Abstract
Dr Thomas E. Starzl, who died on March 4, 2017, was one of the great pioneers of organ transplantation. He was also a pioneer in the field of xenotransplantation. In 1964 he carried out baboon kidney transplants in 6 patients with terminal renal disease for whom no living or deceased donor became available; graft survival was for 19–60 days, the grafts being lost largely through continuous complement activation. Between 1966 and 1974, he carried out one ex vivo liver perfusion and three orthotopic liver transplants using chimpanzees as sources of organs; graft survival was for <14 days. In 1992 and 1993, his team carried out baboon liver transplantation in two patients with cirrhosis from hepatitis B infection; graft survival was for 70 and 26 days, respectively. This early clinical experience is briefly discussed. Towards the end of his life, Dr Starzl was somewhat disillusioned by what he considered excessive regulation of medical research in the US, and believed that new advances were now likely to take place in countries such as China, where the regulatory framework is less developed.
Keywords: Baboon, Chimpanzee, Kidney, Liver, Thomas E. Starzl, Xenotransplantation
Introduction
Tom Starzl (Figure 1), who sadly died on March 4, 2017, just a week before his 91st birthday, was one of the greatest pioneers in transplantation. He made several major contributions to the development of the field, which he summarized in his informative autobiography (1). For these contributions, he was awarded the US National Medal of Science and, shared with Sir Roy Calne, the Lasker-DeBakey Award for Clinical Medical Science (2).
Figure 1.

Thomas E. Starzl MD, PhD
In the early 1960s, Dr Starzl introduced an immunosuppressive regimen in which he combined therapy with azathioprine with corticosteroids, later adding antithymocyte globulin, that transformed clinical kidney allotransplantation from a relatively unsuccessful therapy to one associated with increasing success (3). He was one of the first to document hyperacute rejection of a kidney allograft (4), and also to report the development of a coagulopathy after allotransplantation (5) – both pathologic conditions that are of great interest and relevance to those of us in xenotransplantation research today.
He was the first to fully develop the technique of orthotopic liver transplantation (6), carrying out the first successful clinical liver transplant in 1968 (7). After the clinical introduction of cyclosporine by Sir Roy Calne (8), Dr Starzl again combined this agent with corticosteroids to improve the results of organ transplantation further (9). He subsequently comprehensively explored FK 506 (tacrolimus) as an immunosuppressive agent (10), introducing it clinically in 1989 (11). The potency of this agent enabled him to lead the clinical development of intestinal and multi-organ transplantation (12). Based on observations on many of his long-surviving transplant patients, he stressed the importance of mixed hematolymphopoietic chimerism as the basis of immunological tolerance (13,14).
Dr. Starzl was one of the most remarkable men it has been my privilege to meet. He conscientiously documented all of the observations he made both experimentally and clinically, both with regard to progress made and complications that were faced. He retained an encyclopedic knowledge of the transplantation literature. His productivity over the course of his career was legendary, with almost 2,500 publications to his name.
He was also a pioneer in the field of xenotransplantation (to which field Sir Roy also contributed in preclinical models) (reviewed in 15), and it is for this reason that I spent time with him in May and June of 2012, discussing his early contributions, which were recognized in 1999 when he was elected the first Honorary Member of the International Xenotransplantation Association (IXA) (16). In 2012, his memory for the early events of xenotransplantation remained acute.
Initial experience with renal allotransplantation
Having carried out original research in neuroscience for the PhD degree, research which made significant contributions to the field, Dr. Starzl turned his attention to cardiac physiology, and investigated cardiac pacemaking for the treatment of heart block (outlined in his autobiography [1]). He then became interested in metabolism, and that resulted in the development of models of liver transplantation for physiological studies.
It was in the early 1960s that Dr Starzl turned his full attention to organ transplantation. In his clinical kidney allotransplantation program at the University of Colorado in Denver, the maintenance therapy he administered combined azathioprine with corticosteroids. When a kidney was being donated by a living donor (as was usually the case in those days), he believed it was important to begin azathioprine therapy 7–10 days before the transplant. Initially, corticosteroids were not administered until a rejection episode occurred (which in that era occurred in 100% of the patients), but were subsequently given in all cases. With azathioprine alone, mean survival after kidney allotransplantation was increased from 7–14 days to 30–35 days, but with treatment of rejection with corticosteroids, mean survival increased to >70 days.
These data were first presented at a meeting of surgeons from all of the world’s active kidney transplant programs in Washington, DC, in 1963. “Quite a few of the people attending the meeting didn’t believe a damn word I said,” Dr Starzl confided in me. “In clinical allotransplantation (in Denver) we had accumulated a total of at least 30 patients, and about 85% were alive.” However, “our knowledge of the immune system was very basic. The slate was practically blank until Macfarlane Burnet came up with the clonal selection theory, which really is the basis of immunology today. It was not known that passenger leukocytes leave the graft. It was not until 1980 that it was understood that they migrated to the spleen, and it was not for a further 10 years that it was shown that dendritic cells migrate. So we had no knowledge at all of immunology. For example, the function of the lymphocytes had not been determined. We did not know of the antigen-presenting cell, the dendritic cell, which was not discovered until four of five years later.
“It was in this climate of ignorance that these first successful cases were carried out. Remarkably, a number of those early patients with familial donor organs lived for at least 25 years, some even remaining alive today 50 years after the transplant. These are the longest surviving organ transplant recipients in the world, and are now dying of old age”.
Renal xenotransplantation using baboons as a source of organs
On the basis of these encouraging results from clinical allotransplantation, Dr. Starzl began to consider using a xenograft in some cases. The difficulty in obtaining human kidneys – either living or deceased – stimulated this consideration, which was enhanced further by the publication from Keith Reemtsma’s group of the results of kidney transplantation using chimpanzees as ‘donors’ in 6 patients with terminal renal disease (17). (Keith Reemtsma was the Founding Honorary President of the IXA [18].)
The majority of Reemtsma’s patients died within two months from either rejection of the graft or an infectious complication of the immunosuppressive therapy, but one patient survived for nine months, dying suddenly from what was thought to be an electrolyte disturbance. In his 1964 book on kidney transplantation (19), Dr Starzl believed that, because the problem of kidney procurement would greatly limit the widespread application of therapeutic clinical allotransplantation, the introduction of xenotransplantation by Reemtsma was an event of great significance.
With the publication of Reemtsma’s results, Claude Hitchcock, of Hennepin County Hospital in Minnesota, a hospital with a connection with the University of Minnesota, reported that he had previously carried out a clinical kidney transplant using a rhesus monkey as the source of the organs on February 16th, 1963 (20). Drs. Starzl and Hitchcock subsequently pooled their knowledge and resources. “Hitchcock believed that because of its anthropomorphic qualities, use of chimpanzees would cause a public outcry if used on a large scale for organ transplantation. In his opinion, lower nonhuman primates, such as baboons or monkeys, would be more acceptable. Baboons were so plentiful in South Africa that they were hunted as pests, the way people were shooting coyotes in the old American west. We thought that rather than rhesus monkeys, or chimps, the baboon should be tested.”
Dr Starzl planned a definitive clinical trial of baboon kidney transplants. He knew scientists at the Rockefeller Institute in New York City, who were experts in blood typing of nonhuman primates, and so he set up a multidisciplinary team, obtaining baboons from a breeding colony at the Southwest Foundation for Research and Education, San Antonio, Texas. In the majority of the patients, both kidneys were transplanted from the baboon into the patient, as in Reemtsma’s cases (Figure 2).
Figure 2.
Insertion of the pair of baboon kidneys into a patient following the technique of Reemtsma. (Modified from Starzl TE, et al. Transplantation 1964;2:752–776 [21]).
All patients were in the terminal phase of their disease. In the six cases in which baboons were used as donors, suitable familial donors were not available in any case, and cadaveric kidneys were unsuccessfully sought during the period of preoperative observation, in one case for as long as two months (21). The blood types of the patients and their donors were known. Two female and four male East African baboons (Papio doguera) were used, weighing from 15.7 to 24.8 kg. Pathogenic bacteria, parasites, fungi, and viruses were excluded.
In the recipients, splenectomy and bilateral nephrectomy were performed transperitoneally. The xenografts were then placed retroperitoneally through an oblique lower abdominal incision. Azathioprine was started 7–10 days preoperatively, and given at the maximum dose without causing profound leukopenia. Prednisone (150–200 mg/day in divided doses) was later added to the pretreatment regimen, and continued after transplantation. Later in the postoperative course, an effort was made to reduce the quantity of steroids as quickly as possible, but this did not prove to be feasible since rejection occurred even with a very high level of prednisone therapy. In addition, actinomycin C was administered, and local radiation of the graft was either given prophylactically or for the treatment of rejection. Immunologic studies included the measurement of heteroagglutinins (xenogeneic antibodies), anti-A and anti-B isoagglutinins, and erythrocyte survival (22).
Early after transplantation, urine output was excessive, ranging from 8.7L to 24.5L within the first 24 hours, with a mean of 16.5L. (A patient with a kidney xenograft carried out by Hume died as a consequence of an uncontrollable diuresis which totaled 54 liters on the first postoperative day [23].) Survival of the patients ranged from 19 to 60 days (Table 1). As with Reemstma’s series, the majority of deaths were related to rejection or infection.
Table 1.
Clinical organ xenotransplants carried out by Dr Starzl and his colleagues1
Dr Starzl sent tissues to Kendrick Porter, a leading histopathologist at St. Mary’s Hospital in London, for histological examination (24). At first sight, rejection did not appear to be more severe than in an allograft, but on further study Dr. Starzl recognized that there was an additional component of the immune response that could not be controlled, and this was continual complement activation. He therefore did not continue the clinical trial.
“We had nothing in our armamentarium that could control it,” said Dr. Starzl. In 1964, in his book on renal transplantation, he had written: “However, despite failure of all of the baboon and most of the chimpanzee xenotransplants, the temporarily favorable early course of a number of patients with maintenance of life-sustaining renal function for many weeks provided hope that animal donors would be useful when better immunosuppressive measures became available” (19).
In Dr Starzl’s opinion, there seemed to be a definite functional superiority of the chimpanzee grafts, the average of daily creatinine clearance being much higher in three of Reemtsma’s patients than in any of the baboon series. Relief of azotemia was also more complete. Furthermore, rejection episodes in the chimpanzee occurred less frequently, were more completely reversible, and less steroid therapy was required.
Because of the absence of AB antigens on baboon erythrocytes, and because of the possibility that renal tissue is also free of these substances, the possibility existed that baboon-to-human xenografts would not be adversely affected by blood group mismatches. But serial changes of anti-A and anti-B titers in the patients indicated that there were important specific immunologic changes consequent to transplanting AB-mismatched kidneys.
In his 1964 book (19), Dr Starzl made a statement with regard to xenotransplantation that is equally applicable today. “The use of any form of heterotransplantation (xenotransplantation) must be considered as the purest form of investigative effort…. There is, at present, no place for unplanned or casual procedures of this type. What must emerge from a minimal number of cases is a clean body of unassailable factual data upon which to build future progress. There is no other justification for such a surgical experiment.”
Grants and the Markle scholarship
I asked Dr. Starzl how he obtained funding for his experimental work. “I had grants. The NIH was very loose in those days…. about how we spent the money. When we put in for grants, we were moving so rapidly that, when the grant was funded, we already knew the answers; we had already done the experiments we were proposing. When we got the money, we did the next batch of experiments, and then used those results for the next grant, and so the system served us very well for the better part of 50 years.”
In late 1958, Dr Starzl applied for a prestigious Markle scholarship. “This scholarship was different from a grant as it was awarded for a project that might take a lifetime, and only had a small chance of succeeding. Only one in every five of the candidates was successful in their application.”
Remarkably, Dr Starzl’s proposal was to develop liver transplantation in humans, which must have been considered almost science fiction in those early days.
“I had good luck as there wasn’t a single physician or MD on the selection committee, not even a single scientist. The people making the judgments were from various backgrounds. For example, one was head of the Federal Reserve bank, and another was the head of the penal system in Canada. They were from every discipline except medicine or science. They were more interested in assessing the candidate’s personality, character, and leadership potential, rather than assessing the actual proposal. Some of the proposals were utterly preposterous. My own was probably the best example of this.”
Initial experience with liver allotransplantation
Having been awarded a scholarship, Dr Starzl initiated an extremely active experimental program of liver allotransplantation. By 1963, his team was ready to perform liver transplantation in patients, but the results were disappointing (6), necessitating a decision to put the clinical program on hold for several years. In 1969, however, he carried out the first successful clinical procedure (7).
Liver xenotransplantation using chimpanzees as a source of organs
Dr Starzl and his colleagues were again pioneers in the field of clinical liver xenotransplantation, performing one ex vivo chimpanzee liver perfusion and three chimpanzee liver transplants in humans between 1966 and 1974 (Table 1). with the grafts functioning from <1 to 14 days. This ambitious program was driven largely by necessity.
The decision was based to some extent on the encouraging results obtained after renal allotransplantation with a triple drug immunosuppressive regimen (azathioprine, corticosteroids, antilymphocyte globulin). It was also thought that a chimpanzee donor might be selected on the basis of histocompatibility antigens that were shared by both species (25).
“In the 1960s it was very hard to find organ donors,” he told me. “Brain death was an appalling idea to most people, and it was even worse if you had a brain-dead child. We had a sick child (in Denver), so the need for a donor was there.” Dr. Starzl planned to save the child’s life with a liver xenograft. “There is always an element of ambition to get something new done, and so you put together the need and the ambition.”
The first clinical procedure was performed in 1966 when a chimpanzee liver was anastomosed to the femoral vessels of a young child in hepatic coma (Table 1) (26). The graft functioned for 24 hours, during which period the patient’s coma was partially resolved, and clearance of bilirubin and alkaline phosphatase was documented. The loss of the graft was associated with the formation of thrombi in the hepatic artery and several smaller vessels. The result was typical of subsequent experience.
The first orthotopic liver xenotransplant was performed on July 15th, 1966 (25). A serious bleeding diathesis developed but, after 5 hours, spontaneously ceased as suddenly as it had begun. The total operating time was >20 hours. Blood transfusions totaled 3,500ml. The patient woke within a few minutes after returning to the recovery room and seemed to be in an excellent general condition. There were serious difficulties with thrombocytopenia beginning on the second postoperative day. At the time it was assumed that thrombocytes were being sequestered in the xenograft. The patient died almost nine days after insertion of the xenograft.
Two more liver xenotransplants were performed (Table 1) (27,28). In one, the two kidneys from the chimpanzee were perfused sequentially to temporarily reduce the titer of anti-chimpanzee isoagglutinins before the liver was transplanted. “There was some publicity, but the animal rights people did not seem to get to know about the transplants,” explained Dr Starzl. “We had an unusual relationship with the two Denver newspapers. If we asked them to keep things quiet, they generally complied. That would be unheard of today. There was less publicity than with James Hardy’s heart transplant in 1964, in which a chimpanzee was the source of the organ (29). However, we had this moral issue which was the whole idea of using a chimpanzee as a donor. I was just very uneasy about it. The whole idea about this humanoid chimpanzee was something so fundamentally wrong.”
Dr Starzl reminisced. “It was an unnerving experience in a way because one of the chimpanzees was brought to my house. It was dressed in a jumpsuit with little straps around the shoulders. When it got out of the cage it was very polite, and knew how to drink tea from a cup. I was really unnerved because it was acting in such a human fashion, like a pretty good-sized hairy child.”
Dr Starzl did not use chimpanzees again as sources of organs.
In his 1969 book on hepatic transplantation (25), Dr Starzl wrote: “The circumstances under which this and the previous hepatic xenotransplantation were performed were similar. In both cases, the condition of the recipients deteriorated markedly while a search was being conducted for cadaveric organs. Ultimately, the use of a chimpanzee organ was viewed as a desperate therapeutic effort. If xenografts can be made to work, their most urgent need in the future will probably be to replace organs such as the liver and heart, for which long-term interim function cannot be provided by machines or instruments analogous to the artificial kidney”.
Liver xenotransplantation using baboons as a source of organs
In 1981, Dr Starzl relocated from Denver to Pittsburgh, where, following the introduction of cyclosporine, he established what was to become the busiest transplant program in the world. In one year, >600 liver transplants were carried out.
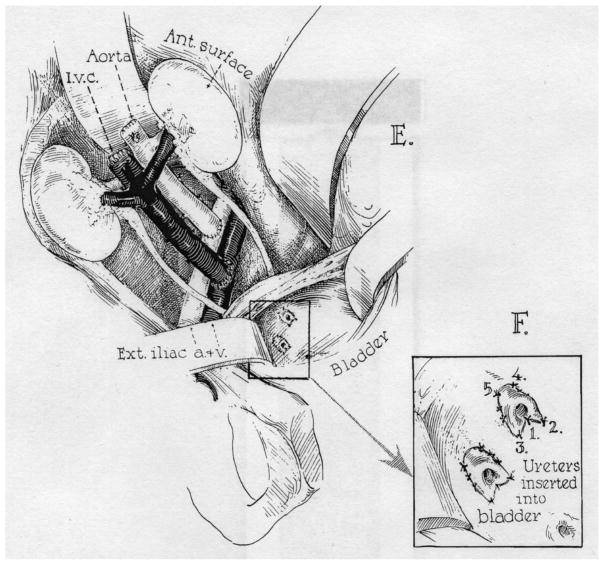
An ability to control both the cellular and humoral components of xenograft rejection in laboratory experiments, together with an organ shortage that placed limits on clinical transplantation services, prompted the Pittsburgh group to undertake clinical liver transplantation from baboons. The use of animal organs was considered to possibly have some advantage with regard to recurrence of disease, such as viral hepatitis, as some viruses are species-specific.
Baboon livers were transplanted in two patients as a means of avoiding hepatitis B (HBV) recurrence (30). The baboons (Papio cynocephalus) were obtained from the Southwest Foundation for Research and Education, San Antonio, Texas. Retrovirus antibody screening revealed the animals to be negative for simian T-lymphotropic virus, human T-cell leukemia virus 1 and 2, simian immunodeficiency virus and other important viruses.
The first of the patients, a 35 years-old man with HBV-associated chronic active hepatitis, also had human immunodeficiency virus (HIV) infection. Although the HIV carrier state was an undesirable factor, the patient was accepted into the HBV xenotransplantation protocol because of his urgent clinical status.
The grafts in the two patients functioned for 70 and 26 days, respectively (Table 1), without evidence of graft infection by the hepatitis virus. The first case, in which the patient was also HIV-positive, can be considered a relative success in that there was little pathologic evidence of rejection in the liver, but this was achieved probably at the expense of over-immunosuppression, the patient dying of overwhelming sepsis.
In this patient, products of hepatic synthesis, including clotting factors, became those of the baboon liver with no obvious adverse effects, as was also shown experimentally (31). Death followed a cerebral and subarachnoid hemorrhage that was caused by an angio-invasive aspergillus infection. However, the underlying cause of death was widespread biliary sludge that formed in the biliary tree. At necropsy, the biliary anastomosis was intact and patent, but the bile duct mucosa was dusky. Biliary sludge occupied the entire intrahepatic biliary tree. This finding was similar to that seen in past allografts with biliary stasis or obstruction. Multiple bile infarcts were present throughout the liver.
This experience demonstrated the feasibility of controlling the rejection of a baboon liver xenograft in a human recipient. The biliary stasis that was the beginning of lethal infectious complications may have been correctable by modifications of surgical technique. In further trials, this group planned to avoid the error of over-immunosuppression.
This first transplant gained a great deal of publicity. Dr. Starzl said, “I think it was mostly positive.” (There were, however, demonstrations outside the hospital by those who opposed the use of baboons for this purpose. Some referred to Dr Starzl as ‘Dr Frankenstarzl’.)
I asked Dr Starzl to comment on the fact that the patient had AIDS. He mentioned that baboons do not have the receptor for AIDS, and they also do not have the receptor for the B-virus. At that time HIV was categorically a contraindication to any kind of organ allotransplantation.
The second case was less successful as the patient did not regain consciousness or renal function during the postoperative period, but again there was little histopathologic evidence of rejection in the transplanted liver.
“We had permission to do four of those cases. We did two and then stopped,” explained Dr Starzl. “The reason we stopped is exactly the same as we stopped back in 1963, and that was that there was an extra element, and we knew what it was, which is complement activation, which didn’t show the slightest sign of going away. The biopsies never showed cell-mediated rejection. With today’s immunosuppressive therapy, baboon organs that have been genetically-engineered to protect them from the human complement response might function long-term in humans. Baboons could be used if we were serious about putting human needs before those of animals, and doing something constructive to protect human health.”
Medical research
When I talked with Dr Starzl in 2012, he was disillusioned by what he considered the excessive regulation of medical research and clinical trials in the US, which “is steadily getting worse”. He felt that regulation was destroying innovation in the US, and was totally different from his early days in transplantation. “I am very much troubled by the difficulty that lays ahead with the regulatory agencies, especially the FDA.” He believed that xenotransplantation would be developed largely in countries such as China, where, in 2012, there was as yet little significant regulation.
Comment
Some of the topics investigated by Dr Starzl have implications for what we have been exploring in pig-to-nonhuman primate models. Today, thrombocytopenia is a major problem after pig-to-baboon liver transplantation, just as it was when a chimpanzee was the source of the liver for a patient. His belief that, when a living human donor was to provide an organ, immunosuppressive therapy should be begun at least a week before the transplant may be relevant to xenotransplantation where, of course, the timing of the operative procedure will always be known. When pigs are the sources of the organs, rather than nonhuman primates, whether criticism of the procedures will be muted remains to be seen.
I believe Dr Starzl was correct in predicting that regulation of medical research in the US (and indeed, in the Western world) would become greater, as I believe it has, but whether clinical xenotransplantation will be introduced first in a country where there is less regulation, such as China, remains to be seen (though clinical pig corneal transplantation has already been undertaken in China).
Abbreviations
- HBV
hepatitis B virus
- HIV
human immunodeficiency virus
References
- 1.Starzl TE. The Puzzle People: Memoirs of a Transplant Surgeon. University of Pittsburgh Press; Pittsburgh & London: 1992. [Google Scholar]
- 2.Lasker. 2012 ( http://www.laskerfoundation.org/awards/2012_c_description_p.htm)
- 3.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with the subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385–3395. [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Lerner R, Dixon FJ, Groth CG, Brettschneider L, Terasaki PI. Shwartzman reaction after renal homotransplantation. New Engl J Med. 1968;278:642–648. doi: 10.1056/NEJM196803212781202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Boehmig HJ, Amemiya H, et al. Clotting changes, including disseminated intravascular coagulation, during rapid renal-homograft rejection. New Engl J Med. 1970;283:383–390. doi: 10.1056/NEJM197008202830801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Marchioro TL, von Kaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–676. (1963b) [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Groth CG, Brettschneider L, et al. Orthotopic homotransplantation of the human liver. Ann Surg. 1968;168:392–415. doi: 10.1097/00000658-196809000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calne RY, Rolles K, White DJG, et al. Cyclosporin A initially as the only immunosuppressant in 34 patients of cadaveric organs: 32 kidneys, 2 pancreas, and 2 livers. Lancet. 1979;2:1033–1036. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 9.Starzl TE, Weil R, III, Iwatsuki S, et al. The use of cyclosporine A and prednisone in cadaver kidney transplantation. Surg Gynecol Obstet. 1980;151:17–26. [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl TE, Makowka L, Todo S, editors. FK 506: a Potential Breakthrough in Immunosuppression. Transplant Proc. 1987;19(Suppl 6):1–103. [PMC free article] [PubMed] [Google Scholar]
- 11.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramanan, Jain A. FK 506 for human liver, kidney, and pancreas transplantation. Lancet. 1989;2:1000–1004. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starzl TE, Rowe MI, Todo S, et al. Transplantation of multiple abdominal viscera. JAMA. 1989;261:1449–1457. [PMC free article] [PubMed] [Google Scholar]
- 13.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starzl TE, Zinkernagel RM. Antigen localization and migration in immunity and tolerance. N Engl J Med. 1998;339:1905–1913. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hara H, Gridelli B, Lin YJ, Marcos A, Cooper DK. Liver xenografts for the treatment of acute liver failure: clinical and experimental experience and remaining immunologic barriers. Liver Transpl. 2008;14:425–434. doi: 10.1002/lt.21476. [DOI] [PubMed] [Google Scholar]
- 16.Cooper DKC, Cramer DV. Report: International Xenotransplantation Association. Xenotransplantation. 2000;7:2. [Google Scholar]
- 17.Reemtsma K, McCracken BH, Schlegel JU, et al. Renal heterotransplantation in man. Ann Surg. 1964;160:384–410. doi: 10.1097/00000658-196409000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardy MA. Keith Reemtsma, a pioneering giant in transplantation dies at 74. Xenotransplantation. 2000;7:163–165. [Google Scholar]
- 19.Starzl TE. Experience in Renal Transplantation. Philadelphia: WB Saunders Co; 1964. Renal heterotransplantation; pp. 262–283. [Google Scholar]
- 20.Hitchcock CR, Kiser JC, Telander RL, Seljeskog EL. Baboon renal grafts. JAMA. 1964;189:934–937. doi: 10.1001/jama.1964.03070120056013. [DOI] [PubMed] [Google Scholar]
- 21.Starzl TE, Marchioro TL, Peters GN, et al. Renal heterotransplantation from baboon to man: experience with 6 cases. Transplantation. 1964;2:752–776. doi: 10.1097/00007890-196411000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirkpatrick CH, Wilson WEC. Experience in Renal Transplantation. Philadelphia: WB Saunders Co; 1964. Immunologic studies of baboon to man renal heterotransplantation; pp. 284–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hume DM. Discussion of Reemtsma paper. Ann Surg. 1964;160:384. [Google Scholar]
- 24.Porter KA. Experience in Renal Transplantation. Philadelphia: WB Saunders Co; 1964. Pathological changes in transplanted kidneys; pp. 299–358. [Google Scholar]
- 25.Starzl TE. Experience in Hepatic Transplantation. Philadelphia: WB Saunders Co; 1969. Orthotopic heterotransplantation; pp. 408–421. [Google Scholar]
- 26.Starzl TE, Marchioro TL, Farts TD, McArdle MJ, Iwasaki Y. Avenues of future research in homotransplantations of the liver. Am J Surg. 1966;112:391–400. doi: 10.1016/0002-9610(66)90209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giles GR, Boehmig HJ, Amemiya H, Halgrimson CG, Starzl TE. Clinical heterotransplantation of the liver. Transplant Proc. 1970;2:506–512. [PMC free article] [PubMed] [Google Scholar]
- 28.Starzl TE, Ishikawa M, Putnam CW, et al. Progress in and deterrents to orthotopic liver transplantation, with special reference to survival, resistance to hyperacute rejection, and biliary duct reconstruction. Transplant Proc. 1974;6:129–139. [PMC free article] [PubMed] [Google Scholar]
- 29.Hardy JD, Chavez CM, Kurrus FD, et al. Heart transplantation in man: developmental studies and report of a case. JAMA. 1964;188:1132–1140. [PubMed] [Google Scholar]
- 30.Starzl TE, Fung J, Tzakis A, et al. Baboon-to-human liver transplantation. Lancet. 1993;341:65–71. doi: 10.1016/0140-6736(93)92553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valdivia LA, Lewis JH, Celli S, et al. Hamster coagulation and serum proteins in rat recipients of hamster xenografts. Transplantation. 1993;56:489–490. doi: 10.1097/00007890-199308000-00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dubernard JM, Bonneau M, Latour M. Hetergrafts in Primates. Fondation Merieux; Villeurbanne: 1974. [Google Scholar]
- 33.Taniguchi S, Cooper DKC. Clinical xenotransplantation: past, present and future. Ann R Coll Surg Engl. 1997;79:13–19. [PMC free article] [PubMed] [Google Scholar]