Abstract
Non-alcoholic fatty liver disease (NAFLD) is the result of the accumulation of adipose tissue deposits in the liver and it is associated with type 2 diabetes. Crocus sativus (saffron) is known for its antioxidant and its potential hypoglycemic effects. We investigated the role of saffron on NAFLD in diabetic rats. Thirty adult male rats were allocated into three groups; control (n=10), which received normal diet; streptozotocin (STZ) group (n=10), which received normal chow diet, 10% fructose in their drinking water and STZ (40 mg/kg body weight; STZ-saffron group (n=10), which followed the same dietary and pharmacological pattern as STZ group and were additionally supplemented with saffron (100 mg/kg/day). Metabolic profile was measured and histopathological examination of the liver was evaluated. STZ group exhibited the highest glucose levels at the end of the experiment (P<0.05), while there was no difference between control and STZ-saffron group (584 vs. 213 mg/dl vs. 209 mg/dl, respectively). STZ group revealed higher percentage of steatosis (5–33%) when compared to the other two groups (P<0.005). Saffron exhibits both hypoglycemic and hepatoprotective actions. Yet, further studies enlightening the exact mechanisms of saffron's mode of actions are required.
Keywords: saffron, non-alcoholic fatty liver, metabolism, diabetes, rats
Introduction
Diabetes mellitus, especially type 2 (T2D) worldwide affects approximately 2.8% of the population, a percentage that is estimated to reach 4.4% by 2030 (1). T2D is a multi-dimensional disease, accompanied with a variety of co-morbidities (2). Although, different drugs are successfully used for controlling blood glucose levels, they are ineffective in reversing or even withholding the progression of certain complications (3). A disease associated with the increased prevalence of T2D for which awareness has been raised over the last decade is non-alcoholic fatty liver disease (NAFLD) (4,5). In patients with T2D, concurrent NAFLD might increase the risk of chronic kidney failure and the overall mortality rate (6). Nonetheless, control of the impaired glucose homeostasis and lipid concentrations inhibits micro-vascular complications and fat deposits accumulation in the liver tissue (7,8). Therefore, the main objective of medical treatment as well as prophylaxis in patients with T2D and NAFLD is metabolic control.
Saffron, the dry stigmas of the plant Crocus Sativus has been used in folklore medicine as a potent drug for asthma, liver disease, pain and dysregulation of the estrus cycle since ancient times (9). Recently, assembling evidence has suggested the use of saffron as an anti-diabetic drug (10,11). The hypoglycemic and hypolipidemic effects of total saffron and its extracts were reported in several animal studies involving experimental diabetes and its complications such as diabetic nephropathy and encephalopathy (12,13).
Aim of our study was to evaluate the potential effect of total saffron on glycemic and lipid control in an experimental model of T2D and to investigate its protective effect in NAFLD, as a complication of diabetes.
Materials and methods
Animals-study design
Twelve-week-old Sprague-Dawley male rats were obtained from Harlan Laboratories S.r.l. (Milan, Italy) and maintained in weather controlled chambers (temperature 23±2°C, humidity 55±5%) under controlled lightning (12 h light per day) and central ventilation (15 air changes/h) for 15 days in order to adapt to the new environment. Rats had free access to food and water throughout the study (10-week). The experimental protocol was approved by the ‘Scientific Committee for the approval of protocols using animals for scientific purposes’ established in the Laboratory for Experimental Surgery and Surgical Research ‘N.S. Christeas’ of Athens Medical School and by the competent Veterinary Directorate of Attica Region. The protocol was in compliance with EU legislation (Directive 2010/63/EU) regarding the use of animals in biomedical science.
Thirty rats (n=30) were randomized initially into 2 experimental groups as follows: Control, animals received normal chow diet and normal drinking water (n=10); T2D group, animals received normal chow diet and 10% fructose in their drinking water for 2 weeks (n=20).
At the end of the 2-week period, the animals of T2D received one intraperitoneal injection of relatively low dose streptozotocin (STZ; 40 mg/kg body weight; Sigma, St. Louis, IL, USA).
STZ was dissolved in citrate buffer (10 mM, pH 4.5), while the animals belonging to the control group received vehicle injection. Up to 5 days following STZ administration, the induction of diabetes in all STZ and fructose treated animals was confirmed by glucose measurement in blood collected from the saphenous vein of the rats using an automatic glucometer (glucometer Wellion® Linus, AgaMatrix Inc., Salem, NH, USA). All animals with blood glucose levels higher than 200 mg/dl after a 12-h fasting period were considered diabetic. After the confirmation of the induction of diabetes, the rats of the experimental group were further randomized into two new groups; STZ group (n=10): Animals received normal chow and water throughout the study and STZ+saffron group (STZ-saffron) (n=10): Rats were supplemented with Crocus sativus (100 mg/kg/day) diluted in their drinking water for 8 weeks. The dosage of Crocus sativus was determined according to previous reports (14,15).
Blood collection-serum measurements
Blood samples were collected at the beginning (t0) of the study, 2 weeks later (t1), after the induction of T2D (t2), as well as one (t3) and 2 months later (t4) (at 9:00 a.m., after a 12-h fast) using capillary tubes introduced into the medial retro-orbital venous plexus under light ether anesthesia.
Lipidemic, glycemic profile and liver function tests were determined in blood serum. The calculation of low-density lipoprotein (LDL) was calculated by the the Friedewald formula.
Serum insulin and adiponectin levels
Serum insulin and adiponectin levels were determined by enzyme-linked immunosorbent assay (ELISA) using commercially available kits. (Rat High Range Insulin ELISA, ALPCO (26-G Keewaydin Drive, Salem, NH, USA) Rat ADP/Acrp30 (Adiponectin) ELISA kit Elabscience Biotechnology, Co., Ltd. (Wuhan, China).
Histopathological examination
Following euthanasia, liver was immediately fixed in 10% formalin at room temperature for 24 h. The tissues were then embedded in paraffin, sectioned and mounted on glass microscope slides. The sections were stained with hematoxylin and eosin and examined using light microscopy by two independent researchers who were blinded to the randomization scheme.
Statistical analysis
Data are expressed as mean ± 1 standard deviation for continuous variables and as frequency (% percentage) for qualitative data. The normality of the distributions was assessed with Kolmogorov-Smirnov's test and graphical methods.
Comparisons between more than two groups were performed with analysis of variance (ANOVA). Kruskal-Wallis's test was utilized as a non-parametric test for multiple group comparisons, using Mann-Whitney's U test for post hoc multiple testing. Comparisons between multiple time points were performed using Repeated Measures ANOVA and Friedman's test with Wilcoxon's Signed Ranks test for post hoc comparisons.
All tests were two-sided
Differences were considered as statistically significant if the null hypothesis could be rejected with >95% confidence interval (P<0.05).
Results
Body weight, lipidemic and glycemic profile and liver function
No significant differences were recorded between the groups at baseline measurements as shown in Table I.
Table I.
Lipidemic, glycemic and metabolic profile serum levels throughout the experiment expressed as mean (SD).
| t0 | t1 | t2 | t3 | t4 | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Weight (g) | |||||
| Control | 218 (6) | 362 (22) | 371 (30)b | 409 (56)d | 423 (76)i |
| STZ | 218 (5) | 364 (25) | 330 (30) | 338 (26) | 307 (33) |
| STZ-saffron | 221 (3) | 367 (12) | 330 (19) | 333 (16) | 300 (24) |
| Glucose(mg/dl) | |||||
| Control | 110 (11) | 148 (10) | 189 (129)c | 187 (136)e | 213 (185) |
| STZ | 115 (10) | 139 (11) | 299 (134) | 378 (118) | 584 (184)j |
| STZ-saffron | 110 (12) | 139 (15) | 350 (155) | 237 (113)f | 209 (99) |
| Cholesterol (mg/d) | |||||
| Control | 107 (20) | 77 (7) | 72 (8) | 72 (19)g | 74 (15) |
| STZ | 107 (13) | 74 (10) | 75 (9) | 89 (9) | 93 (14)k |
| STZ-saffron | 109 (23) | 74 (8) | 73 (9) | 86 (18) | 87 (17) |
| HDL (mg/dl) | |||||
| Control | 69 (6) | 68 (6) | 69 (7) | 69 (7) | 71 (5) |
| STZ | 68 (3) | 68 (3) | 68 (3) | 68 (3) | 67 (3) |
| STZ-saffron | 68 (5) | 68 (5) | 68 (6) | 69 (5) | 67 (5) |
| Triglycerides (mg/dl) | |||||
| Control | 104 (21) | 87 (27) | 92 (97) | 80 (18)h | 110 (40) |
| STZ | 104 (230) | 96 (22) | 89 (42) | 129 (52) | 1,55l (52) |
| STZ-saffron | 87 (25) | 114 (11)a | 89 (37) | 125 (54) | 130 (47) |
| Insulin (ng/dl) | |||||
| Control | 1.8 (0.4) | 1.8 (0.2) | 2.2 (0.7) | 2 (0.2) | 2.6 (0.8) |
| STZ | 2 (0.4) | 1.8 (0.2) | 2 (0.3) | 2 (0.3) | 2.4 (0.9) |
| STZ-saffron | 1.9 (0.3) | 2.1 (0.3)a | 1.9 (0.2) | 2.4 (0.5) | 2.3 (0.7) |
| Adiponectin (ng/dl) | |||||
| Control | 2.4 (1.5) | 2.9 (2.5) | 4.2 (3.2) | 4.5 (2) | |
| STZ | 1.6 (1.1) | 5.7 (2) | 1.4 (0.2) | 12.5 (13) | |
| STZ-saffron | 2.3 (1.8) | 1.5 (1) | 1.5 (0.7) | 5.6 (5.6) |
STZ -saffron group vs. STZ group and STZ-saffron group vs. control, P<0.05
Control group vs. STZ-saffron group and control group vs. STZ group, P<0.05
STZ-saffron group vs. STZ group, P<0.05
STZ group vs. control group and STZ group vs. STZ-saffron group, P<0.05
STZ group vs. control group, P<0.05. SD, standard deviation; STZ, streptozotocin.
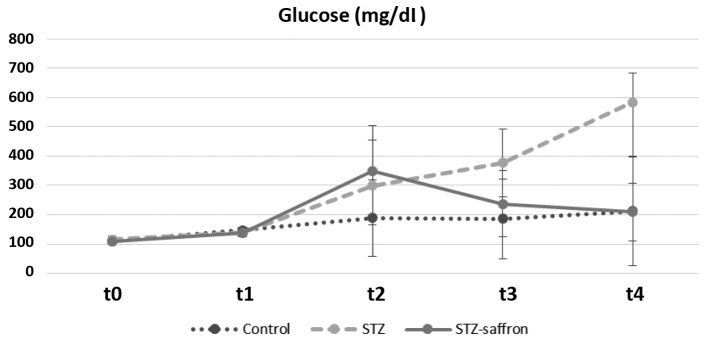
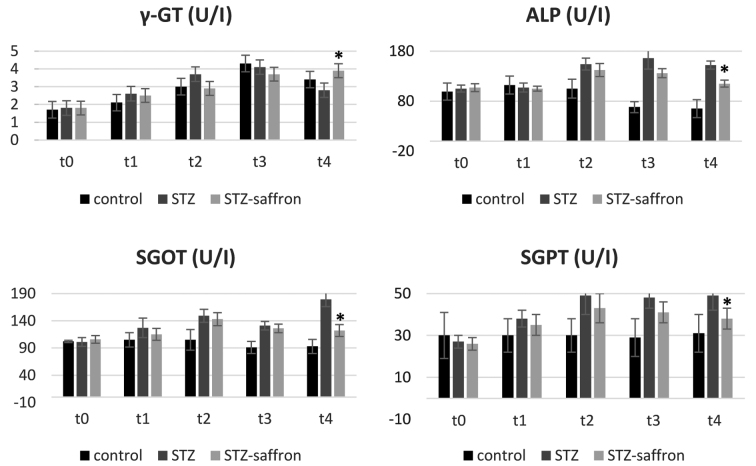
At the end of the study (t4), body weight levels remained increased in control animals when compared to both STZ and STZ-saffron groups (P<0.005, in all cases). Laboratory analysis revealed higher serum glucose concentration in STZ group as compared to the two other studied groups (P<0.005 in all cases). STZ group had increased serum total cholesterol and triglyceride levels in comparison to the control group (P<0.05 for both serum total cholesterol and triglyceride levels) (Table I). Regarding the group receiving Crocus sativus (STZ-saffron), statistically significant differences were observed in comparisons between all time points except t2 vs. t3. In this group, the animals presented increased body weight levels from baseline to t1 while these levels had declined gradually from t1 up to the end of the study. Serum glucose levels in STZ-saffron group were increased in t2 as compared to both baseline and t1 levels, while these levels were lower in t3 and t4 compared to t2 (Fig. 1). t1 and t2 serum total cholesterol levels were lower in comparison to baseline values. At the end of the study serum alanine aminotransferase and aspartate aminotransferase levels were significantly lower in STZ-saffron than STZ group (P<0.001; Fig. 2)
Figure 1.
Glucose serum levels alternations throughout the study among groups. STZ, streptozotocin.
Figure 2.
Effect of saffron on liver function test results. *STZ-saffron group vs. STZ group (P<0.001). γ-GT, γ-glutamyl transpeptidase; ALP, alkaline phospatase; SGOT, serum glutamil oxaloacetic transaminase; SGPT, serum glutamic-pyruvic transaminase.
Serum insulin and adiponectin measurements
No significant differences were recorded for insulin and adiponectin levels in STZ and STZ-saffron groups between different measurements during time (Table I).
Liver histopathology
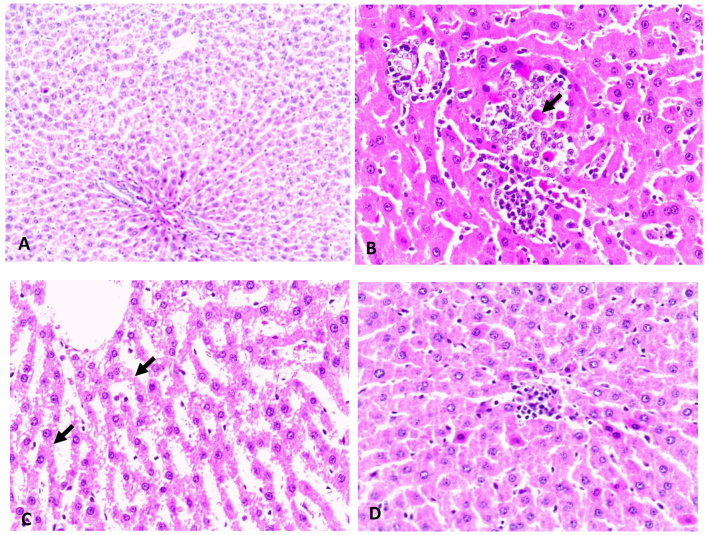
Hematoxylin-eosin-stained liver samples obtained from the STZ group revealed statistically significant higher number of cases (5–33%) with signs of steatosis when compared to the control and STZ-saffron groups where no steatosis was observed (P<0.005). As regards the presence of visible Mallory's hyaline, hepatic tissue stained samples obtained from STZ group showed an increased number of cases when compared to the control and STZ-saffron groups (P<0.005 in both cases; Fig. 3).
Figure 3.
Hematoxylin and eosin (H&E) staining of liver tissue. (A) Control group; normal histological apperance of liver in control rats. H&E magnification, ×40, (B) STZ group; intralobular mononuclear inflammatory infiltrations and Mallory bodies (arrow) due to degeneration of hepatocytesin diabetic rats. H&E magnification, ×120, (C) STZ group; diffuse microvesicular degeneration of hepatocytes (arrow) in diabetic rats. H&E magnification, ×120, (D) STZ-saffron group; mild mononuclear inflammatory aggregation in saffron group rats. H&E magnification, ×120.
Discussion
Crocus sativus is a regimen that has been currently put under the spotlight for its hypoglycemic, anti-oxidant and anti-inflammatory effects (16). Despite the fact that the hypothesis for saffron's glucose-lowering capacity was initially published in 1998, the first reports on the role of saffron specifically on T2D and its manifestations are dating back a decade (17,18). Our results suggest that saffron does not only exert antidiabetic actions, but it also protects the liver damage induced by D2M, both in terms of hepatic histology and liver function.
Diabetes induction was achieved by a two-sided approach. On the one hand, animals received orally fructose through drinking water and on the other hand they received an intraperitoneal injection of STZ. This combined model of low dose STZ and fructose administration simulates better T2D than a single shot of a high dose of STZ, which resembles more T1D (19). Likewise, fructose alone would not achieve the desirable levels of hyperglycemia. It should also be pointed out that STZ is correlated with weight loss in rats, in contrast to the apparent weight gain in the control group (20).
Regarding the establishment of NAFLD, STZ is among others a suitable candidate for achieving a model of liver steatosis (21). Specifically, STZ induces hyperglycemia and insulin resistance, which eventually lead to an increased fat deposition in the hepatic tissue (22). In addition to the action of STZ, fructose supplementation can promote oxidative stress through increased intestinal translocation of bacterial endotoxin, which leads to secretion of endotoxin in portal blood and subsequently, causes activation of Kupffer cells in the liver (14).
In our study, we approached the potent effect of saffron on glucose metabolism in two dimensions; Diabetes per se and NAFLD as a collateral manifestation. In the former case, Crocus sativus effect on tissue sensitivity to insulin is well-documented. Our results are in accordance with the existing literature data (11,15,23). The decrease in glucose levels in the STZ-saffron group was dramatic, thus resulting in glucose levels similar to the of the control group at the end of the experiment. Although, crocin and crocetin-two of the main compounds of saffron- have been extendedly studied regarding their hypoglycemic potency, it is illustrated in our study that these substances keep these properties, even when not separately administered (24,25). The tenacious glucose-lowering effect of saffron is indicative of a synergistic action of its extracts, commonplace in herbs (26). Based on the fact that insulin levels were not increased in the STZ-saffron group, we rather suggest that the effect of saffron is due to its ability to increase insulin sensitivity. Apart from the observations regarding enhancement of insulin sensitivity, in 2012, a possible mechanism for saffron's net effect on this particular pathway was suggested by Kang et al (27). More specifically, they studied glucose metabolism of differentiated C2C12 skeletal muscle cells and proposed that the improvement of insulin resistance was achieved via both an insulin-independent pathway (activation of AMPK/ACC and MAPKs pathway) and an insulin-dependent one (PI3-kinase/Akt and mTOR pathway) (27). Additional to the insulin-resistance enhancing properties of saffron, Rajaei et al advocated that cocretin -one of saffron's compartments- upregulated insulin secretion from pancreatic β-cells thanks to its antioxidant effect which led to a decrease of ROS levels focally in Langerhan's cells (15).
Saffron's role on NAFLD may be justified through its glucose-lowering action and its antioxidant properties. Low glucose levels are associated with a negative feedback to SREBP-1c and ChREBP activity, halting, in this way, lipogenesis (4,28). Moreover, saffron's proven antioxidant effects contribute to lowering focal oxidative stress level in the hepatic tissue as an outcome of T2D, diminishing, as such, the progress of NAFLD (11). The fact that there were observed lower glucose levels in serum and remission of T2D is in favor of a glucose-dependent mechanism of action of saffron on NAFLD. Its antioxidant and anti-inflammatory properties suggest a concomitant glucose-independent pathway (14). There is still to be investigated which of these two mechanisms is more efficient and prominent in the case of NAFLD and whether they are acting synergistically or interfering in certain cross-links. As it concerns the relatively decreased hepatic enzymes in the STZ-saffron animals (Fig. 2), it can be hypothesized that saffron exerts a protective effect against STZ toxicity and/or diabetes induced liver steatosis.
When taking into account the implication of saffron on T2D and NAFLD, it is obvious that it exhibits a pattern similar to this of insulin-sensitizing agents like ppar-gamma agonists. Drugs of this category are broadly used for the treatment of T2D and have been investigated in the treatment of NAFLD in patients with and without diabetes (7). It is a matter of concern if the pathway of ppar-gamma has a distinct role in the beneficial attributes of saffron.
Insulin's role in T2D and in the mechanism of action of saffron is well-described (27,29). There is a wide range of evidence showing that there is a firm correlation between saffron and insulin resistance (15,30). Although, an impressive decrease in glucose levels was marked, no respective decrease of insulin levels was seen. It is assumed that an increase of insulin sensitivity is the determinant factor.
Our study, despite its novelty, possesses certain limitations. Small number of animals and administration of saffron via water not allowing to determine the pharmacokinetics and its metabolism.
Available data on the hypoglycemic effect of saffron are promising, yet the most preferable dosage remains an issue of debate. There is a fluctuation in the dosage preferred in different animal experiments ranging from 20 to 100 mg/kg (31). As a consequence, elucidation of the exact dosage of saffron or its components, in order to accomplish the highest therapeutic index and efficacy.
The aforementioned results clearly indicate that saffron administration protects the liver from the toxic effects of STZ-induced diabetes. At the moment, only certain hypothesis can be expressed regarding the exact mechanism of saffron's action in fatty liver. Hence, the original observations described in this paper seem to be very important in terms of potential therapeutic effects of crocus in human diabetes with or without fatty liver disease. Nonetheless, further research is required in order to elucidate the above hypothesis.
Acknowledgements
We wish to thank Mr. Panagiotis Tsakiropoulos, Mr. Nikolaos Tsakiropoulos, Mrs. Esmeralda Ntousi for their kind assistance in laboratory techniques. This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Glossary
Abbreviations
- T2D
type 2 diabetes
- NAFLD
non-alcoholic fatty liver disease
- STZ
streptozotocin
References
- 1.Rathmann W, Giani G. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:2568–2569, author reply 2569. doi: 10.2337/diacare.27.10.2568. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part. 1998;1:Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15: 539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Baxter M. Treatment of type 2 diabetes: A structured management plan. Adv Ther. 2008;25:106–114. doi: 10.1007/s12325-008-0017-y. [DOI] [PubMed] [Google Scholar]
- 4.Leite NC, Villela-Nogueira CA, Cardoso CR, Salles GF. Non-alcoholic fatty liver disease and diabetes: From physiopathological interplay to diagnosis and treatment. World J Gastroenterol. 2014;20:8377–8392. doi: 10.3748/wjg.v20.i26.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 6.Schindhelm RK, Heine RJ, Diamant M. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:e94. doi: 10.2337/dc07-0982. author reply e95. [DOI] [PubMed] [Google Scholar]
- 7.Abd El-Kader SM, El-Den Ashmawy EM. Non-alcoholic fatty liver disease: The diagnosis and management. World J Hepatol. 2015;7:846–858. doi: 10.4254/wjh.v7.i6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdeen MB, Chowdhury NA, Hayden MR, Ibdah JA. Nonalcoholic steatohepatitis and the cardiometabolic syndrome. J Cardiometab Syndr. 2006;1:36–40. doi: 10.1111/j.0197-3118.2006.05523.x. [DOI] [PubMed] [Google Scholar]
- 9.Bathaie SZ, Bolhasani A, Hoshyar R, Ranjbar B, Sabouni F, Moosavi-Movahedi AA. Interaction of saffron carotenoids as anticancer compounds with ctDNA, Oligo (dG.dC)15, and Oligo (dA.dT)15. DNA Cell Biol. 2007;26:533–540. doi: 10.1089/dna.2007.0598. [DOI] [PubMed] [Google Scholar]
- 10.Azimi P, Ghiasvand R, Feizi A, Hariri M, Abbasi B. Effects of Cinnamon, Cardamom, Saffron, and Ginger Consumption on Markers of Glycemic Control, Lipid Profile, Oxidative Stress, and Inflammation in Type 2 Diabetes Patients. Rev Diabet Stud. 2014;11:258–266. doi: 10.1900/RDS.2014.11.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samarghandian S, Borji A, Delkhosh MB, Samini F. Safranal treatment improves hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic rats. J Pharm Pharm Sci. 2013;16:352–362. doi: 10.18433/J3ZS3Q. [DOI] [PubMed] [Google Scholar]
- 12.Samarghandian S, Azimi-Nezhad M, Samini F. Ameliorative effect of saffron aqueous extract on hyperglycemia, hyperlipidemia, and oxidative stress on diabetic encephalopathy in streptozotocin induced experimental diabetes mellitus. BioMed Res Int. 2014;2014:920857. doi: 10.1155/2014/920857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hazman Ö, Bozkurt MF. Anti-inflammatory and Antioxidative Activities of Safranal in the Reduction of Renal Dysfunction and Damage that Occur in Diabetic Nephropathy. Inflammation. 2015;38:1537–1545. doi: 10.1007/s10753-015-0128-y. [DOI] [PubMed] [Google Scholar]
- 14.Imajo K, Yoneda M, Kessoku T, Ogawa Y, Maeda S, Sumida Y, Hyogo H, Eguchi Y, Wada K, Nakajima A. Rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Int J Mol Sci. 2013;14:21833–21857. doi: 10.3390/ijms141121833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajaei Z, Hadjzadeh MA, Nemati H, Hosseini M, Ahmadi M, Shafiee S. Antihyperglycemic and antioxidant activity of crocin in streptozotocin-induced diabetic rats. J Med Food. 2013;16:206–210. doi: 10.1089/jmf.2012.2407. [DOI] [PubMed] [Google Scholar]
- 16.Bailey CJ, Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care. 1989;12:553–564. doi: 10.2337/diacare.12.8.553. [DOI] [PubMed] [Google Scholar]
- 17.Hemmati M, Zohoori E, Mehrpour O, Karamian M, Asghari S, Zarban A, Nasouti R. Anti-atherogenic potential of jujube, saffron and barberry: Anti-diabetic and antioxidant actions. EXCLI J. 2015;14:908–915. doi: 10.17179/excli2015-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zafar M, Naeem-Ul-Hassan Naqvi S. Effects of STZ-Induced diabetes on the relative weights of kidney, liver and pancreas in albino rats: A comparative study. Int J Morphol. 2010;28:135–142. doi: 10.4067/S0717-95022010000100019. [DOI] [Google Scholar]
- 19.Skovsø S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Investig. 2014;5:349–358. doi: 10.1111/jdi.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye JH, Chao J, Chang ML, Peng WH, Cheng HY, Liao JW, Pao LH. Pentoxifylline ameliorates non-alcoholic fatty liver disease in hyperglycaemic and dyslipidaemic mice by upregulating fatty acid β-oxidation. Sci Rep. 2016;6:33102. doi: 10.1038/srep33102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boskabady MH, Farkhondeh T. Antiinflammatory, Antioxidant, and Immunomodulatory Effects of Crocus sativus L. and its Main Constituents. Phytother Res. 2016;30:1072–1094. doi: 10.1002/ptr.5622. [DOI] [PubMed] [Google Scholar]
- 22.Escribano J, Alonso GL, Coca-Prados M, Fernandez JA. Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Lett. 1996;100:23–30. doi: 10.1016/0304-3835(95)04067-6. [DOI] [PubMed] [Google Scholar]
- 23.Shirali S, Bathaie S Zahra, Nakhjavani M. Effect of crocin on the insulin resistance and lipid profile of streptozotocin-induced diabetic rats. Phytother Res. 2013;27:1042–1047. doi: 10.1002/ptr.4836. [DOI] [PubMed] [Google Scholar]
- 24.Mousavi SH, Tayarani NZ, Parsaee H. Protective effect of saffron extract and crocin on reactive oxygen species-mediated high glucose-induced toxicity in PC12 cells. Cell Mol Neurobiol. 2010;30:185–191. doi: 10.1007/s10571-009-9441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xi L, Qian Z, Xu G, Zheng S, Sun S, Wen N, Sheng L, Shi Y, Zhang Y. Beneficial impact of crocetin, a carotenoid from saffron, on insulin sensitivity in fructose-fed rats. J Nutr Biochem. 2007;18:64–72. doi: 10.1016/j.jnutbio.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Zhou X, Seto SW, Chang D, Kiat H, Razmovski-Naumovski V, Chan K, Bensoussan A. Synergistic Effects of Chinese Herbal Medicine: A Comprehensive Review of Methodology and Current Research. Front Pharmacol. 2016;7:201. doi: 10.3389/fphar.2016.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang C, Lee H, Jung ES, Seyedian R, Jo M, Kim J, Kim JS, Kim E. Saffron (Crocus sativus L.) increases glucose uptake and insulin sensitivity in muscle cells via multipathway mechanisms. Food Chem. 2012;135:2350–2358. doi: 10.1016/j.foodchem.2012.06.092. [DOI] [PubMed] [Google Scholar]
- 28.Nascimbeni F, Aron-Wisnewsky J, Pais R, Tordjman J, Poitou C, Charlotte F, Bedossa P, Poynard T, Clément K, Ratziu V, LIDO study Group Statins, antidiabetic medications and liver histology in patients with diabetes with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000075. doi: 10.1136/bmjgast-2015-000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bajerska J, Mildner-Szkudlarz S, Podgórski T, Oszmatek-Pruszyńska E. Saffron (Crocus sativus L.) powder as an ingredient of rye bread: An anti-diabetic evaluation. J Med Food. 2013;16:847–856. doi: 10.1089/jmf.2012.0168. [DOI] [PubMed] [Google Scholar]
- 30.Asri-Rezaei S, Tamaddonfard E, Ghasemsoltani-Momtaz B, Erfanparast A, Gholamalipour S. Effects of crocin and zinc chloride on blood levels of zinc and metabolic and oxidative parameters in streptozotocin-induced diabetic rats. Avicenna J Phytomed. 2015;5:403–412. [PMC free article] [PubMed] [Google Scholar]
- 31.Hemmati M, Asghari S, Zohoori E, Karamian M. Hypoglycemic effects of three Iranian edible plants; jujube, barberry and saffron: Correlation with serum adiponectin level. Pak J Pharm Sci. 2015;28:2095–2099. [PubMed] [Google Scholar]