Abstract
Gallbladder carcinoma (GBC) is the most common biliary tract cancer and exhibits poor patient prognosis. Previous studies have identified that long non-coding RNAs (lncRNAs) serve important regulatory roles in cancer biology. Alterations in lncRNAs are associated with several types of cancer. However, the contribution of lncRNAs to GBC remains unclear. To investigate the lncRNAs that are potentially involved in GBC, lncRNA profiles were identified in three pairs of human GBC and corresponding peri-carcinomatous tissue samples using microarray analysis. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was used to validate the microarray data. In order to elucidate potential functions, Gene Ontology, Kyoto Encyclopedia of Genes and Genomes analysis, and network analysis were used to determine relevant signaling pathways. Abundant RNA probes were used, and 1,758 lncRNAs and 1,254 mRNAs were detected to be differentially expressed by the microarray. Compared with para-carcinoma tissue, numerous lncRNAs were markedly upregulated or downregulated in GBC. The results demonstrated that the lncRNAs that were downregulated in GBC were more numerous compared with the lncRNAs that were upregulated. Among them, RP11-152P17.2-006 was the most upregulated, whereas CTA-941F9.9 was the most downregulated. The RT-qPCR results were consistent with the microarray data. Pathway analysis indicated that five pathways corresponded to the differentially expressed transcripts. It was demonstrated that lncRNA expression in GBC was markedly altered, and a series of novel lncRNAs associated with GBC were identified. The results of the present study suggest that the functions of lncRNAs are important in GBC development and progression.
Keywords: computational biology, expression profile, gallbladder neoplasms, long non-coding RNA, gene microarray analysis
Introduction
Gallbladder carcinoma (GBC) is the most common biliary tract cancer and a common type of cancer of the gastrointestinal tract (1). The incidence and distribution of GBC differs by region (2,3); for example, the prevalence of GBC in South and East Asia is increased compared with that in Europe and the Americas (4). Surgical resection is the only treatment for GBC; however, the majority of patients are not candidates for curative resection when they are diagnosed, and patients with advanced stages of the disease are likely to exhibit recurrences following surgery (5). Although there have been advances in chemotherapy, the prognosis of GBC remains poor (6). The mean survival ranges between 5.2 and 24.4 months (7–10). The majority of patients with GBC succumb to metastasis and recurrence following surgery. Although previous studies have reported that accumulated genomic damage promotes GBC progression (11,12), the underlying molecular mechanisms of GBC progression remain unclear.
Previous studies have identified non-coding RNAs (ncRNAs) as principal components of the human transcriptome (13,14). According to their length, ncRNAs may be divided into two types: Long ncRNAs (lncRNAs) and small regulatory RNAs. lncRNAs have lengths of between 200 bp and 100 kb (15). Increasing evidence in previous years has determined that ncRNAs serve important regulatory roles in cellular physiological processes and diseases (16), including Huntington's disease (17), Alzheimer's disease (18), and glioma, lung, colorectal, breast cancer and hepatocellular cancer (19–22). Accordingly, lncRNA dysregulation, including metastasis-associated lung adenocarcoma transcript 1, colon cancer-associated transcript 1, homeobox transcript antisense RNA (HOTAIR) and low expression in tumor, is also associated with GBC (23–26). Although numerous lncRNAs have been discovered over the previous decade, the biological functions of lncRNAs in GBC and their underlying molecular mechanisms remain unclear. Using microarray analysis, 654 lncRNAs and 1,057 mRNAs that were markedly aberrantly expressed in GBC and paired peri-carcinomatous tissue samples were identified. The results were validated using the reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and were consistent with the data analysis of the profiles. Co-expression networks of lncRNAs and mRNAs, as well as Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses, were used to determine the biological processes for lncRNAs. The results of the present study demonstrated that aberrantly expressed lncRNAs may serve a role in the occurrence and development of GBC.
Materials and methods
Ethics statement
All aspects of the present study were approved by the Zhongshan Hospital Research Ethics Committee (Zhongshan Hospital, Fudan University, Shanghai, China). All patients provided written informed consent for the use of their surgical specimens in the present study in accordance with the Committee's regulations. The clinical characteristics of the patients with GBC are presented in Table I. Tissue specimens were selected randomly from Zhongshan Hospital, Fudan University (Shanghai, China) between April and September 2014.
Table I.
Clinical characteristics of 23 gallbladder carcinoma cases.
| Patient no. | Age, years | Gender | T | N | M | TNM stage |
|---|---|---|---|---|---|---|
| P1 | 69 | F | 3 | 0 | 0 | IIIA |
| P2 | 51 | M | 2 | 0 | 0 | II |
| P3 | 62 | M | 3 | 1 | 0 | IIIB |
| P3 | 50 | F | 3 | 0 | 0 | IIIA |
| P4 | 40 | M | 1 | 0 | 0 | I |
| P5 | 50 | F | 3 | 0 | 0 | IIIA |
| P6 | 41 | F | 1b | 0 | 0 | I |
| P7 | 50 | F | 3 | 0 | 0 | IIIA |
| P8 | 71 | F | 4 | 1 | 0 | IVA |
| P9 | 60 | F | 4 | 2 | 1 | IVB |
| P10 | 51 | F | 1b | 0 | 0 | I |
| P11 | 57 | F | 1b | 0 | 0 | I |
| P12 | 65 | F | 3 | 0 | 0 | IIIA |
| P13 | 66 | M | 3 | 1 | 0 | IIIB |
| P14 | 57 | F | 2 | 2 | 0 | IVB |
| P15 | 78 | F | 1b | 0 | 0 | I |
| P16 | 68 | F | 2 | 0 | 0 | II |
| P17 | 41 | M | 3 | 1 | 0 | IIIB |
| P18 | 49 | M | 3 | 0 | 0 | IIIA |
| P19 | 76 | M | 3 | 1 | 0 | IIIB |
| P20 | 67 | F | 3 | 0 | 0 | IIIA |
| P21 | 62 | F | 4 | 2 | 1 | IVB |
| P22 | 66 | M | 3 | 0 | 0 | IIIA |
| P23 | 75 | M | 3 | 0 | 0 | IIIA |
T, tumor; N, node; M, metastasis; P, patient; F, female; M, male.
Extraction of total RNA and RT-qPCR
Paired tissues of GBC and peri-carcinomatous tissues from each patient were quick-frozen in liquid nitrogen immediately following resection and stored at −80°C. Total RNA was isolated using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocol. A BioPhotometer® plus 6132 instrument (Eppendorf, Hamburg, Germany) was used to measure RNA concentrations. Total RNA (1 µg) was reverse transcribed using Moloney murine leukaemia virus reverse transcriptase (Fermentas; Thermo Fisher Scientific, Inc.). The lncRNA expression levels were determined using RT-qPCR (27) with an Applied Biosystems® 7500 fast real-time PCR thermal cycler instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.), and three replicate PCRs were performed. The PCR kit was purchased from Tiangen Biotech Co., Ltd. (Beijing, China) and 250 ng cDNA was added to each PCR tube. The primers used in the present study are listed in Table II. β-Actin and 18s were used as an endogenous reference (the β-actin primers were purchased from Sangon Biotech Co., Ltd., Shanghai, China). The amplification conditions were as follows: Reverse transcription reaction at 42°C for 30 min per cycle. The PCR cycling conditions were as follows: Enzyme activation at 95°C for 10 sec per 40 cycles, and annealing and extension at 60°C for 32 sec.
Table II.
Primers used for reverse transcription-quantitative polymerase chain reaction.
| lncRNA | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| β-actin | CCAAGCAGCATGAAGATCAA | TCTGCTGGAAGGTGCTGAG |
| 18s | TTGGTCTGTTTAGCGAGGTG | ACGCTGAGCCAGTCAGTGTA |
| CRNDE | CAAATGGAAGCCAGAGGAAA | ATTCAGCACAAGGCAAGGAT |
| CTA-941F9.9 | CTCCGTTTCTTCTCTGAGACTTC | GAGGCACTTCCTTGTGACTT |
lncRNA, long non-coding RNA; CRNDE, colorectal neoplasia differentially expressed.
lncRNA and mRNA microarray expression profiling
Microarray hybridization was carried out by the CapitalBio Corporation (Shanghai, China) on behalf of the present study. Ribosomal RNA was removed from total RNA using an mRNA-ONLY™ Eukaryotic mRNA Isolation kit (Epicentre; Illumina, Madison, WI, USA), according to the manufacturer's protocol, and discarded. Each mRNA sample was transcribed into complementary RNA (cRNA) containing cyanine-3-cytidine 5′-triphosphate fluorescent labels (Agilent Technologies, Inc., Santa Clara, CA, USA). Klenow enzyme labeling strategy was adopted following reverse transcription using CbcScript II reverse transcriptase. Labeled cDNA was produced by Eberwine's linear RNA amplification method and subsequent enzymatic reaction. This procedure has been previously described, and the procedure has been improved by using CapitalBio cRNA Amplification and Labeling kit (CapitalBio, Beijing, China) for producing higher yields of labeled cDNA (28). The labeled cRNAs were hybridized on the Human lncRNA array V4.0 (4×180K, Agilent Technologies, Inc.), which contained the global profiles of 108,458 transcripts (78,243 ncRNAs and 30,215 coding RNAs). The microarrays were washed with two consecutive solutions (0.2% SDS, 2xSSC at 42°C for 5 min, and 0.2xSSC for 5 min at room temperature) and scanned using an Agilent G2505C Microarray Scanner system (Agilent Technologies, Inc.), according to the manufacturer's protocol. The raw data were analyzed using Feature Extraction software (version 10.7.1.1; Agilent Technologies, Inc.) and then normalized using percentile normalization. Aberrantly expressed lncRNAs and mRNAs (fold-change ≥2.0 or ≤0.5; P<0.05) were selected for further study. To identify lncRNAs and mRNAs expression patterns, hierarchical clustering was performed on six tissue samples using Cluster 3.0 (Stanford University School of Medicine, Stanford, CA, USA) and Treeview 2.0 (Baryshnikova Lab, Princeton University, NJ, USA). The results were compared with the microarray data deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) [gene sequence expression series (GSE) accession number GSE62335, www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE62335].
Functional group analysis
To identify the biological functions of the 1,057 aberrantly expressed mRNAs determined as described above, GO and KEGG analyses were used to determine the signaling pathways. The mRNA data were uploaded into the Database for Annotation, Visualization and Integrated Discovery (david.abcc.ncifcrf.gov/tools.jsp) prior to analyzing the enrichment. Fisher's exact test was used to determine the significance of the GO term enrichment for differentially expressed genes. P<0.05 was considered to indicate a statistically significant difference. A false discovery rate (FDR) <0.05 determined the significance of the association of the pathways with the conditions.
Co-expression network construction
An lncRNA-mRNA co-expression network was constructed to determine their association, and the algorithm was as follows: i) The data were preprocessed for median gene expression values of all transcripts from the same coding gene, without special treatment for lncRNAs; ii) data for aberrantly expressed lncRNAs and mRNAs were removed following screening; iii) Pearson's correlation coefficient (PCC) was calculated and the R value was used to calculate the PCC between lncRNAs and mRNAs; and iv) the data were screened and data for which the PCC was >0.99 were selected. The gene co-expression network was constructed using Cytoscape software version 3.1.1 (U.S. National Institute of General Medical Sciences, Washington, DC, USA).
In the network, yellow nodes represented the lncRNAs and green nodes represented the mRNAs. Circular nodes represented lncRNAs and diamond nodes represented the mRNAs. Continuous lines indicated a positive association and dashed lines indicated a negative association.
Cell culture
The human GBC cell line SGC-996 was provided by the Tumor Cytology Research Unit (Medical College, Tongji University, Shanghai, China). Human GBC cell line GBC-SD was purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM, Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 mg/ml streptomycin and 100 units/ml penicillin. Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2.
siRNA transfection
RP5-899B16.2-specific siRNAs and non-silencing negative control siRNA were purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China; product ID siP01001). The target sequences of RP5-899B16.2 were 5′-GGAUAGAUACAUUGACACUdTdT-3′ (siRNA-1) and 5′-GGAUAGAAUCAGGUUCCAUdTdT-3′ (siRNA-2). SGC-996 and GBC-SD cells were transfected with Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol.
Cell viability
Transfected and non-transfected SGC-996 cells and GBC-SD cells were seeded into 96-well plates at a concentration of 3×103 cells/well and 2.5×103 cells/well, respectively. Between days 1 and 5, 10 µl MTT solution with solvent PBS was added to each well of one plate, followed by incubation at 37°C for 4 h. The absorbance at 490 nm was measured using a microplate reader. The assay was performed in triplicate.
Statistical analysis
All statistical analyses were performed using SPSS software (version 18.0; SPSS, Inc., Chicago, IL, USA). For comparison, a two-tailed Student's t-test was performed, when appropriate. Data in Fig. 2 were presented as log2-transformed median fold-changes in expression ± standard error, and results in Fig. 5 were presented as the mean ± standard error of the mean. All histograms were constructed using Prism for Windows (version 5.0; GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
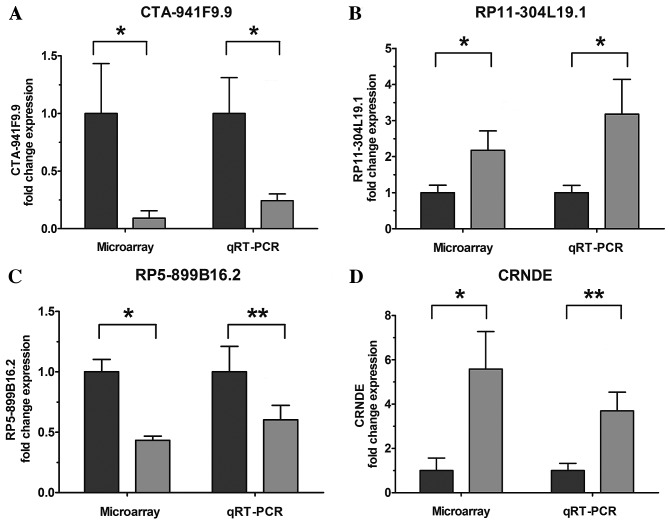
Figure 2.
Comparison between the microarray data and RT-qPCR results. (A) CTA-941F9.9, (B) RP5-899B16.2, (C) RP11-304L19.1 and (D) CRNDE were determined to be differentially expressed in gallbladder carcinoma samples (grey bars) compared with wild-type samples (black bars) in three patients using microarray analysis, and validated using RT-qPCR in tissues of 23 patients. Results are presented as log2-transformed median fold-changes in expression ± standard error across patients for lncRNA validation. Validation of the lncRNAs indicated that the microarray data were consistent with the RT-qPCR results. *P<0.05, **P<0.01. RT-qPCR, reverse transcription-polymerase chain reaction; CRNDE, colorectal neoplasia differentially expressed.
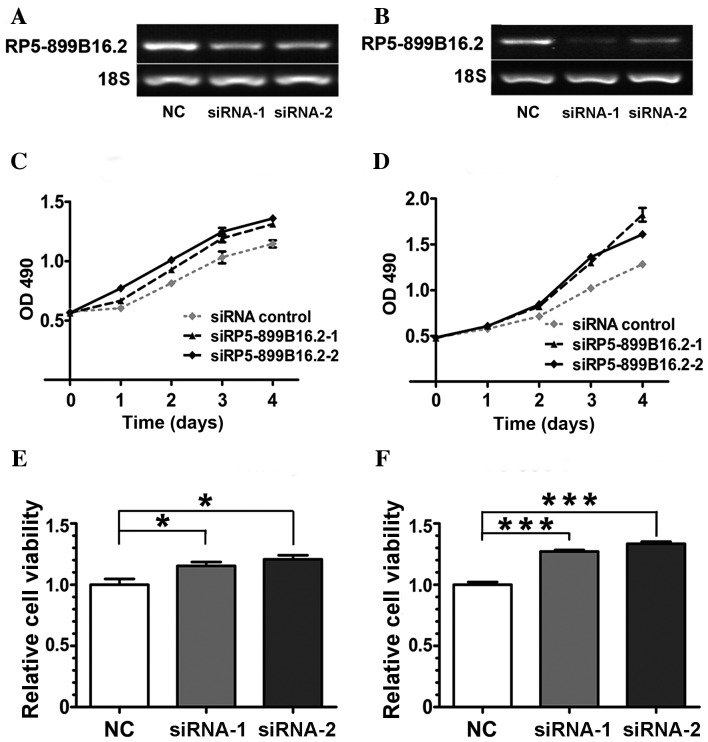
Figure 5.
RP5-899B16.2 silencing affects the viability of gallbladder carcinoma cell lines. Expression of RP5-899B16.2 in (A) GBC-SD and (B) SGC-996 cell lines transfected with control siRNA or two siRNAs targeting RP5-899B16.2 (siRNA-1 and siRNA-2). 18S was used as the internal control. MTT assay of (C) GBC-SD and (D) SGC-996 cells transfected with NC, siRNA-1 and siRNA-2. Relative cell viability with respect to NC-treated cells (set as 1) of the (E) GBC-SD and (F) SGC-996 cell lines 96 h following siRNA transfection. siRNA-1: siRP5-899B16.2-1; siRNA-2: siRP5-899B16.2-2; *P<0.05; ***P<0.001. siRNA, small interfering RNA; NC, negative control; OD, optical density.
Results
lncRNA and mRNA expression profiles in GBC
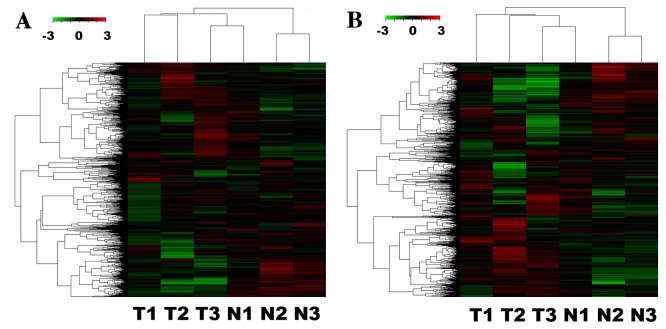
To analyze the expression profiles of lncRNAs between tumor tissue and PCT (para-carcinoma tissue), fold-changes (tumor tissue vs. PCT), P-values and FDRs were used to assess the normalized expression of genes. According to the microarray data, <17,032 lncRNAs and <22,848 mRNAs expressed in the three pairs of GBC and PCT were detected, which included 654 lncRNAs (229 upregulated and 425 downregulated) and 1,057 mRNAs (394 upregulated and 663 downregulated) with markedly different expression. In addition, 112 lncRNAs and 184 mRNAs were identified as consistently upregulated in all three GBC groups, whereas 283 lncRNAs and 427 mRNAs were consistently downregulated. The 30 most marked differentially expressed lncRNAs and mRNAs identified using microarray analysis are presented in Tables III and IV. The hierarchical clustering analysis dendrograms indicated that the samples exhibited associations among the lncRNA and mRNA expression patterns (Fig. 1A and B). Among the aberrantly expressed lncRNA transcripts, RP11-152P17.2-006 (log2 fold-change tumor/wild-type=10.57) was the most markedly upregulated, whereas the expression of CTA-941F9.9 (log2 fold-change tumor/wild-type=−15.00) was the most markedly downregulated. Subsequently, the results were compared with the microarray data deposited in the GEO, which demonstrated that 1,212 lncRNAs and 1,213 mRNAs were aberrantly expressed between five pairs of GBC and adjacent non-tumor samples (fold-change ≥1.25, P<0.05 and FDR<0.05). Three pairs of analogous samples were further analyzed, and 654 lncRNAs and 1,057 mRNAs aberrantly expressed in three GBC tissues compared with the adjacent non-tumor samples were identified (fold-change ≥2.0, P<0.05 and FDR<0.05), which used a different fold-change threshold compared with previous results. If the fold-change was defined to be ≥1.5, the aberrant expression gene numbers were 1,565 lncRNAs and 2,321 mRNAs.
Table III.
Microarray analysis of the 30 most aberrantly regulated lncRNAs in three pairs of gallbladder carcinoma tissues.
| Probe name | lncRNA ID | Gene symbol | P-value | FC | Regulation | Chromosome |
|---|---|---|---|---|---|---|
| p11051 | ENST00000422971.1 | CTA-941F9.9 | 0.017 | 15.00 | Down | 22(−): 46000311–46001501 |
| p7473 | ENST00000591222.1 | NA | 0.014 | 13.99 | Down | 17(+): 66186024–66188943 |
| p3312 | ENST00000551672.1 | NA | 0.021 | 13.56 | Down | 12(−): 80849274–80852604 |
| p13773 | ENST00000514158.1 | CTC-454M9.1 | 0.009 | 13.45 | Down | 5(+): 88185276–88237187 |
| p14911 | ENST00000449672.1 | AOAH-IT1 | 0.016 | 12.84 | Down | 7(−): 36637439–36639726 |
| p21662 | TCONS_00029197 | NA | 0.025 | 12.24 | Down | 21(+): 17979036–17979674 |
| p20932 | TCONS_00004443 | NA | 0.013 | 11.75 | Down | 2(−): 130324222–130351825 |
| p35139_v4 | ENST00000515376.1 | NA | 0.023 | 11.23 | Down | 4(+): 174451610–174512475 |
| p35137_v4 | ENST00000512246.1 | NA | 0.026 | 11.14 | Down | 4(+): 174451608–174462981 |
| p2150 | ENST00000436715.1 | H19 | 0.019 | 11.02 | Down | 11(−): 2016668–2017801 |
| p1587 | ENST00000454837.1 | ANTXRLP1 | 0.033 | 10.94 | Down | 10(−): 47620144–47640809 |
| p15883 | ENST00000520594.1 | NA | 0.039 | 10.57 | Up | 8(−): 106797231–107072695 |
| p16513 | ENST00000594708.1 | NA | 0.027 | 9.97 | Down | 9(−): 72808912–72873782 |
| p36138_v4 | TCONS_00010091 | NA | 0.025 | 9.44 | Down | 5(+): 130589969–130593098 |
| p43626_v4 | XR_430247.1 | NA | 0.040 | 9.30 | Down | 19(+): 42060081–42061684 |
| p22857 | TCONS_00009823 | NA | 0.042 | 9.29 | Down | 5(−): 178365677–178368084 |
| p43270_v4 | XR_429785.1 | NA | 0.000 | 9.12 | Down | 16(+): 85196766–85199924 |
| p15702 | ENST00000523786.1 | NA | 0.002 | 8.87 | Down | 8(−): 57432677–57472056 |
| p15932 | ENST00000517869.1 | NA | 0.033 | 8.87 | Down | 8(−): 126934766–126963394 |
| p7444 | ENST00000580515.1 | BZRAP1-AS1 | 0.026 | 8.87 | Down | 17(+): 56406298–56429790 |
| p35142_v4 | ENST00000515345.1 | NA | 0.029 | 8.51 | Down | 4(+): 174451624–174458566 |
| p33555 | ENST00000460744.1 | NA | 0.022 | 8.06 | Down | 3(+): 111011565–111261149 |
| RNA33675|snoRNA_scaRNA_271_77 | RNA33675| snoRNA_scaRNA_271_77 | NA | 0.012 | 7.72 | Down | NA |
| p38655_v4 | ENST00000458974.1 | NA | 0.024 | 7.63 | Down | 14(+): 101364256–101364333 |
| p12993 | ENST00000509866.1 | NA | 0.036 | 7.62 | Down | 4(+): 174451612–174458842 |
| p13228 | ENST00000503568.1 | NA | 0.004 | 7.61 | Down | 5(−): 74343543–74348468 |
| p34010_v4 | ENST00000415582.1 | NA | 0.048 | 7.55 | Up | 1(−): 201969228–201970411 |
| RNA143553|tRNA_470_66 | RNA143553|tRNA_470_66 | NA | 0.018 | 7.45 | Down | NA |
| p4096 | ENST00000436329.1 | GPC6-AS1 | 0.020 | 7.44 | Down | 13(−): 94806446–94840245 |
| p40958_v4 | XR_427898.1 | NA | 0.028 | 7.43 | Down | 6(−): 25015198–25036372 |
lncRNA, long non-coding RNA; ID, identification no.; FC, fold-change; NA, not annotated.
Table IV.
Microarray analysis of the 30 most aberrantly regulated mRNAs in three pairs of gallbladder carcinoma tissues.
| Probe name | Ensembl ID | Gene symbol | P-value | FC | Regulation | Chromosome |
|---|---|---|---|---|---|---|
| A_23_P390700 | ENST00000550305 | CNTN1 | 0.037 | 21.52 | Down | 12: 41414156–41414215 |
| A_23_P134347 | ENST00000542995 | CPVL | 0.003 | 18.15 | Down | 7: 29105730–29105671 |
| A_33_P3265749 | ENST00000370934 | PTGER3 | 0.020 | 14.64 | Down | 1: 71478048–71477989 |
| A_23_P168993 | ENST00000345060 | ADRB3 | 0.049 | 13.94 | Down | 8: 37821117–37821058 |
| A_23_P150457 | ENST00000438354 | LYVE1 | 0.028 | 12.52 | Down | 11: 10580104–10580045 |
| A_24_P236935 | ENST00000424910 | KLK6 | 0.006 | 12.00 | Up | 19: 51466784–51466725 |
| A_33_P3397865 | ENST00000291901 | TNNT1 | 0.019 | 11.51 | Up | 19: 55644255–55644196 |
| A_33_P3290239 | NA | DUOXA1 | 0.017 | 11.51 | Up | 15: 45411363–45411304 |
| A_33_P3275801 | ENST00000373960 | DES | 0.031 | 11.51 | Down | 2: 220291400–220291459 |
| A_24_P261760 | ENST00000356986 | KLRG1 | 0.007 | 11.49 | Down | 12: 9147774–9147833 |
| A_33_P3254844 | ENST00000401731 | CEACAM7 | 0.005 | 11.48 | Up | 19: 42178513–42178454 |
| A_33_P3256997 | ENST00000398984 | NA | 0.029 | 11.05 | Down | 11: 59980755–59980696 |
| A_33_P3265739 | ENST00000306666 | PTGER3 | 0.021 | 10.98 | Down | 1: 71436629–71436570 |
| A_33_P3248405 | ENST00000536164 | NRK | 0.038 | 10.96 | Down | X: 105139249–105139308 |
| A_23_P145718 | ENST00000483864 | AOAH | 0.017 | 10.92 | Down | 7: 36570069–36561708 |
| A_33_P3294533 | ENST00000321728 | PRKCB | 0.040 | 10.62 | Down | 16: 24231495–24231554 |
| A_23_P339588 | ENST00000338313 | TAGAP | 0.011 | 10.37 | Down | 6: 159460015–159459956 |
| A_24_P40626 | ENST00000318160 | GREM2 | 0.013 | 10.08 | Down | 1: 240654036–240653977 |
| A_21_P0010449 | ENST00000422971 | XLOC_014399 | 0.012 | 10.05 | Down | 22: 46000392–46000333 |
| A_33_P3406196 | ENST00000344825 | KLRD1 | 0.036 | 9.97 | Down | 12: 10462019–10462078 |
| A_24_P156490 | ENST00000286627 | KCNMA1 | 0.006 | 9.84 | Down | 10: 78644826–78644767 |
| A_33_P3225760 | ENST00000412923 | PCDH18 | 0.014 | 9.29 | Down | 4: 138453085–138453026 |
| A_33_P3220015 | ENST00000390341 | NA | 0.028 | 9.25 | Down | 7: 38339480–38339421 |
| A_23_P64898 | ENST00000538029 | KLRG1 | 0.047 | 9.24 | Down | 12: 9162594–9162653 |
| A_21_P0005574 | NA | XLOC_006224 | 0.028 | 9.12 | Down | 7: 123284900–123284959 |
| A_33_P3240512 | ENST00000377474 | KCTD12 | 0.043 | 8.94 | Down | 13: 77454410–77454351 |
| A_33_P3249872 | ENST00000262722 | FBLN1 | 0.031 | 8.54 | Down | 22: 45959173–45959232 |
| A_23_P145606 | ENST00000320658 | CHRM2 | 0.041 | 8.51 | Down | 7: 136700790–136700849 |
| A_33_P3257027 | ENST00000377614 | FGF7 | 0.025 | 8.51 | Down | 15: 49776810–49776869 |
| A_23_P342641 | ENST00000536707 | SLC44A5 | 0.021 | 8.42 | Up | 1: 75679412–75677201 |
ID, identification no.; FC, fold-change; NA, not annotated.
Figure 1.
Differences in the lncRNA and mRNA expression profiles between gallbladder carcinoma and non-tumorous tissues. (A) Results from hierarchical clustering demonstrate distinguishable (A) lncRNA and (B) mRNA expression profiling among samples. Red indicates increased relative expression and green indicates decreased relative expression. lncRNA, long non-coding RNA. T, tumor; N, wild-type.
Validation of the microarray data using RT-qPCR
To validate the microarray analysis results, four upregulated/downregulated lncRNAs were randomly selected for validation using RT-qPCR. The results demonstrated that lncRNAs RP11-304L19.1 and colorectal neoplasia differentially expressed (CRNDE) were upregulated and CTA-941F9.9 and RP5-899B16.2 were downregulated in the tumor samples compared with PCT samples (Fig. 2). These RT-qPCR results were consistent with the microarray data.
GO and KEGG pathway analyses
To investigate potential gene and gene product enrichment in biological processes, cellular components and molecular functions, GO analysis was performed with the differentially expressed mRNAs. An FDR ≤0.05 (Bonferroni correction) was used to determine significant changes in the differentially expressed gene list and the GO annotation list. The results suggested that a number of functional signaling pathways were enriched, including those involved in the regulation of cell activation, external side of the plasma membrane, membrane rafts and regulation of immune system processes. In addition, the cell surfaces exhibited the most increased enrichment of GO terms within GBC (Fig. 3A). Pathway analyses indicated that five pathways corresponded to differentially expressed transcripts and were the human immunodeficiency virus-induced T cell apoptosis, T cytotoxic cell-surface molecules, T helper cell-surface molecules, primary immunodeficiency and the T cell receptor signaling pathways (Fig. 3B).
Figure 3.
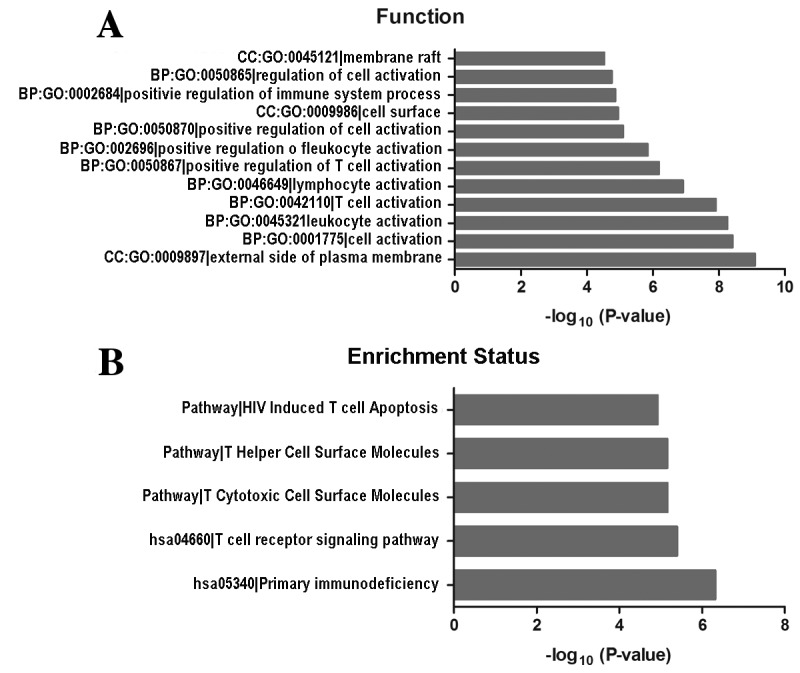
(A) GO analysis and (B) Kyoto Encyclopedia of Genes and Genomes pathway analysis of differentially expressed long non-coding RNAs in gallbladder carcinoma. GO, Gene Ontology; HIV, human immunodeficiency virus; hsa, Homo sapiens.
Construction of a co-expression network
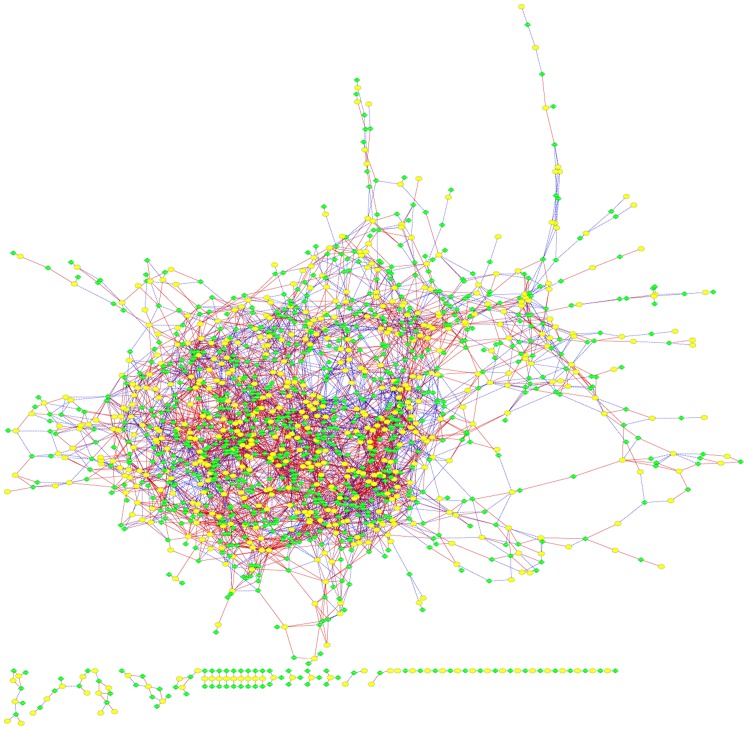
To explore the association between lncRNAs differentially expressed in GBC and targeted mRNAs, a co-expression network was constructed. Using PCC ≥0.99 and P<0.0001, 5,679 pairs of co-expressed lncRNAs and mRNAs composed of 922 mRNAs (87.2% of all differentially expressed mRNAs) and 602 lncRNAs (92.0% of all differentially expressed lncRNAs) were identified, and 3,366 pairs demonstrated a positive association (Fig. 4). The results suggested that the lncRNA-mRNA pairs exhibiting the most marked positive correlation coefficiency included XR_429508.1 and CD3γ. ENST00000451584.1 and transcription factor activator protein 2Aα exhibited the most marked negative correlation coefficiency. This co-expression network indicated that 1 lncRNA may target ≤29 coding genes and that one coding gene may associate with ≤3 lncRNAs. For example, this network indicated that CRNDE was associated with cell division cycle-associated 7-like (CDCA7L) and solute carrier 44A5 (SLC44A5).
Figure 4.
lncRNA-mRNA network constructed based on the correlation analysis between the differentially expressed lncRNAs and mRNAs. Yellow nodes represent lncRNAs and green nodes represent the target mRNAs. Red continuous lines represent a positive association, and blue dashed lines represent a negative association. lncRNA, long non-coding RNA.
Silencing of RP5-899B16.2 increased GBC cell viability
To further confirm the functional relevance of lncRNA expression, SGC-996 and GBC-SD cell lines were transfected with RP5-899B16.2-specific siRNAs and non-silencing negative control siRNA. The RT-qPCR results indicated that RP5-899B16.2 expression was markedly downregulated at the mRNA level in the two transfected cells compared with the control (Fig. 5A and B).
The frequent dysregulation of RP5-899B16.2 in GBC tissues and cell lines suggested that this gene may serve an important role in GBC. To investigate the effects of STMN1 deficiency, an MTT assay was performed and cell viability curves were constructed. It was demonstrated that the viability of the GBC cell lines increased markedly following the silencing of RP5-899B16.2 (Fig. 5C-F).
Discussion
The carcinogenesis of GBC is a complex process (1). Despite advances in research in recent decades, its pathogenesis remains unclear, and further studies are required. The association between lncRNAs and tumors has been frequently investigated: Previous studies have indicated that lncRNAs serve important roles in regulating gene expression and are associated with cancer development (29,30), including HOTAIR in breast cancer (22), digestive system cancer (31) and urothelial cancer (32). The maternally expressed gene 3 was downregulated in several cancers and was demonstrated to inhibit tumor growth (33,34). CRNDE was upregulated in colorectal adenoma, adenocarcinoma and glioma (35,36). The majority of the aforementioned lncRNAs were also identified in the present study. However, studies on the association of lncRNA expression with GBC are limited (23–26). Therefore, it is important to understand the association between lncRNA expression and GBC to identify its pathogenesis. These results suggested that lncRNAs are potential targets for novel therapies.
In the present study, lncRNA and mRNA expression profiles in GBC were investigated using microarray chips. The results demonstrated that >100 lncRNA expression levels were changed compared with matched adjacent non-cancerous tissues. Currently, to the best of our knowledge, there are no studies on lncRNA expression profiles in GBC. The discrepancy between the data of the present study and GEO data may be due to differences in tumor tissues. Furthermore, the platform that was used was the Human lncRNA array V4.0 (4×180K), whereas the platform for the GEO data was the Affymetrix Human Gene 2.0 ST Array. The difference between the two platforms in array content, gene coverage availability, specific exon or splice junction probes, labeling systems and systematic lncRNA classification may lead to the difference in results. Therefore, novel lncRNAs were identified outside the scope of the GEO data. This avoided the elimination of lncRNAs that serve important roles in GBC.
In the previous decade, studies have suggested that a number of lncRNAs contribute to important functions, including the regulation of gene expression, serve an important role in cell development and metabolism, and are aberrant in a variety of diseases (13,37). To elucidate the underlying molecular mechanisms of lncRNA function, a co-expression network was further constructed by combining aberrantly expressed lncRNAs and mRNAs. Multiple lncRNAs were demonstrated to be markedly associated with mRNAs. CRNDE is an intergenic lncRNA located on chromosome 16, which is also overexpressed in colorectal carcinomas, gliomas and leukemias (35,38). CDCA7L is a target gene for cellular Myc proto-oncogene protein (c-Myc) that is involved in cell proliferative and apoptotic signaling pathways (39). However, SLC44A5 codes for a choline transporter-like protein that is associated with cell proliferation. Therefore, it was hypothesized that CRNDE may be a direct or indirect target gene of CDCA7L and SLC44A5. This lncRNA and mRNA co-expression network provides a strong foundation for predicting the function of lncRNAs. The malfunction of regulating this co-expression network may be an important step for the development and progression of GBC. The underlying molecular mechanisms of GBC progression remain unclear. Furthermore, GO analysis and pathway analysis were used to investigate the biological functions of lncRNA in the occurrence and development of GBC. More research is required to elucidate the functions of lncRNAs.
In conclusion, the expression profiles of lncRNAs and mRNAs in GBC with microarray analyses were determined, and 654 lncRNAs and 1,057 mRNAs were identified; the majority of which were novel identifications in GBC. Additionally, an lncRNA-mRNA co-expression network was constructed and it was demonstrated that CRNDE was associated with the c-Myc protein CDCA7L. lncRNAs may function by interacting with mRNAs or proteins in GBC. Accurate signaling pathways warrant further study and are critical for identifying novel methods for the early diagnosis and treatment of GBC. Further functional studies may provide potential therapeutic targets or molecular biomarkers of GBC. The results of the present study suggest useful evidence for investigating potential therapeutic targets for GBC.
Acknowledgements
The Microarray data are publicly accessible via the Gene Expression Omnibus, no. GSE74048. The present study was supported by the National Natural Science Foundation of China (grant no. 81272728).
Glossary
Abbreviations
- GBC
gallbladder carcinoma
- lncRNA
long non-coding RNA
- RT-qPCR
reverse transcription-quantitative polymerase chain reaction
- GO
Gene Ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- PCT
para-carcinoma tissue
References
- 1.Zhu AX, Hong TS, Hezel AF, Kooby DA. Current management of gallbladder carcinoma. Oncologist. 2010;15:168–181. doi: 10.1634/theoncologist.2009-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller G, Jarnagin WR. Gallbladder carcinoma. Eur J Surg Oncol. 2008;34:306–312. doi: 10.1016/j.ejso.2007.07.206. [DOI] [PubMed] [Google Scholar]
- 3.Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: Geographical distribution and risk factors. Int J Cancer. 2006;118:1591–1602. doi: 10.1002/ijc.21683. [DOI] [PubMed] [Google Scholar]
- 4.Misra S, Chaturvedi A, Misra NC, Sharma ID. Carcinoma of the gallbladder. Lancet Oncol. 2003;4:167–176. doi: 10.1016/S1470-2045(03)01021-0. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi K, Chijiiwa K, Saiki S, Nishihara K, Takashima M, Kawakami K, Tanaka M. Retrospective analysis of 70 operations for gallbladder carcinoma. Br J Surg. 1997;84:200–204. doi: 10.1002/bjs.1800840217. [DOI] [PubMed] [Google Scholar]
- 6.Grobmyer SR, Lieberman MD, Daly JM. Gallbladder cancer in the twentieth century: Single institution's experience. World J Surg. 2004;28:47–49. doi: 10.1007/s00268-003-7131-4. [DOI] [PubMed] [Google Scholar]
- 7.Wu XS, Shi LB, Li ML, Ding Q, Weng H, Wu WG, Cao Y, Bao RF, Shu YJ, Ding QC, et al. Evaluation of two inflammation-based prognostic scores in patients with resectable gallbladder carcinoma. Ann Surg Oncol. 2014;21:449–457. doi: 10.1245/s10434-013-3292-z. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett DL, Fong Y, Fortner JG, Brennan MF, Blumgart LH. Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann Surg. 1996;224:639–646. doi: 10.1097/00000658-199611000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butte JM, Matsuo K, Gönen M, D'Angelica MI, Waugh E, Allen PJ, Fong Y, DeMatteo RP, Blumgart L, Endo I, et al. Gallbladder cancer: Differences in presentation, surgical treatment, and survival in patients treated at centers in three countries. J Am Coll Surg. 2011;212:50–61. doi: 10.1016/j.jamcollsurg.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Cziupka K, Partecke LI, Mirow L, Heidecke CD, Emde C, Hoffmann W, Siewert U, van den Berg N, von Bernstorff W, Stier A. Outcomes and prognostic factors in gallbladder cancer: A single-centre experience. Langenbecks Arch Surg. 2012;397:899–907. doi: 10.1007/s00423-012-0950-8. [DOI] [PubMed] [Google Scholar]
- 11.Ooi A, Suzuki S, Nakazawa K, Itakura J, Imoto I, Nakamura H, Dobashi Y. Gene amplification of Myc and its coamplification with ERBB2 and EGFR in gallbladder adenocarcinoma. Anticancer Res. 2009;29:19–26. [PubMed] [Google Scholar]
- 12.Nagahashi M, Ajioka Y, Lang I, Szentirmay Z, Kasler M, Nakadaira H, Yokoyama N, Watanabe G, Nishikura K, Wakai T, et al. Genetic changes of p53, K-ras, and microsatellite instability in gallbladder carcinoma in high-incidence areas of Japan and Hungary. World J Gastroenterol. 2008;14:70–75. doi: 10.3748/wjg.14.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 14.An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagano T, Fraser P. No-nonsense functions for long noncoding RNAs. Cell. 2011;145:178–181. doi: 10.1016/j.cell.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, Finch CE, St Laurent G, III, Kenny PJ, Wahlestedt C, et al. Expression of a noncoding RNA is elevated in Alzheimer's disease and drives rapid feed-forward regulation of beta-secretase. Nat Med. 2008;14:723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson R. Long non-coding RNAs in Huntington's disease neurodegeneration. Neurobiol Dis. 2012;46:245–254. doi: 10.1016/j.nbd.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Zhang P, Wang L, Piao HL, Ma L. Long non-coding RNA HOTAIR in carcinogenesis and metastasis. Acta Biochim Biophys Sin (Shanghai) 2014;46:1–5. doi: 10.1093/abbs/gmt117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vikram R, Ramachandran R, Abdul KS. Functional significance of long non-coding RNAs in breast cancer. Breast Cancer. 2014;21:515–521. doi: 10.1007/s12282-014-0554-y. [DOI] [PubMed] [Google Scholar]
- 21.Tu ZQ, Li RJ, Mei JZ, Li XH. Down-regulation of long non-coding RNA GAS5 is associated with the prognosis of hepatocellular carcinoma. Int J Clin Exp Pathol. 2014;7:4303–4309. [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu XS, Wang XA, Wu WG, Hu YP, Li ML, Ding Q, Weng H, Shu YJ, Liu TY, Jiang L, et al. MALAT1 promotes the proliferation and metastasis of gallbladder cancer cells by activating the ERK/MAPK pathway. Cancer Biol Ther. 2014;15:806–814. doi: 10.4161/cbt.28584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma M, Kong X, Weng M, Zhang MD, Qin YY, Gong W, Zhang WJ, Quan ZW. Long non-coding RNA-LET is a positive prognostic factor and exhibits tumor-suppressive activity in gallbladder cancer. Mol Carcinogen. 2015;54:1397–1406. doi: 10.1002/mc.22215. [DOI] [PubMed] [Google Scholar]
- 25.Ma MZ, Li CX, Zhang Y, Weng MZ, Zhang MD, Qin YY, Gong W, Quan ZW. Long non-coding RNA HOTAIR, a c-Myc activated driver of malignancy, negatively regulates miRNA-130a in gallbladder cancer. Mol Cancer. 2014;13:156. doi: 10.1186/1476-4598-13-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma MZ, Chu BF, Zhang Y, Weng MZ, Qin YY, Gong W, Quan ZW. Long non-coding RNA CCAT1 promotes gallbladder cancer development via negative modulation of miRNA-218-5p. Cell Death Dis. 2015;6:e1583. doi: 10.1038/cddis.2014.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta DeltaC(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Patterson TA, Lobenhofer EK, Fulmer-Smentek SB, Collins PJ, Chu TM, Bao W, Fang H, Kawasaki ES, Hager J, Tikhonova IR, et al. Performance comparison of one-color and two-color platforms within the MicroArray Quality Control (MAQC) project. Nat Biotechnol. 2006;24:1140–1150. doi: 10.1038/nbt1242. [DOI] [PubMed] [Google Scholar]
- 29.Khachane AN, Harrison PM. Mining mammalian transcript data for functional long non-coding RNAs. PLoS One. 2010;5:e10316. doi: 10.1371/journal.pone.0010316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan YF, Feng L, Zhang XQ, Song LJ, Liang HX, Li ZQ, Tao FB. Role of long non-coding RNAs in gene regulation and oncogenesis. Chin Med J (Engl) 2011;124:2378–2383. [PubMed] [Google Scholar]
- 31.Cheng Y, Jutooru I, Chadalapaka G, Corton JC, Safe S. The long non-coding RNA HOTTIP enhances pancreatic cancer cell proliferation, survival and migration. Oncotarget. 2015;6:10840–10852. doi: 10.18632/oncotarget.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heubach J, Monsior J, Deenen R, Niegisch G, Szarvas T, Niedworok C, Schulz WA, Hoffmann MJ. The long noncoding RNA HOTAIR has tissue and cell type-dependent effects on HOX gene expression and phenotype of urothelial cancer cells. Mol Cancer. 2015;14:108. doi: 10.1186/s12943-015-0371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Zhou Y, Mehta KR, Danila DC, Scolavino S, Johnson SR, Klibanski A. A pituitary-derived MEG3 isoform functions as a growth suppressor in tumor cells. J Clin Endocrinol Metab. 2003;88:5119–5126. doi: 10.1210/jc.2003-030222. [DOI] [PubMed] [Google Scholar]
- 34.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graham LD, Pedersen SK, Brown GS, Ho T, Kassir Z, Moynihan AT, Vizgoft EK, Dunne R, Pimlott L, Young GP, et al. Colorectal neoplasia differentially expressed (CRNDE), a novel gene with elevated expression in colorectal adenomas and adenocarcinomas. Genes Cancer. 2011;2:829–840. doi: 10.1177/1947601911431081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellis BC, Molloy PL, Graham LD. CRNDE: A long non-coding RNA involved in CanceR, neurobiology and DEvelopment. Front Genet. 2012;3:270. doi: 10.3389/fgene.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS. Non-coding RNAs: Regulators of disease. J Pathol. 2010;220:126–139. doi: 10.1002/path.2638. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Sun S, Pu JK, Tsang AC, Lee D, Man VO, Lui WM, Wong ST, Leung GK. Long non-coding RNA expression profiles predict clinical phenotypes in glioma. Neurobiol Dis. 2012;48:1–8. doi: 10.1016/j.nbd.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Goto Y, Hayashi R, Muramatsu T, Ogawa H, Eguchi I, Oshida Y, Ohtani K, Yoshida K. JPO1/CDCA7, a novel transcription factor E2F1-induced protein, possesses intrinsic transcriptional regulator activity. Biochim Biophys Acta. 2006;1759:60–68. doi: 10.1016/j.bbaexp.2006.02.004. [DOI] [PubMed] [Google Scholar]