | 8 | ||
| Infiltrate | 4 | 5 | 5 | 14 |
P<0.05. SGK1, serum- and glucocorticoid-regulated kinase.
SGK1 expression is negatively correlated with overall AEG survival rate
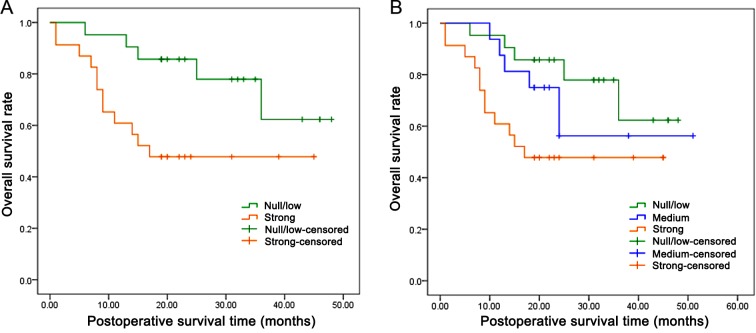
In order to assess the potential consequence of higher SGK1 expression on patients with AEG, the overall cumulative survival rate in patients was compared with different levels of SGK1 expression. A total of 58 patients (2 patients were not available) were followed up for survival analysis over a period of 60 months. Because of the limited follow-up time, the survival rate of the two groups was >25% and the median survival time could not be calculated. However, the mean survival time for patients with AEG with strong SGK1-positive staining was 26.08 months, significantly lower compared with the SGK1-negative group (39.10 months) (P=0.027; Fig. 2A, Table III). Additionally, further analysis demonstrated that the patients with weak and moderate SGK1-positive staining also exhibited a significantly shorter mean survival time of 36.50 months, as compared with the SGK1-negative group (P=0.049; Fig. 2B, Table III). In addition, univariate and multivariate prognosis analyses were performed using the Cox's proportional hazards regression model to analyze if higher expression of SGK1 was an independent prognostic factor for the overall survival of patients. As illustrated in Table IV, univariate prognosis analysis revealed that the overall survival of patients with AEG was significantly associated with a higher expression level of SGK1 (P=0.023) and poorer lymph node stage (P=0.020). However, the multivariate Cox's proportional hazards model revealed no significant association between overall survival and the lymph node status (P=0.074) or the expression of SGK1 (P=0.135) (Table IV), suggesting that the status of SGK1 expression is not an independent prognostic factor in patients with AEG.
Figure 2.
Kaplan-Meier survival analysis of the association between the expression of SGK1 and the overall survival rate of patients with adenocarcinoma of esophagogastric junction. SGK1 with (A) strong (P=0.027) or (B) medium (P=0.049) SGK1-positive immunochemistry staining were associated with a poorer overall survival rate. SGK1, serum- and glucocorticoid-regulated kinase.
Table III.
Means and medians for the survival time (months) of patients with adenocarcinoma of esophagogastric junction with expression of SGK1.
| Mean 95% confidence interval | Median | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SGK1 | Estimationa | SEa | Lower bound | Upper bound | Estimationa | SEa | Lower bound | Upper bound | P-value |
| Null/low | 39.100 | 3.371 | 32.493 | 45.706 | N/A | N/A | N/A | N/A | |
| Strong | 26.087 | 3.845 | 18.551 | 33.623 | 17.000 | N/A | N/A | N/A | |
| Overall | 32.862 | 2.881 | 27.216 | 38.508 | N/A | N/A | N/A | N/A | 0.027b |
| Null/low | 39.100 | 3.371 | 32.493 | N/A | N/A | N/A | N/A | ||
| Medium | 36.500 | 5.533 | 25.654 | N/A | N/A | N/A | N/A | ||
| Strong | 26.087 | 3.845 | 18.551 | 17.000 | N/A | N/A | N/A | ||
| Overall | 35.218 | 2.685 | 29.955 | N/A | N/A | N/A | N/A | 0.049c | |
Estimation is limited to the longest survival time.
indicate the comparison of overall survival time between the null/low and strong groups, and between the null/low, medium and strong groups, respectively. SE, standard error; N/A, not available; SGK1, serum- and glucocorticoid-regulated kinase.
Table IV.
Univariate and multivariate analysis showing overall survival in patients with adenocarcinoma of esophagogastric junction.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Covariates | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Gender | 1.676 (0.567–4.567) | 0.350 | ||
| Age | 0.997 (0.429–2.429) | 0.994 | ||
| Pathological type | 0.659 (0.401–1.401) | 0.112 | ||
| Differentiation | 1.659 (0.867–3.867) | 0.127 | ||
| Invasion depth | 1.804 (0.694–4.694) | 0.226 | ||
| Lymph node | 2.066 (1.121–3.121) | 0.020* | 1.366 (0.461–4.461) | 0.074 |
| TNM | 4.231 (0.987–18.987) | 0.052* | ||
| SGK1 | 1.838 (1.088–3.088) | 0.023* | 1.598 (0.872–2.872) | 0.135 |
SGK1, serum- and glucocorticoid-regulated kinase; CI, confidence interval; HR, hazard ratio; TNM, tumor node metastasis stage.
Discussion
To the best of our knowledge, the present study is the first to examine the expression of SGK1 and investigate its association with the clinicopathological characteristics and prognosis of patients with AEG. In the present study, it was established that SGK1 expression is significantly higher in the cancerous tissue of patients with AEG as compared with adjacent tissue and the healthy control group. Furthermore, the analysis indicates that a higher expression level of SGK1 was correlated with multiple clinicopathological factors including cancer cell differentiation, lymph node metastasis and the overall survival rate of patients with AEG. These results provide the first evidence that high SGK1 expression may be a novel risk factor for the progression of AEG, and may also serve as a prognostic biomarker for this increasingly prevalent cancer.
SGK1 has been demonstrated to share >50% sequence similarity with another serine/threonine kinase protein kinase B (Akt), a downstream target of the PI3K signaling pathway (18,19). Additionally, SGK1 is able to be fully activated through phosphorylation at Thr256 by phosphoinositide dependent protein kinase-1 (PDK) 1 and Ser422 by PDK2 similar to Akt, suggesting that SGK1 may be involved in the parallel signaling pathways that Akt is involved in (20). Considering the extensively distributed and essential function of PI3K-AKT pathway in the progression of various cancers (21), the aberrant expression of SGK1 in cancerous tissues was expected. For the first time, the results of the present study have demonstrated that SGK1 is highly expressed in AEG, which is similar to its expression in colon, breast and lung cancer (10–12). However, decreased expression of SGK1 has also been reported in certain cancers, including prostate and ovarian cancer, and adrenocortical tumor (14–16). These controversial results may be due to the specificity of different types of cancer, particularly considering that these three cancer types with a low expression of SGK1 are all associated with the endocrinal system, which may contribute to the lower expression of SGK1 through certain unidentified mechanisms. These controversial results also encourage further investigations in other cancer types from the same body system, such as esophageal and gastric cancer, which may be helpful to clarify the expression profile of SGK1 and its significance in the progression of cancer.
In the present study, it was identified that a higher expression level of SGK1 is associated with the poor differentiation and worse prognosis of patients with AEG. This result is consistent with the majority of previous studies that demonstrated that SGK1 was increased in the cancerous tissue of multiple cancers such as lung, breast and colorectal cancer. Using SGK1 knockout mice, Nasir et al (10) demonstrated that SGK1 deficiency increased the resistance of mice to chemical-induced carcinogenesis. Combined with the results from the study performed by Abbruzzese et al (12), which revealed that higher SGK1 mRNA expression was associated with the prognostic indicators of lung cancer, including tumor size and clinical stage as classified in non-small cell lung cancer, the results of the present study suggest that a higher expression of SGK1 is an etiological agent and/or prognosis indicator of AEG.
In the current study, the correlation of SGK1 expression with the overall survival of patients with AEG was also analyzed. The results demonstrated that elevated SGK1 staining was associated with a poorer prognosis in patients. Thus, SGK1 upregulation may be a poor prognosis indicator in patients with AEG. An increase in postoperative visitation for patients with high SGK1 expression may identify the recurrence and metastasis of AEG at an early stage, which has important significance for increasing the detection rate and early treatment of AEG. Survival follow-up outcome analysis identified that the mortality rate for patients with high expression of SGK1 was almost 0% after 2 years. It was also noted that ~50% of the patients were followed for <36 months, thus further follow-up is required and more significant results may be achieved once the clinical data is more complete.
Although the molecular mechanism underlying the association between higher SGK1 expression and poor differentiation and worse prognosis has not been demonstrated in the present study, a number of aspects of activated SGK1 provide a plausible basis for the potential higher risk of increased SGK1 in cancerous tissue. Firstly, it has been previously demonstrated that SGK1 is associated with the progression of inflammation (22–24), and inflammation per se has been demonstrated to be associated with the development of multiple types of cancer (25,26). A previous study demonstrated that active SGK1 decreases the production of pro-inflammatory cytokines, such as tumor necrosis factor, interleukin (IL)-12, and IL-1 (23). Due to the essential roles of the pro-inflammatory cytokines in the modulation of cancer progression, such as the role of IL-1 in priming interferon γ-producing tumor-antigen-specific CD8+ T cells, higher expression of SGK1 could inhibit the activation of specific immune responses to cancer cells, promoting immune evasion. Secondly, the activation of SGK1 has been revealed to phosphorylate forkhead box protein O3 (FoxO3), an established potent tumor suppressor, through inducing cell cycle arrest and apoptosis in multiple cancers (27–29). Higher expression of SGK1-phosphorylated FoxO3 results in its export from the nucleus to the cytoplasm, possibly deactivating the anti-tumorigenesis property of FoxO3 (30). Thirdly, activation of SGK1 has been reported to promote the expression of pro-matrix metalloproteinase 9 expression (31), which may aid in explaining the pro-metastasis effects of higher expressed SGK1 in patients with AEG. Lastly, activated SGK1 has been demonstrated to promote epithelial cell survival under conditions of cellular stress, such as serum-deprivation and chemotherapy treatment (30,32), increased SGK1 expression may promote the progression of AEG through this mechanism.
In conclusion, the present study has demonstrated for the first time that the expression of SGK1 is significantly increased in cancerous AEG tissue, and that upregulated SGK1 is associated with a more aggressive and poor prognosis in patients with AEG. Although the pathogenesis mechanism underlying the role of SGK1 in AEG warrants further investigation in vivo and in vitro, the results of the current study indicate that SGK1 is a novel potential target for the early clinical diagnosis, intervention therapy, and prognosis of AEG.
Acknowledgements
The present study was supported by the Program for the Innovation Team of Science and Technology in Universities of Henan Province (grant no. 15IRTSTHN024), the Development Program for the Key Laboratories of Henan Province (grant no. 2015SPT003) and the National Institute of Dental and Craniofacial Research (grant no. DE023633; received by HW).
References
- 1.Engstrom PF, Arnoletti JP, Benson AB, III, Chen YJ, Choti MA, Cooper HS, Covey A, Dilawari RA, Early DS, Enzinger PC, et al. NCCN clinical practice guidelines in oncology: Colon cancer. J Natl Compr Canc Netw. 2009;7:778–831. doi: 10.6004/jnccn.2009.0056. [DOI] [PubMed] [Google Scholar]
- 2.Liu K, Zhang W, Chen X, Chen X, Yang K, Zhang B, Chen Z, Zhou Z, Hu J. Comparison on clinicopathological features and prognosis between esophagogastric junctional adenocarcinoma (Siewert II/III Types) and distal gastric adenocarcinoma: Retrospective cohort study, a single institution, high volume experience in china. Medicine (Baltimore) 2015;94:e1386. doi: 10.1097/MD.0000000000001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vial M, Grande L, Pera M. Epidemiology of adenocarcinoma of the esophagus, gastric cardia, and upper gastric third. Recent Results Cancer Res. 2010;182:1–17. doi: 10.1007/978-3-540-70579-6_1. [DOI] [PubMed] [Google Scholar]
- 4.Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: Understanding the rising incidence of this disease. Semin Radiat Oncol. 2013;23:3–9. doi: 10.1016/j.semradonc.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siewert JR, Feith M, Stein HJ. Biologic and clinical variations of adenocarcinoma at the esophago-gastric junction: Relevance of a topographic-anatomic subclassification. J Surg Oncol. 2005;90:139–146. doi: 10.1002/jso.20218. [DOI] [PubMed] [Google Scholar]
- 6.Lang F, Böhmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho) physiological significance of the serum-and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 7.Lang F, Cohen P. Regulation and physiological roles of serum-and glucocorticoid-induced protein kinase isoforms. Sci STKE. 2001;2001:re17. doi: 10.1126/stke.2001.108.re17. [DOI] [PubMed] [Google Scholar]
- 8.Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol Cell Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang F, Henke G, Embark HM, Waldegger S, Palmada M, Böhmer C, Vallon V. Regulation of channels by the serum and glucocorticoid-inducible kinase-implications for transport, excitability and cell proliferation. Cell Physiol Biochem. 2003;13:41–50. doi: 10.1159/000070248. [DOI] [PubMed] [Google Scholar]
- 10.Nasir O, Wang K, Föller M, Gu S, Bhandaru M, Ackermann TF, Boini KM, Mack A, Klingel K, Amato R, et al. Relative resistance of SGK1 knockout mice against chemical carcinogenesis. IUBMB Life. 2009;61:768–776. doi: 10.1002/iub.209. [DOI] [PubMed] [Google Scholar]
- 11.Sommer EM, Dry H, Cross D, Guichard S, Davies BR, Alessi DR. Elevated SGK1 predicts resistance of breast cancer cells to Akt inhibitors. Biochem J. 2013;452:499–508. doi: 10.1042/BJ20130342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbruzzese C, Mattarocci S, Pizzuti L, Mileo AM, Visca P, Antoniani B, Alessandrini G, Facciolo F, Amato R, D'Antona L, et al. Determination of SGK1 mRNA in non-small cell lung cancer samples underlines high expression in squamous cell carcinomas. J Exp Clin Cancer Res. 2012;31:4. doi: 10.1186/1756-9966-31-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talarico C, D'Antona L, Scumaci D, Barone A, Gigliotti F, Fiumara CV, Dattilo V, Gallo E, Visca P, Ortuso F, et al. Preclinical model in HCC: The SGK1 kinase inhibitor SI113 blocks tumor progression in vitro and in vivo and synergizes with radiotherapy. Oncotarget. 2015;6:37511–37525. doi: 10.18632/oncotarget.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rauhala HE, Porkka KP, Tolonen TT, Martikainen PM, Tammela TL, Visakorpi T. Dual-specificity phosphatase 1 and serum/glucocorticoid-regulated kinase are downregulated in prostate cancer. Int J Cancer. 2005;117:738–745. doi: 10.1002/ijc.21270. [DOI] [PubMed] [Google Scholar]
- 15.Chu S, Rushdi S, Zumpe ET, Mamers P, Healy DL, Jobling T, Burger HG, Fuller PJ. FSH-regulated gene expression profiles in ovarian tumours and normal ovaries. Mol Hum Reprod. 2002;8:426–433. doi: 10.1093/molehr/8.5.426. [DOI] [PubMed] [Google Scholar]
- 16.Ronchi CL, Sbiera S, Leich E, Tissier F, Steinhauer S, Deutschbein T, Fassnacht M, Allolio B. Low SGK1 expression in human adrenocortical tumors is associated with ACTH-independent glucocorticoid secretion and poor prognosis. J Clin Endocrinol Metab. 2012;97:E2251–E2260. doi: 10.1210/jc.2012-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pirker R, Pereira JR, von Pawel J, Krzakowski M, Ramlau R, Park K, de Marinis F, Eberhardt WE, Paz-Ares L, Störkel S, et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: Analysis of data from the phase 3 FLEX study. Lancet Oncol. 2012;13:33–42. doi: 10.1016/S1470-2045(11)70318-7. [DOI] [PubMed] [Google Scholar]
- 18.Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol. 1993;13:2031–2040. doi: 10.1128/MCB.13.4.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI3-kinase-stimulated signaling pathway. EMBO J. 1999;18:3024–3033. doi: 10.1093/emboj/18.11.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi T, Cohen P. Activation of serum-and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J. 1999;339:319–328. doi: 10.1042/bj3390319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mundi PS, Sachdev J, McCourt C, Kalinsky K. AKT in cancer: New molecular insights and advances in drug development. Br J Clin Pharmacol. 2016;82:943–956. doi: 10.1111/bcp.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou H, Gao S, Duan X, Liang S, Scott DA, Lamont RJ, Wang H. Inhibition of serum-and glucocorticoid-inducible kinase 1 enhances TLR-mediated inflammation and promotes endotoxin-driven organ failure. FASEB J. 2015;29:3737–3749. doi: 10.1096/fj.15-270462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu Z, Lamont GJ, Lamont RJ, Uriarte SM, Wang H, Scott DA. Resolvin D1, resolvin D2 and maresin 1 activate the GSK3β anti-inflammatory axis in TLR4-engaged human monocytes. Innate Immun. 2016;22:186–195. doi: 10.1177/1753425916628618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatelia K, Singh K, Singh R. TLRs: Linking inflammation and breast cancer. Cell Signal. 2014;26:2350–2357. doi: 10.1016/j.cellsig.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 26.Gao S, Brown J, Wang H, Feng X. The role of glycogen synthase kinase 3-β in immunity and cell cycle: Implications in esophageal cancer. Arch Immunol Ther Exp (Warsz) 2014;62:131–144. doi: 10.1007/s00005-013-0263-9. [DOI] [PubMed] [Google Scholar]
- 27.Dehner M, Hadjihannas M, Weiske J, Huber O, Behrens J. Wnt signaling inhibits Forkhead box O3a-induced transcription and apoptosis through up-regulation of serum-and glucocorticoid-inducible kinase 1. J Biol Chem. 2008;283:19201–19210. doi: 10.1074/jbc.M710366200. [DOI] [PubMed] [Google Scholar]
- 28.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 29.You H, Jang YJ, You-Ten AI, Okada H, Liepa J, Wakeham A, Zaugg K, Mak TW. p53-dependent inhibition of FKHRL1 in response to DNA damage through protein kinase SGK1. Proc Natl Acad Sci USA. 2004;101:14057–14062. doi: 10.1073/pnas.0406286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol Cell Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borst O, Schaub M, Walker B, Schmid E, Münzer P, Voelkl J, Alesutan I, Rodríguez JM, Vogel S, Schoenberger T, et al. Pivotal role of serum-and glucocorticoid-inducible kinase 1 in vascular inflammation and atherogenesis. Arterioscler Thromb Vasc Biol. 2015;35:547–557. doi: 10.1161/ATVBAHA.114.304454. [DOI] [PubMed] [Google Scholar]
- 32.Mikosz CA, Brickley DR, Sharkey MS, Moran TW, Conzen SD. Glucocorticoid receptor-mediated protection from apoptosis is associated with induction of the serine/threonine survival kinase gene, sgk-1. J Biol Chem. 2001;276:16649–16654. doi: 10.1074/jbc.M010842200. [DOI] [PubMed] [Google Scholar]