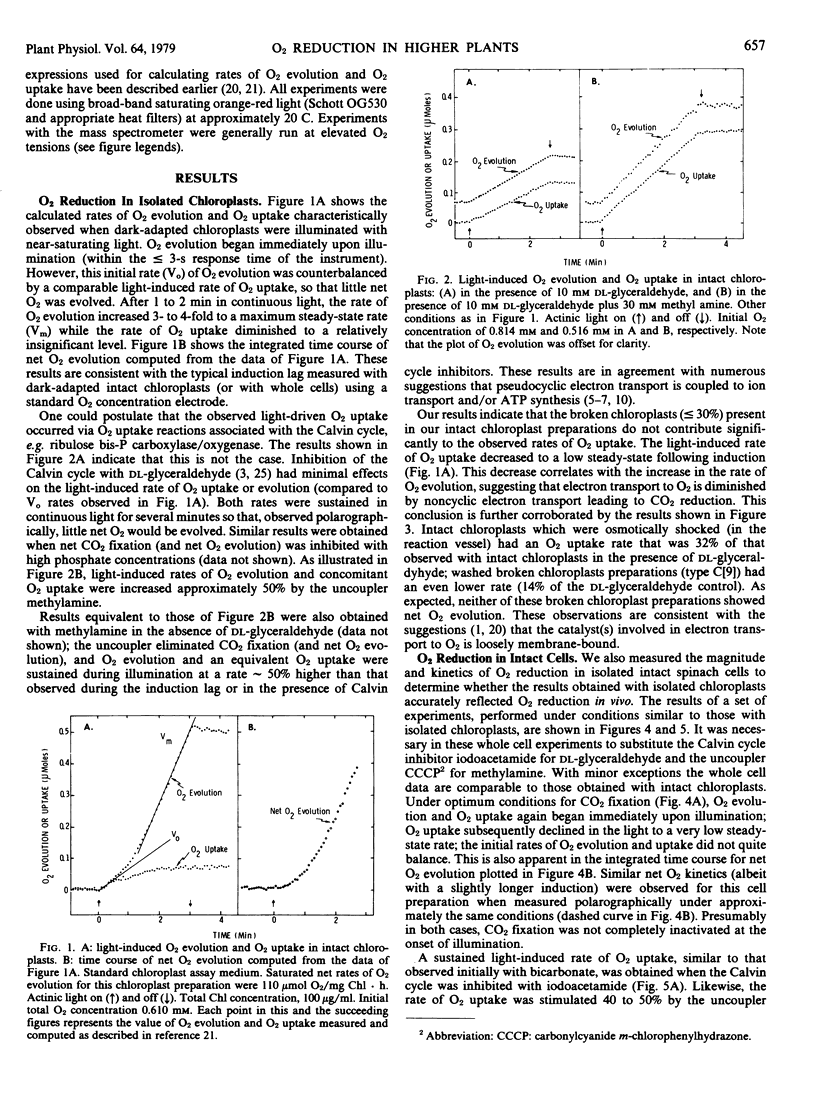
Abstract
The time course of light-induced O2 exchange by isolated intact chloroplasts and cells from spinach was determined under various conditions using isotopically labeled O2 and a mass spectrometer. In dark-adapted chloroplasts and cells supplemented with saturating amounts of bicarbonate, O2 evolution began immediately upon illumination. However, this initial rate of O2 evolution was counterbalanced by a simultaneous increase in the rate of O2 uptake, so that little net O2 was evolved or consumed during the first ∼ 1 minute of illumination. After this induction (lag) phase, the rate of O2 evolution increased 3- to 4-fold while the rate of O2 uptake diminished to a very low level. Inhibition of the Calvin cycle, e.g. with dl-glyceraldehyde or iodoacetamide, had negligible effects on the initial rate of O2 evolution or O2 uptake; both rates were sutained for several minutes, and about balanced so that no net O2 was produced. Uncouplers had an effect similar to that observed with Calvin cycle inhibitors, except that rates of O2 evolution and photoreduction were stimulated 40 to 50%.
These results suggest that higher plant phostosynthetic preparations which retain the ability to reduce CO2 also have a significant capacity to photoreduce O2. With near-saturating light and sufficient CO2, O2 reduction appears to take place primarily via a direct interaction between O2 and reduced electron transport carriers, and occurs principally when CO2-fixation reactions are suboptimal, e.g. during induction or in the presence of Calvin cycle inhibitors. The inherent maximum endogenous rate of O2 reduction is approximately 25 to 50% of the maximum rate of noncyclic electron transport coupled to CO2 fixation. Although the photoreduction of O2 is coupled to ion transport and/or phosphorylation, this process does not appear to supply significant amounts of ATP directly during steady-state CO2 fixation in strong light.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamberger E. S., Avron M. Site of action of inhibitors of carbon dioxide assimilation by whole lettuce chloroplasts. Plant Physiol. 1975 Oct;56(4):481–485. doi: 10.1104/pp.56.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J. A., Osmond C. B., Lorimer G. H. Fixation of O(2) during Photorespiration: Kinetic and Steady-State Studies of the Photorespiratory Carbon Oxidation Cycle with Intact Leaves and Isolated Chloroplasts of C(3) Plants. Plant Physiol. 1978 Dec;62(6):954–967. doi: 10.1104/pp.62.6.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORTI G., JAGENDORF A. T. Photosynthetic phosphorylation in the absence of redox dyes: oxygen and ascorbate effects. Biochim Biophys Acta. 1961 Dec 9;54:322–330. doi: 10.1016/0006-3002(61)90372-9. [DOI] [PubMed] [Google Scholar]
- Forti G., Gerola P. Inhibition of photosynthesis by azide and cyanide and the role of oxygen in photosynthesis. Plant Physiol. 1977 May;59(5):859–862. doi: 10.1104/pp.59.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. O. Nomenclature for isolated chloroplasts. Nat New Biol. 1972 Jan 26;235(56):125–126. doi: 10.1038/newbio235125a0. [DOI] [PubMed] [Google Scholar]
- Heber U. Energy coupling in chloroplasts. J Bioenerg Biomembr. 1976 Jun;8(3):157–172. doi: 10.1007/BF00748961. [DOI] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Huber S., Edwards G. The effect of oxygen on CO2 fixation by mesophyll protoplast extracts of C3 and C4 plants. Biochem Biophys Res Commun. 1975 Nov 3;67(1):28–34. doi: 10.1016/0006-291x(75)90278-8. [DOI] [PubMed] [Google Scholar]
- Kaiser W. The effect of hydrogen peroxide on CO2 fixation of isolated intact chloroplasts. Biochim Biophys Acta. 1976 Sep 13;440(3):476–482. doi: 10.1016/0005-2728(76)90035-9. [DOI] [PubMed] [Google Scholar]
- MEHLER A. H. Studies on reactions of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch Biochem Biophys. 1951 Aug;33(1):65–77. doi: 10.1016/0003-9861(51)90082-3. [DOI] [PubMed] [Google Scholar]
- Ozbun J. L., Volk R. J., Jackson W. A. Effects of Light and Darkness on Gaseous Exchange of Bean Leaves. Plant Physiol. 1964 Jul;39(4):523–527. doi: 10.1104/pp.39.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson C. O., Myers J. Photosynthetic Production of Hydrogen Peroxide by Anacystis nidulans. Plant Physiol. 1973 Jan;51(1):104–109. doi: 10.1104/pp.51.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R. J., Kok B. Photoreduction of O(2) Primes and Replaces CO(2) Assimilation. Plant Physiol. 1976 Sep;58(3):336–340. doi: 10.1104/pp.58.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R., Kok B., Ollinger O. Kinetics and Apparent K(m) of Oxygen Cycle under Conditions of Limiting Carbon Dioxide Fixation. Plant Physiol. 1978 Jun;61(6):915–917. doi: 10.1104/pp.61.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. N-tetramethyl-rho-phenylenediamine as a catalyst of photophosphorylation. Biochim Biophys Acta. 1966 Feb 7;112(2):204–212. doi: 10.1016/0926-6585(66)90321-9. [DOI] [PubMed] [Google Scholar]
- Servaites J. C. Rapid isolation of mesophyll cells from leaves of soybean for photosynthetic studies. Plant Physiol. 1977 Apr;59(4):587–590. doi: 10.1104/pp.59.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabas A. R., Walker D. A. Inhibition of spinach phosphoribulokinase by DL-glyceraldehyde. Biochem J. 1976 Mar 1;153(3):613–619. doi: 10.1042/bj1530613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolove P. M., Marsho T. V. Slow fluorescence quenching of type A chloroplasts. Relationship to electron-flow with CO2 as acceptor. FEBS Lett. 1977 Mar 15;75(1):28–32. doi: 10.1016/0014-5793(77)80045-8. [DOI] [PubMed] [Google Scholar]
- Steiger H. M., Beck E. Oxygen Concentration in Isolated Chloroplasts during Photosynthesis. Plant Physiol. 1977 Dec;60(6):903–906. doi: 10.1104/pp.60.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]


