Abstract
The metastatic lymph node status (N classification) is an important prognostic factor for patients with colorectal cancer (CRC). The aim of the present study was to evaluate and compare the prognostic assessment of three different lymph node staging methods, namely standard lymph node (pN) staging, metastatic lymph node ratio (LNR) and log odds of positive lymph nodes (LODDS) in CRC patients who undergo curative resection (R0). Data were retrospectively collected from 192 patients who had undergone R0 resection. Kaplan-Meier survival curves, Cox proportional hazards model and accuracy of the three methods (pN, LNR and LODDS) were compared to evaluate the prognostic effect. Univariate analysis demonstrated that pN, LNR and LODDS were all significantly correlated with survival (P=0.001, P<0.001 and P<0.001, respectively). The final result of the 3-step multivariate analysis demonstrated that LODDS was superior to the other two N categories. Patients in the same pN or LNR classifications may be classified into different LODDS stages with different prognoses. Thus, LODDS may be a meaningful prognostic indicator and superior to the pN and LNR classifications in CRC patients who undergo curative (R0) resection.
Keywords: colorectal cancer, lymph node, lymph node ratio, log odds of positive lymph nodes, survival
Introduction
Over 1.3 million individuals are annually diagnosed with colorectal cancer (CRC) worldwide, and approximately half of CRC patients eventually succumb to the disease (1). The metastatic lymph node status (N classification) is currently considered the most reliable prognostic indicator for patients with radically resected CRC (2,3). In 1997 and 2002, the International Union Against Cancer (UICC) and the American Joint Committee on Cancer (AJCC) proposed a classification for N categories that was based on the number of metastatic lymph nodes (4). Currently, this UICC/AJCC N classification is used most widely for CRC staging. According to the guidelines of the AJCC/UICC, a minimum of 12 lymph nodes must be resected and assessed to adequately evaluate lymph node status. The positive nodes category (pN), which is based on the number of involved lymph nodes, may be affected by the adequacy of the lymph nodes retrieved or examined (5) and it is affected by age, site of disease, T stage, extensiveness of lymphadenectomy performed by the surgeon and diligence of the pathologist (6–8). Unfortunately, despite the AJCC recommendation stating that ≥12 lymph nodes must be examined, the median number of examined lymph nodes was low, ranging between 6 and 13 (9). If the number of retrieved lymph nodes is <12, the pN category for those patients may be inaccurate due to what is referred to as ‘stage migration’ or ‘Will Rogers phenomenon’ (10).
Recently, a new lymph node ratio (LNR)-based system has been proposed, representing the ratio of the metastatic and total retrieved lymph nodes. A number of studies have proven that the LNR classification is superior to the UICC/AJCC number-based pN classification, mainly because it is not as significantly affected by the total number of retrieved nodes (11–16). However, when the ratio of the metastatic lymph nodes is 0, it is so regardless of the number of the total retrieved lymph nodes. It is the same as the pN0 classification, in the sense that there are no positive lymph nodes detected. A proportion of CRC patients have no lymph node metastasis; those patients will not benefit from the LNR classification in terms of predicting outcomes. The log odds of positive lymph nodes (LODDS), another novel prognostic indicator, was recently introduced (17) and it is defined as the log of the ratio between the number of positive and the number of negative lymph nodes. The prognostic significance of LODDS in gastric, pancreatic and breast cancer was previously investigated (18–22) and certain studies have indicated the superiority of LODDS over LNR in terms of prognostic significance in colon cancer (17,23,24). The value of LODDS remains unclear, and studies comparing the prognostic value among the pN, LNR and LODDS classifications for CRC following R0 resection have been sparse. Thus, a study was designed with the aim of evaluating LODDS as a prognostic factor for CRC and comparing its prognostic value with those of the pN and LNR classifications by analyzing a series of 192 patients submitted to curative (R0) resection.
Patients and methods
Patients and follow-up
A total of 192 CRC patients who underwent curative (R0) resection were recruited for the present study at Zhongnan Hospital of Wuhan University (Wuhan, China) between January, 2007 and October, 2010. The exclusion criteria included stage IV disease, administration of neoadjuvant chemotherapy, endoscopic mucosal resection, synchronous or metachronous primary cancer in other organs, patients for whom lymph node information was unavailable and those for whom a complete follow-up was unavailable. All the patients were followed up after surgery at 3-, 6- or 12-month intervals. The follow-up of the entire study population was continued until either death or October, 2015. The median follow-up period was 65 months (range, 4–106 months) for all patients.
Definition of the three N classifications
Lymph node involvement was classified according to the seventh edition of the tumor-node-metastasis (TNM) classification system of UICC/AJCC (N0, no metastasis; N1, 1–3 metastatic lymph nodes; and N2, >3 metastatic lymph nodes) (25). LNR is defined as the ratio of the metastatic and the total retrieved lymph nodes. LODDS was estimated as follows: LODDS = log [(pnod + 0.5)/(tnod-pnod + 0.5)] (23), where pnod is the number of positive lymph nodes and tnod is the total number of retrieved lymph nodes; 0.5 is added to both the numerator and the denominator to avoid singularity. All the nodes were separately dissected from the specimen at the end of the procedure by the surgeon.
Statistical computing and analyses
To investigate the optimal categorization of LNR and LODDS, the Classification and Regression Trees technique (CART) (26) was used to determine high discriminating cut-off points in the statistical R software package, version 3.2.1 (The R Foundation for Statistical Computing, Vienna, Austria). Statistical analyses were performed using SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA). Several clinicopathological characteristics were compared using the Pearson's χ2 test or Fisher's exact test. Univariate analysis of survival was performed using the Kaplan-Meier method to estimate survival rates in patient subgroups and the log-rank test was used to test differences among the survival curves of different patient groups. A multivariate analysis was conducted by Cox proportional hazards models. The 3-step multivariate analysis was applied to identify the N classification most significantly correlated with prognosis. In step 1 of the multivariate analysis, all the significant factors in the univariate analysis were included, as well as pN classification, excluding LNR and LODDS. In step 2, LNR classification was also included, but not LODDS. In step 3 of the multivariate analysis, all three N classifications were included. To elucidate how LODDS is superior to the other two N classifications, scatter plots were constructed. The cut-off points were determined using the R statistical software package, version 3.2.1., and statistical analyses were performed using SPSS software, version 17.0. For all analyses, differences with P-values <0.05 were considered statistically significant.
Results
Cut-off values and associations between clinicopathological characteristics and LNR/LODDS
Of the 192 patients, 113 (58.9%) were male and 79 (41.1%) were female, and the median age was 59 years (range, 23–90 years). The median follow-up period was 65 months (range, 4–106 months) for all patients. The overall 5-year survival for the whole group of patients was 71.5%. Among the 192 patients, the median number of retrieved lymph nodes was 9 (range, 1–34) and the median number of metastatic lymph nodes in node-positive patients was 1.1 (range, 1–18).
The optimal cut-off LNR and LODDS values were calculated according to CART. As regards LNR, patients were grouped as follows: LNR1, ratio <0.10; LNR2, ratio 0.10–0.33; and LNR3, ratio ≥0.34. The 5-year survival rates in the LNR1, LNR2 and LNR3 groups were 82.9, 56.8 and 45.7%, respectively. Furthermore, the patients were classified into three LODDS groups as follows: LODDS1 (LODDS<-0.82); LODDS2 (−0.82≤LODDS2<-0.57); and LODDS3 (LODDS≥-0.57). The 5-year survival rates of LODDS1, LODDS2 and LODDS3 patients were 84.8, 70.6 and 43.0%, respectively. The 5-year survival rates of pN0, pN1 and pN2 (pN classification) patients were 81.2, 59.8 and 56.3% respectively.
The associations between clinicopathological characteristics and the LNR/LODDS in this study are shown in Table I. LNR classification was connected with pN classification (P<0.001) and preoperative serum carcinoembryonic antigen (CEA) level (P<0.05). The proportion of higher pN classification increased with the procession of LNR classification, as did the CEA level. This association was also observed in the LODDS classification (P<0.001 and P<0.05, respectively). In addition, LODDS was significantly associated with age (P<0.05).
Table I.
Associations between clinicopathological characteristics and LNR/LODDS (n=192).
| LNR, n (%) | LODDS, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | LNR1(n=124) | LNR2(n=33) | LNR3(n=35) | P-value | LODDS1(n=120) | LODDS2(n=17) | LODDS3(n=55) | P-value |
| Gender | 0.400 | 0.695 | ||||||
| Male | 75 (61) | 16 (49) | 22 (63) | 72 (60) | 11 (65) | 30 (55) | ||
| Female | 49 (39) | 17 (51) | 13 (37) | 48 (40) | 6 (35) | 25 (45) | ||
| Age (years) | 0.429 | 0.041 | ||||||
| <60 | 72 (58) | 18 (55) | 16 (46) | 71 (59) | 12 (71) | 23 (42) | ||
| ≥60 | 52 (42) | 15 (45) | 19 (54) | 49 (41) | 5 (29) | 32 (58) | ||
| Location | 0.071 | 0.399 | ||||||
| Left colon | 12 (10) | 9 (27) | 5 (14) | 13 (11) | 2 (12) | 11 (20) | ||
| Right colon | 33 (26) | 5 (15) | 5 (14) | 31 (26) | 3 (17) | 9 (16) | ||
| Rectum | 79 (64) | 19 (58) | 25 (72) | 76 (63) | 12 (71) | 35 (64) | ||
| Max (cm) | 0.905 | 0.366 | ||||||
| <5 | 94 (76) | 26 (79) | 26 (74) | 88 (73) | 15 (88) | 43 (78) | ||
| ≥5 | 30 (24) | 7 (21) | 9 (26) | 32 (27) | 2 (12) | 12 (22) | ||
| Differentiation | 0.059 | 0.141 | ||||||
| High | 35 (28) | 6 (18) | 5 (14) | 33 (27) | 4 (23) | 9 (16) | ||
| Moderate | 75 (61) | 19 (58) | 20 (57) | 72 (60) | 11 (65) | 31 (57) | ||
| Poor | 14 (11) | 8 (24) | 10 (29) | 15 (13) | 2 (12) | 15 (27) | ||
| T stage | 0.075 | 0.152 | ||||||
| T1 | 3 (2) | 0 (0) | 0 (0) | 2 (2) | 0 (0) | 1 (2) | ||
| T2 | 40 (32) | 5 (15) | 7 (20) | 4 (33) | 2 (12) | 10 (18) | ||
| T3 | 44 (36) | 9 (27) | 16 (46) | 43 (36) | 6 (35) | 20 (36) | ||
| T4 | 37 (30) | 19 (58) | 12 (34) | 35 (29) | 9 (53) | 24 (44) | ||
| pN | <0.001 | <0.001 | ||||||
| N0 | 108 (87) | 0 (0) | 0 (0) | 100 (83) | 4 (24) | 4 (7) | ||
| N1 | 16 (13) | 29 (88) | 23 (66) | 20 (17) | 13 (76) | 35 (64) | ||
| N2 | 0 (0) | 4 (12) | 12 (34) | 0 (0) | 0 (0) | 16 (29) | ||
| Nodes retrieved | 0.784 | 0.071 | ||||||
| <12 | 79 (64) | 20 (61) | 24 (69) | 70 (58) | 14 (82) | 39 (71) | ||
| ≥12 | 45 (36) | 13 (39) | 11 (31) | 50 (42) | 3 (18) | 16 (29) | ||
| CEAa (ng/ml) | 0.038 | 0.024 | ||||||
| <5 | 80 (74) | 18 (60) | 13 (50) | 77 (75) | 10 (67) | 24 (52) | ||
| ≥5 | 28 (26) | 12 (40) | 13 (50) | 26 (25) | 5 (33) | 22 (48) | ||
Some values were missing for this variable. LNR, lymph node ratio; LODDS, log odds of positive lymph nodes; Max, maximum tumor size; pN, positive nodes; CEA, preoperative serum carcinoembryonic antigen.
Survival analysis
In the univariate analysis (Table II), age, preoperative serum CEA level, T stage, pN classification, LNR classification and LODDS classification were found to be significantly correlated with prognosis (P<0.05). The survival curves of patients according to pN, LNR and LODDS classifications are presented in Fig. 1 (P=0.001, P<0.001 and P<0.001, respectively), and exhibit a good discriminatory ability among groups for all three N classifications.
Table II.
Univariate survival analysis results of 192 CRC patients.
| Variables | N(n=192) | 5-year OS rate (%) | 95% CI | P-value |
|---|---|---|---|---|
| Gender | 0.696 | |||
| Male | 113 | 70.4 | 70.3–70.3 | |
| Female | 79 | 73.1 | 73.0–73.0 | |
| Age (years) | 0.002 | |||
| <60 | 106 | 80.8 | 80.7–80.7 | |
| ≥60 | 86 | 60.1 | 60.0–60.0 | |
| Location | 0.438 | |||
| Left colon | 26 | 61.0 | 60.8–61.8 | |
| Right colon | 43 | 71.0 | 70.9–71.9 | |
| Rectum | 123 | 73.9 | 73.8–74.8 | |
| Max (cm) | 0.631 | |||
| <5 | 146 | 70.2 | 70.1–70.1 | |
| ≥5 | 46 | 75.8 | 75.7–75.7 | |
| Differentiation | 0.686 | |||
| High | 46 | 78.3 | 78.2–78.2 | |
| Moderate | 114 | 69.6 | 69.5–69.5 | |
| Poor | 32 | 68.5 | 68.3–68.3 | |
| T stage | 0.005 | |||
| T1 | 3 | 100.0 | 100.0–100.0 | |
| T2 | 52 | 86.5 | 86.4–86.4 | |
| T3 | 69 | 68.1 | 68.0–68.0 | |
| T4 | 68 | 62.0 | 61.9–62.9 | |
| Nodes retrieved | 0.982 | |||
| <12 | 123 | 71.3 | 70.5–72.5 | |
| ≥12 | 69 | 71.9 | 71.8–72.8 | |
| CEAa (ng/ml) | 0.001 | |||
| <5 | 111 | 81.9 | 81.8–82.8 | |
| ≥5 | 53 | 55.9 | 55.8–56.8 | |
| pN | 0.001 | |||
| N0 | 108 | 81.2 | 81.1–81.1 | |
| N1 | 68 | 59.8 | 59.7–59.7 | |
| N2 | 16 | 56.3 | 56.1–56.1 | |
| LNR | <0.001 | |||
| LNR1 | 124 | 82.9 | 82.8–83.8 | |
| LNR2 | 33 | 56.8 | 56.6–57.6 | |
| LNR3 | 35 | 45.7 | 45.5–45.5 | |
| LODDS | <0.001 | |||
| LODDS1 | 120 | 84.8 | 84.7–84.7 | |
| LODDS2 | 17 | 70.6 | 70.4–70.4 | |
| LODDS3 | 55 | 43.0 | 42.9–43.9 |
Some values were missing for this variable. CRC, colorectal cancer; OS, overall survival; CI, confidence interval; HR, hazard ratio; Max, maximum tumor size; CEA, preoperative serum carcinoembryonic antigen; pN, positive nodes; LNR, lymph node ratio; LODDS, log odds of positive lymph nodes.
Figure 1.
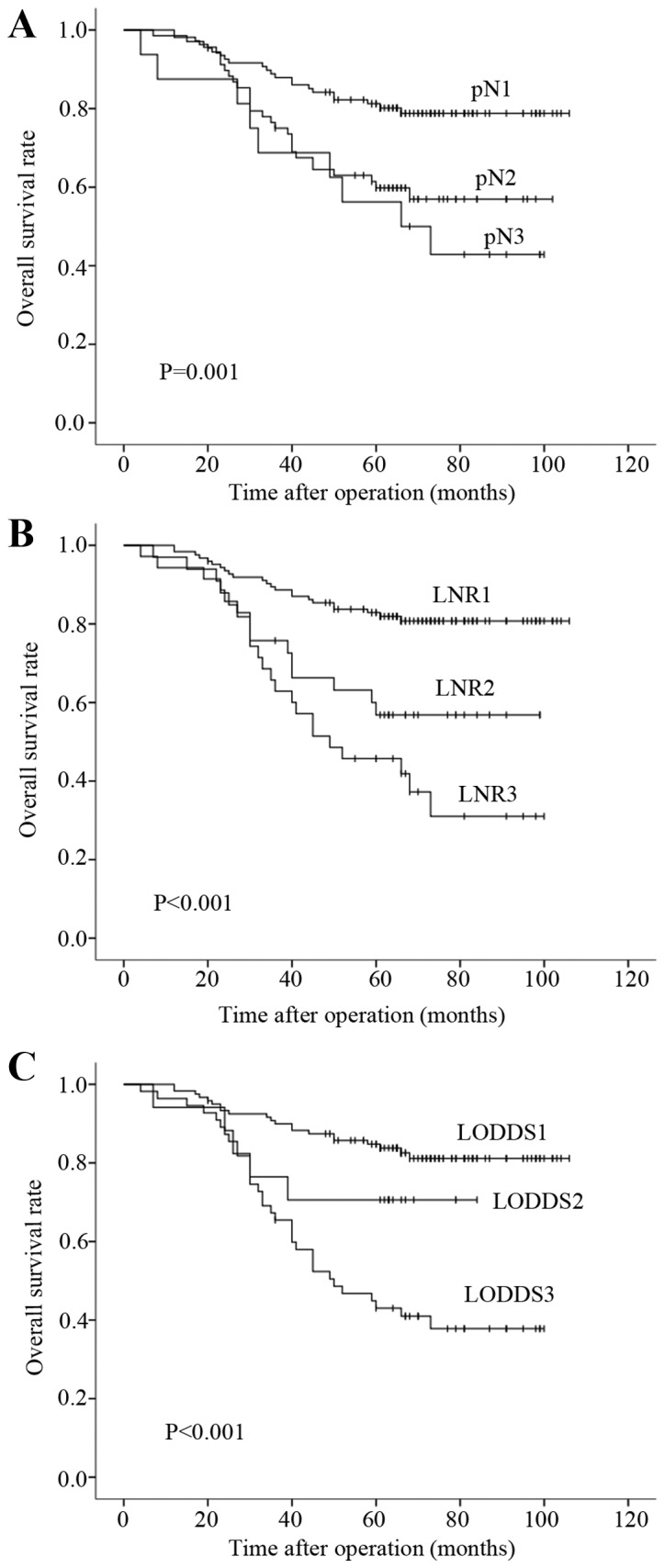
Kaplan-Meier curves of overall survival of the three classifications: Positive nodes category (pN), ratio of the metastatic and total retrieved lymph nodes (LNR) and log odds of positive lymph nodes (LODDS). (A) Advanced pN was associated with poor overall survival. (B) Advanced LNR was associated with poor overall survival. (C) Advanced LODDS was associated with poor overall survival.
3-step multivariate analysis
In step 1 of the multivariate analysis, pN classification was confirmed to be an independent prognostic factor. However, in step 2, LNR classification became significant, while pN classification disappeared. Furthermore, when all the three N classifications were included in step 3 of the multivariate analysis, the pN and LNR classifications were substituted by the LODDS classification (Table III).
Table III.
Multivariate analysis of factors by Cox proportional hazards models.
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age (years) | 1.866 | 1.044–3.044 | 0.035 | 1.669 | 0.935–2.935 | 0.083 | 1.587 | 0.885–2.885 | 0.121 |
| CEAa | 1.979 | 1.100–3.100 | 0.023 | 1.76 | 0.966–3.966 | 0.065 | 1.759 | 0.969–3.969 | 0.063 |
| T stage | 1.492 | 1.018–2.018 | 0.04 | 1.439 | 0.982–2.982 | 0.062 | 1.431 | 0.983–2.983 | 0.061 |
| pN | 1.589 | 1.053–2.053 | 0.027 | – | – | – | – | – | – |
| LNR | – | – | – | 1.746 | 1.252–2.252 | 0.001 | – | – | – |
| LODDS | – | – | – | – | – | – | 1.761 | 1.287–2.287 | <0.001 |
Some values were missing for this variable. HR, hazard ratio; CI, confidence interval; CEA, preoperative serum carcinoembryonic antigen; pN, positive nodes; LNR, lymph node ratio; LODDS, log odds of positive lymph nodes.
Scatter plots of the associations among the three N classifications
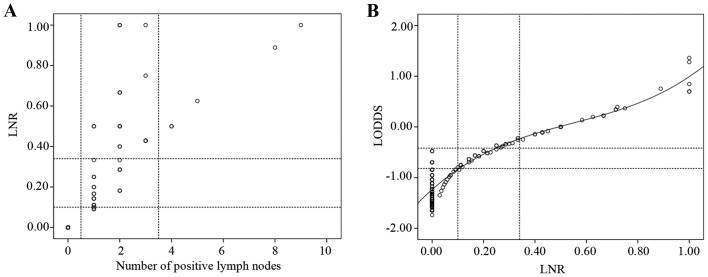
To demonstrate that the LODDS classification is superior to the pN classification, scatter plots were constructed (Fig. 2). When observing the distribution of the number of metastatic nodes and the LNR of patients whose number of retrieved lymph nodes was <12, LNR was able to discriminate among patients with different prognoses in the pN1 stage (Fig. 2A). The correlation between the ratio of metastatic nodes and LODDS was significant (Fig. 2B). When the LNR was <0.4, it increased more slowly than LODDS. The most noteworthy result was that, when the LNR was 0, the value of LODDS was still heterogeneous.
Figure 2.
Scatter plots of the associations between different lymph node classifications. (A) Distribution of the number of positive nodes and the ratio of the metastatic and total retrieved lymph nodes (LNR) of patients with a number of retrieved lymph nodes <12. (B) Distribution of log odds of positive lymph nodes (LODDS) and LNR.
Discussion
A series of 192 CRC patients submitted to curative (R0) resection were analyzed in this study. To the best of our knowledge, this study was the first to demonstrate that the LODDS classification is the best prognostic factor in Chinese CRC patients undergoing curative (R0) resection compared with the pN and LNR N classifications.
The primary limitation of the number-based UICC/AJCC pN classification is that the accuracy of predicting prognosis is significantly affected by the total number of retrieved nodes (15). Previous studies have investigated the prognostic significance of LNR in stage III colon cancer (5,27–29) and stage III rectal cancer (30) and suggested the LNR is useful because it provides information regarding the number of retrieved lymph nodes. Although the LNR classification has been proven to be superior to the pN classification, there are limitations when the ratio of the metastatic to the total retrieved lymph nodes is 0. LODDS, a novel indicator of predicting lymph node status, may provide a new means for improving the accuracy of N classification for prognostic assessment.
Previous studies have indicated the superiority of LODDS over LNR in terms of prognostic significance in colon cancer (17,23,24). However, the available data remain limited. The aim of this study was to compare the prognostic value of pN, LNR and LODDS in CRC patients who underwent R0 resection.
The LNR classification was correlated with the pN classification (P<0.001) and preoperative serum CEA level (P<0.05). The proportion of patients with a higher pN classification increased with the procession of the LNR classification. When the LNR classification increased, the proportion of patients with preoperative serum CEA level ≥5 ng/ml increased gradually. To some extent, this was in accordance with the study of Nozoe et al (31), which demonstrated that the proportion of lymph node metastasis was significantly higher in the high-CEA group compared with that in low-CEA group (P=0.012). This association was also observed in the LODDS classification. Additionally, patients in the low age group were mainly in the lower LODDS classification, while the gap was narrowed in the higher age group; this result may be attributed to the poor immunity of older patients.
Our univariate analysis demonstrated that each node classification system had a relevant prognostic significance. To investigate whether one N classification was superior to the others, a multistep multivariate analysis was used. For example, to compare the LODDS classification with the pN and LNR classifications, a 3-step multivariate analysis was performed. In step 1 of the multivariate analysis, pN classification was one of the independent prognostic factors, whereas in step 2 the pN classification was substituted by the LNR classification. In addition, a 3-step multivariate analysis was performed, including all three N classifications (pN, LNR and LODDS) and the LODDS retained its significance (model 3). The results indicated that the LODDS classification was superior to the pN and LNR classifications.
To validate the superiority of the LODDS classification and address its contribution to the accuracy of prognostic assessment, several scatter plots were constructed. The scatter plot presenting the distribution of the number of metastatic nodes and LNR of patients with a number of retrieved lymph nodes of <12, LNR was able to discriminate among patients with different prognosis in the pN1 stage (Fig. 2A). For example, when the number of positive lymph nodes was 1, it was classified as pN1 stage using the pN classification, while it was divided into LODDS1, LODDS2 and LODDS3 using the LODDS classification. LNR and LODDS were closely correlated (Fig. 2B). When the ratio of node metastasis was <0.4, it increased more slowly compared with LODDS. It is intriguing that, when the ratio of node metastasis was 0 or 1, the value of LODDS was still heterogeneous. LODDS is more efficient in discriminating patients with different survival, indicating that the LODDS system has the potential to discriminate among patients with the same LNR classification with different prognosis, particularly those whose ratio of node metastasis is 0 or 1.
Wang et al (24) investigated 24,477 patients with stage III colon cancer who were registered in the SEER database to compare the LNR and LODDS classifications, and revealed that LODDS was a better prognostic factor than LNR. The LODDS system was a highly reliable staging system with strong predictive ability for non-metastatic colon cancer patients (17,23). In the present study, the LODDS classification was found to be superior to the pN and LNR classifications in Chinese patients with CRC undergoing R0 curative resection for the first time. Song et al (32) revealed that, for Chinese patients with CRC, the LNR classification was more suitable compared with the pN and LODDS classifications for prognostic assessment. Several reasons may have contributed to these different results: i) The cut-off points acquired from different statistical methods for subclassification were different; ii) the proportion of colon and rectal cancer patients was different; and iii) the time interval between the date of the last patient undergoing curative resection to the follow-up deadline (October, 2015) was different.
This study has some limitations, as it was retrospective in nature and included a relatively limited number of patients from one hospital in China. Larger-sample studies and international multicentric research in CRC are required in the near future.
In contrast to the UICC/AJCC pN and LNR classifications, the LODDS system accurately estimates prognosis, minimizing the bias of limited lymph node dissection and examination. In conclusion, the results of the present study suggest that LODDS may be a meaningful prognostic factor, which is superior to the pN and LNR classifications in CRC patients who have undergone R0 resection. Therefore, incorporating the LODDS classification into the CRC staging system may enable clinicians to assess the prognosis of patients more accurately.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (No. 81071825), the Doctoral Fund of Ministry of Education of China (No. 20120141130010).
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: Systematic review. J Natl Cancer Inst. 2007;99:433–441. doi: 10.1093/jnci/djk092. [DOI] [PubMed] [Google Scholar]
- 3.Ovrebo K, Rokke O. Extended lymph node dissection in colorectal cancer surgery. Reliability and reproducibility in assessments of operative reports. Int J Colorectal Dis. 2010;25:213–222. doi: 10.1007/s00384-009-0829-5. [DOI] [PubMed] [Google Scholar]
- 4.Greene FL. Current TNM staging of colorectal cancer. Lancet Oncol. 2007;8:572–573. doi: 10.1016/S1470-2045(07)70185-7. [DOI] [PubMed] [Google Scholar]
- 5.Park IJ, Choi GS, Jun SH. Nodal stage of stage III colon cancer: The impact of metastatic lymph node ratio. Ann Surg Oncol. 2009;100:240–243. doi: 10.1002/jso.21273. [DOI] [PubMed] [Google Scholar]
- 6.Ahmadi O, Stringer MD, Black MA, McCall JL. Clinico-pathological factors influencing lymph node yield in colorectal cancer and impact on survival: Analysis of New Zealand cancer registry data. J Surg Oncol. 2015;111:451–458. doi: 10.1002/jso.23848. [DOI] [PubMed] [Google Scholar]
- 7.Gonsalves WI, Kanuri S, Tashi T, Aldoss I, Sama A, Al-Howaidi I, Ganta A, Kalaiah M, Thota R, Krishnamurthy J, et al. Clinicopathologic factors associated with lymph node retrieval in resectable colon cancer: A Veterans' Affairs Central Cancer Registry (VACCR) database analysis. J Surg Oncol. 2011;104:667–671. doi: 10.1002/jso.21886. [DOI] [PubMed] [Google Scholar]
- 8.Wong KP, Poon JT, Fan JK, Law WL. Prognostic value of lymph node ratio in stage III colorectal cancer. Colorectal Dis. 2011;13:1116–1122. doi: 10.1111/j.1463-1318.2010.02435.x. [DOI] [PubMed] [Google Scholar]
- 9.Wong SL, Ji H, Hollenbeck BK, Morris AM, Baser O, Birkmeyer JD. Hospital lymph node examination rates and survival after resection for colon cancer. JAMA. 2007;298:2149–2154. doi: 10.1001/jama.298.18.2149. [DOI] [PubMed] [Google Scholar]
- 10.Feinstein AR, Sosin DM, Wells CK. The will rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312:1604–1608. doi: 10.1056/NEJM198506203122504. [DOI] [PubMed] [Google Scholar]
- 11.Ceelen W, Van Nieuwenhove Y, Pattyn P. Prognostic value of the lymph node ratio in stage III colorectal cancer: A systematic review. Ann Surg Oncol. 2010;17:2847–2855. doi: 10.1245/s10434-010-1158-1. [DOI] [PubMed] [Google Scholar]
- 12.Huh JW, Kim YJ, Kim HR. Ratio of metastatic to resected lymph nodes as a prognostic factor in node-positive colorectal cancer. Ann Surg Oncol. 2010;17:2640–2646. doi: 10.1245/s10434-010-1015-2. [DOI] [PubMed] [Google Scholar]
- 13.Lu YJ, Lin PC, Lin CC, Wang HS, Yang SH, Jiang JK, Lan YT, Lin TC, Liang WY, Chen WS, et al. The impact of the lymph node ratio is greater than traditional lymph node status in stage III colorectal cancer patients. World J Surg. 2013;37:1927–1933. doi: 10.1007/s00268-013-2051-4. [DOI] [PubMed] [Google Scholar]
- 14.Moug SJ, Saldanha JD, McGregor JR, Balsitis M, Diament RH. Positive lymph node retrieval ratio optimises patient staging in colorectal cancer. Br J Cancer. 2009;100:1530–1533. doi: 10.1038/sj.bjc.6605049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu HB, Zhang LY, Li YF, Zhou ZW, Keshari RP, Xu RH. Ratio of metastatic to resected lymph nodes enhances to predict survival in patients with stage III colorectal cancer. Ann Surg Oncol. 2011;18:1568–1574. doi: 10.1245/s10434-010-1528-8. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg R, Engel J, Bruns C, Heitland W, Hermes N, Jauch KW, Kopp R, Pütterich E, Ruppert R, Schuster T, et al. The prognostic value of lymph node ratio in a population-based collective of colorectal cancer patients. Ann Surg. 2010;251:1070–1078. doi: 10.1097/SLA.0b013e3181d7789d. [DOI] [PubMed] [Google Scholar]
- 17.Arslan NC, Sokmen S, Canda AE, Terzi C, Sarioglu S. The prognostic impact of the log odds of positive lymph nodes in colon cancer. Colorectal Dis. 2014;16:O386–O392. doi: 10.1111/codi.12702. [DOI] [PubMed] [Google Scholar]
- 18.Aurello P, Petrucciani N, Nigri GR, La Torre M, Magistri P, Tierno S, D'Angelo F, Ramacciato G. Log odds of positive lymph nodes (LODDS): What are their role in the prognostic assessment of gastric adenocarcinoma? J Gastrointest Surg. 2014;18:1254–1260. doi: 10.1007/s11605-014-2539-8. [DOI] [PubMed] [Google Scholar]
- 19.La Torre M, Nigri G, Petrucciani N, Cavallini M, Aurello P, Cosenza G, Balducci G, Ziparo V, Ramacciato G. Prognostic assessment of different lymph node staging methods for pancreatic cancer with R0 resection: PN staging, lymph node ratio, log odds of positive lymph nodes. Pancreatology. 2014;14:289–294. doi: 10.1016/j.pan.2014.05.794. [DOI] [PubMed] [Google Scholar]
- 20.Qiu MZ, Qiu HJ, Wang ZQ, Ren C, Wang DS, Zhang DS, Luo HY, Li YH, Xu RH. The tumor-log odds of positive lymph nodes-metastasis staging system, a promising new staging system for gastric cancer after D2 resection in China. PLoS One. 2012;7:e31736. doi: 10.1371/journal.pone.0031736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Z, Xu Y, de Li M, Wang ZN, Zhu GL, Huang BJ, Li K, Xu HM. Log odds of positive lymph nodes: A novel prognostic indicator superior to the number-based and the ratio-based N category for gastric cancer patients with R0 resection. Cancer. 2010;116:2571–2580. doi: 10.1002/cncr.24989. [DOI] [PubMed] [Google Scholar]
- 22.Vinh-Hung V, Verschraegen C, Promish DI, Cserni G, Van de Steene J, Tai P, Vlastos G, Voordeckers M, Storme G, Royce M. Ratios of involved nodes in early breast cancer. Breast Cancer Res. 2004;6:R680–R688. doi: 10.1186/bcr934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persiani R, Cananzi FC, Biondi A, Paliani G, Tufo A, Ferrara F, Vigorita V, D'Ugo D. Log odds of positive lymph nodes in colon cancer: A meaningful ratio-based lymph node classification system. World J Surg. 2012;36:667–674. doi: 10.1007/s00268-011-1415-x. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Hassett JM, Dayton MT, Kulaylat MN. The prognostic superiority of log odds of positive lymph nodes in stage III colon cancer. J Gastrointest Surg. 2008;12:1790–1796. doi: 10.1007/s11605-008-0651-3. [DOI] [PubMed] [Google Scholar]
- 25.Edge S, Byrd DR, Compton C, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th. Springer; New York: 2010. [Google Scholar]
- 26.Breiman L, Friedman J, Stone CJ, Olshen RA. Classification and Regression Trees. Chapman and Hall/CRC; New York: 1984. [Google Scholar]
- 27.Chen SL, Steele SR, Eberhardt J, Zhu K, Bilchik A, Stojadinovic A. Lymph node ratio as a quality and prognostic indicator in stage III colon cancer. Ann Surg. 2011;253:82–87. doi: 10.1097/SLA.0b013e3181ffa780. [DOI] [PubMed] [Google Scholar]
- 28.Hong KD, Lee SI, Moon HY. Lymph node ratio as determined by the 7th edition of the American Joint Committee on Cancer staging system predicts survival in stage III colon cancer. J Surg Oncol. 2011;103:406–410. doi: 10.1002/jso.21830. [DOI] [PubMed] [Google Scholar]
- 29.Lee HY, Choi HJ, Park KJ, Shin JS, Kwon HC, Roh MS, Kim C. Prognostic significance of metastatic lymph node ratio in node-positive colon carcinoma. Ann Surg Oncol. 2007;14:1712–1717. doi: 10.1245/s10434-006-9322-3. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi H, Mochizuki H, Kato T, Mori T, Kameoka S, Shirouzu K, Saito Y, Watanabe M, Morita T, Hida J, et al. Lymph node ratio is a powerful prognostic index in patients with stage III distal rectal cancer: A Japanese multicenter study. Int J Colorectal Dis. 2011;26:891–896. doi: 10.1007/s00384-011-1173-0. [DOI] [PubMed] [Google Scholar]
- 31.Nozoe T, Rikimaru T, Mori E, Okuyama T, Takahashi I. Increase in both CEA and CA19-9 in sera is an independent prognostic indicator in colorectal carcinoma. J Surg Oncol. 2006;94:132–137. doi: 10.1002/jso.20577. [DOI] [PubMed] [Google Scholar]
- 32.Song YX, Gao P, Wang ZN, Tong LL, Xu YY, Sun Z, Xing CZ, Xu HM. Which is the most suitable classification for colorectal cancer, log odds, the number or the ratio of positive lymph nodes? PLoS One. 2011;6:e28937. doi: 10.1371/journal.pone.0028937. [DOI] [PMC free article] [PubMed] [Google Scholar]