Abstract
Neuropeptide hormone oxytocin has roles in social bonding, energy metabolism, and wound healing contributing to good physical, mental and social health. It was previously shown that feeding of a human commensal microbe Lactobacillus reuteri (L. reuteri) is sufficient to up-regulate endogenous oxytocin levels and improve wound healing capacity in mice. Here we show that oral L. reuteri-induced skin wound repair benefits extend to human subjects. Further, dietary supplementation with a sterile lysate of this microbe alone is sufficient to boost systemic oxytocin levels and improve wound repair capacity. Oxytocin-producing cells were found to be increased in the caudal paraventricular nucleus [PVN] of the hypothalamus after feeding of a sterile lysed preparation of L. reuteri, coincident with lowered blood levels of stress hormone corticosterone and more rapid epidermal closure, in mouse models. We conclude that microbe viability is not essential for regulating host oxytocin levels. The results suggest that a peptide or metabolite produced by bacteria may modulate host oxytocin secretion for potential public or personalized health goals.
Keywords: Bacteria, Postbiotic, Stress, Corticosterone, Thymus, Wound healing
1. Introduction
In a series of prior studies using mice we have shown that the prototype probiotic bacterium Lactobacillus reuteri (L. reuteri) imparts an array of health benefits (Erdman and Poutahidis, 2014; Ibrahim et al., 2014; Lakritz et al., 2014; Levkovich et al., 2013; Poutahidis et al., 2013a,b, 2014, 2015; Varian et al., 2016a, b, 2014). Aside from its previously described positive contributions in the heath of oral (Iniesta et al., 2012; Martin-Cabezas et al., 2016) and gastrointestinal (GI) tract mucosa (Cruchet et al., 2015; Gao et al., 2015; Liu et al., 2012; Schreiber et al., 2009; Varian et al., 2016a; Walter et al., 2011), the probiotic has systemic effects on the immune and hormonal profile of the host animal (Buffington et al., 2016; Erdman and Poutahidis, 2014; Ibrahim et al., 2014; Lakritz et al., 2014; Lee et al., 2016; Levkovich et al., 2013; Livingston et al., 2010; Poutahidis et al., 2013a,b, 2014, 2015; Simon et al., 2015; Varian et al., 2016a,b, 2014). Mice that consume L. reuteri live longer (Ibrahim et al., 2014; Varian et al., 2016a) and resist obesity (Fak and Backhed, 2012; Poutahidis et al., 2013b; Qiao et al., 2015; Varian et al., 2016b, 2014) and age-associated atrophic changes of skeletal muscle (Varian et al., 2016a), testis (Poutahidis et al., 2014), and thyroid (Varian et al., 2014) and thymus glands (Varian et al., 2016a). They also have healthy fur coats (Erdman and Poutahidis, 2014; Levkovich et al., 2013), heal their skin wounds faster (Poutahidis et al., 2013a) and are resistant to intestinal (Varian et al., 2016a), mammary gland, liver, and lung cancer (Lakritz et al., 2014; Poutahidis et al., 2015).
Prompted by the observation that mice consuming L. reuteri consistently exhibit increased grooming and maternal care behaviors (Ibrahim et al., 2014), we discovered that the probiotic increases the plasma levels of oxytocin (Ibrahim et al., 2014; Poutahidis et al., 2013a; Varian et al., 2016b). This unprecedented probiotic effect has been proven essential for several of the systemic beneficial effects of L. reuteri as exemplified by experiments using oxytocin deficient mice and immune cell transfers (Erdman and Poutahidis, 2014; Ibrahim et al., 2014; Poutahidis et al., 2013a; Varian et al., 2016b). Interestingly, many of the published beneficial health effects of oxytocin overlap with recognized L. reuteri health-promoting attributes. Indeed, as with L. reuteri (Britton et al., 2014; Collins et al., 2016; Poutahidis et al., 2013a,b; Varian et al., 2016a,b, 2014; Zhang et al., 2015b), the exogenous administration of oxytocin has been shown to accelerate wound healing (Gavrilenko et al., 2003; Gouin et al., 2010; Vitalo et al., 2009), counteract obesity (Barengolts, 2016; Blevins and Baskin, 2015), suppress uncontrolled inflammation (Wang et al., 2015) and prevent muscle wasting (Costa et al., 2014; Elabd et al., 2014) and bone loss (Colaianni et al., 2014; Sun et al., 2016; Tamma et al., 2009).
A role for oxytocin in promoting good mental health, including social bonding, a general sense of well-being, and improved learning and memory is emerging (Carter, 2014; Donaldson and Young, 2008; Feldman et al., 2016). At the same time, recent evidence supporting the presence of a gut-microbiota-brain axis (Dinan et al., 2013; Kelly et al., 2016; Sherwin et al., 2016) suggests that probiotic bacteria can influence emotions, mood, anxiety and depression (Dinan et al., 2013; Kelly et al., 2016; Sherwin et al., 2016). This places oxytocin (Carter, 2014; Donaldson and Young, 2008; Feldman et al., 2016) and probiotic bacteria among novel approaches for preventing or even treating neuropsychiatric disorders (Dinan et al., 2013; Kelly et al., 2016; Sherwin et al., 2016). Hence, their name psychobiotics (Dinan et al., 2013).
Apart from findings showing that L. reuteri upregulates oxytocin and enhances maternal care behaviors (Ibrahim et al., 2014; Poutahidis et al., 2013a; Varian et al., 2016b), until recently there were no other data suggesting that this bacterium could be considered among suspected psychobiotics (Dinan et al., 2013). However, a recent study in mice (Buffington et al., 2016) supports this notion and expands prior findings connecting maternal obesity-induced gut microbiota dysbiosis with detrimental effects in the offspring. A gut microbiota that is poor in L. reuteri associates with adverse social behavior effects in offspring mice. Conversely, supplementary consumption of L. reuteri upregulates oxytocin in the hypothalamus, stimulates the mesolimbic dopamine reward system and promotes prosocial behavior (Buffington et al., 2016). This matches earlier multigenerational findings involving feeding of special diets and L. reuteri ATCC 6475 in mice (Poutahidis et al., 2015).
Among the newly recognized actions of oxytocin, its connection with the immune system, is particularly important (Wang et al., 2015). Indeed, oxytocin contributes to the maturation and selection of T-lymphocytes in the thymus (Hansenne et al., 2009, 2005; Wang et al., 2015), which is integral for immune system homeostasis and overall health. We have recently reported that mice consuming L. reuteri retain a sizable thymus in adulthood and that this effect is mediated by the probiotic-induced upregulation of the Forkhead Box N1 (FoxN1) in thymic epithelial cells (Varian et al., 2016a). FoxN1 is essential for normal thymus development and a competent host immune system as exemplified by mice deficient in FoxN1 (athymic nude mice), which lack functional T-lymphocytes (Nehls et al., 1994; Romano et al., 2013; Zhang et al., 2012), and as a result are highly susceptible to infections and developing cancer.
Another well-established effect of oxytocin in the immune system relates with its ability to down-regulate neutrophilic proinflammatory responses (Al-Amran and Shahkolahi, 2013; Biyikli et al., 2006; Iseri et al., 2005a,b; Petersson et al., 2001; Wang et al., 2015). Studies using L. reuteri consumption in mice consistently show a downregulation of circulating neutrophils in the context of maintaining homeostasis during wound repair and slim physique (Poutahidis et al., 2013a; Varian et al., 2016a,b). Ways in which L. reuteri contributes to reducing neutrophils and consequently lowering systematic inflammatory tone are particularly important in the light of recent evidence that neutrophils are an important cellular component of chronic smoldering subclinical systemic inflammation that associates with increased risk for carcinogenesis, metabolic disorders and cardiovascular disease (Coffelt, 2016; Fc, 2016; Manda-Handzlik and Demkow, 2015; Seijkens et al., 2014). However, while oxytocin has apparent beneficial effects, repeated pharmaceutical oxytocin administration may have unwanted effects under certain circumstances in humans. It may be anxiogenic and promote aggressiveness towards others that are being thought as outsiders or competitors (De Dreu et al., 2011). Also, in patients suffering from borderline personality disorder, oxytocin decreased trust and cooperation (Bartz et al., 2011).
Given the challenges raised regarding the efficacy and safety of exogenous oxytocin administration (Lefevre and Sirigu, 2016; Leng and Ludwig, 2016), the likelihood that microbial supplementation may endogenously increase oxytocin levels appears appealing. In order to go deeper into the mechanistic analysis of L. reuteri mode of action, including oxytocin induction and eventually therapeutic strategies, however, a basic question arises. Is it required to ingest live L. reuteri organisms to achieve up-regulation of oxytocin and associated health benefits?
In the present study, we show that oral dosing of L. reuteri leads to improved wound repair capacity in mouse models and human subjects. We provide direct evidence that dietary L. reuteri lysate is sufficient for wound healing improvements and increases the number of oxytocin-positive cells in the caudal portion of the paraventricular nucleus of the hypothalamus (PVN) in mice. The increase in oxytocin-positive cells in the PVN is correlated with lowered circulating levels of stress hormone corticosterone in mice consuming L. reuteri. Microbe-induced oxytocin is necessary for the induction of important beneficial host effects, including proficient wound repair. Finally, sterile L. reuteri lysate prepared by sonication is sufficient for achieving up-regulation of oxytocin and health benefits in mice, suggesting that these phenomena are triggered by a bacterial component, rather than live probiotic bacteria.
2. Methods
2.1. Animal models
Female outbred Swiss stock CD-1 female mice (Charles River, Wilmington MA), C57BL/6 wild type (wt), oxytocin-wt [ot-wt] and oxytocin-knockout (ot-ko) B6;129S-Oxttm1Wsy/J mice (purchased initially from Jackson labs; Bar Harbor, ME) were used in three separate experiments. Mice were housed and handled in Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited facilities using techniques and diets including L. reuteri as specifically approved by Massachusetts Institute of Technology’s (MIT) Committee on Animal Care (CAC). Mice were housed under standard 12:12 light cycle conditions with lights on at 7AM. Mice were fed a standard control chow Purina RMH3000.
Mouse models were bred in-house to achieve experimental groups. Mice were randomly assigned to experimental groups, and group housed with 4–5 mice per cage. Each experiment included 5–11 animals per group as specifically enumerated within the text. Mice received in their drinking water L. reuteri ATCC-PTA-6475 originally isolated from human breast milk.
2.2. L. reuteri administration in mouse models
In each experiment, subsets of mice received in their drinking water a strain of L. reuteri ATCC-PTA-6475 cultivated as described elsewhere (Poutahidis et al., 2013a; Saulnier et al., 2011). Live organisms were supplied at a starting dosage of 3.5–5.0 × 105 - organisms/mouse/day in drinking water (Lakritz et al., 2014), using oral dosage extrapolated from humans consuming daily chewable L. reuteri DSM17938 tablets (BioGaia Protectis). Live bacterial counts in water bottles were calculated to be 1.4 × 106 colony forming units (CFU) per mouse on day 1, 4.1 × 105 CFU on day 2, and 1.1 × 105 CFU on day 3. Fresh drinking water for both groups of animals was replaced twice weekly throughout the experiments. L. reuteri was detectable by PCR in feces and bowel of mice undergoing the live bacteria dosing regimen, as described in detail in Lakritz et al. (2014).
2.3. Wound repair assay in mice
To test putative roles for microbes or microbe lysate-induced wound healing, as previously described in detail (Poutahidis et al., 2013a), mice underwent a standardized 2.0 mm dorsal cutaneous biopsy procedure under general inhalant isoflurane anesthesia with perioperative buprenorphine injectable analgesia. The mid-dorsal surgical procedures involved first shaving the biopsy site (Suppl. Fig. 1), with alternating betadine and ethanol scrubs according to institutional policy, and finally the biopsy using a 2.0 mm cutaneous skin punch biopsy tool [Miltex Inc, York, PA USA]. Mice were examined at six days after biopsy based upon significant differences that emerged in earlier studies (Poutahidis et al., 2013a). Mice entered experiments at eight weeks of age. Four weeks later [three weeks after start of L. reuteri or sham treatment plus six days of post-biopsy observation], at the conclusion of the study mice were euthanized with CO2 overdose and wound areas were examined postmortem. Specifically, formalin-fixed, routinely-processed, paraffinized, flat wounded skin tissues were used for wound area measurements before being embedded in paraffin blocks. Direct microscopy with a Nikon eclipse 50i microscope and a Nikon DS-5M-L1 digital camera was used to examine and photograph wounds in paraffinized gross skin specimens. The wound areas were subscribed and measured in images using the ImageJ image processing and analysis program (NIH, Bethesda, MD). Results were recorded as image pixels.
2.4. Human subject trials
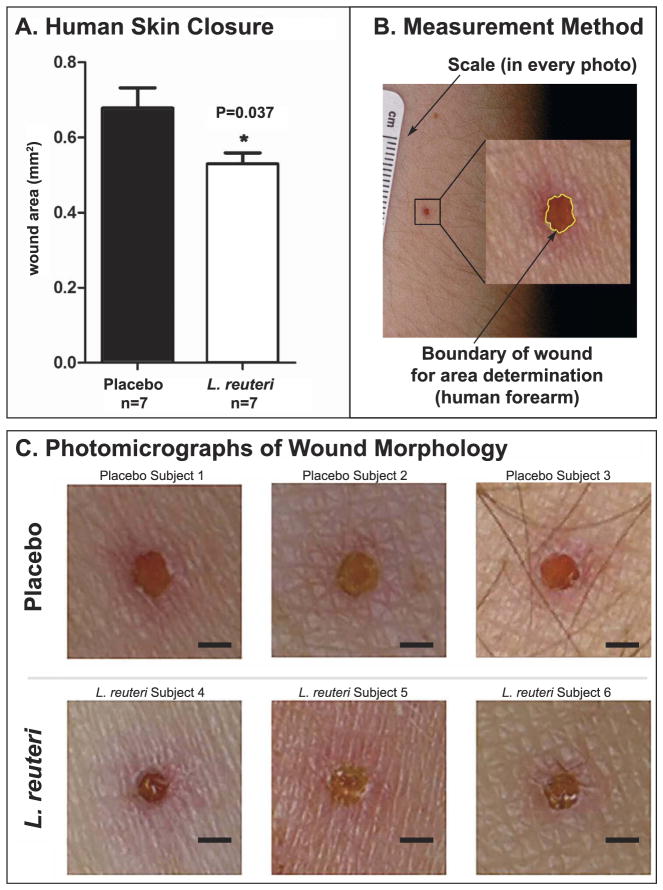
Fourteen healthy female volunteer subjects in a double blind placebo controlled study consumed chewable L. reuteri DSM17938 supplements (BioGaia Protectis) or placebo 60 mg vitamin C twice daily for three weeks before undergoing a full-thickness biopsy of forearm skin at MIT’s Clinical Research Center. Female subjects only were selected in order to minimize differences between sexes, and to simultaneously gather data for skin appearance in females. Subjects (N = 7 assigned randomly per treatment group) had a mean age of 29 years (range, 19–42y) and included individuals with diverse ethnicity (Suppl. Table 1). Human skin wounds were photographed under standardized conditions at time points before and immediately after biopsy plus d3 after biopsy (Suppl. Fig. 2). Individuals measuring the wounds and those collecting data were blind to the identity of experimental treatment groups.
The wound areas were subscribed (inset in Fig. 2A) and the subscribed area was measured in each image using ImageJ (NIH, Bethesda, MD). Results were recorded in pixels, scaled and transformed in mm2 using a standard scale originally contained in the images. To achieve that, wound area measurements were in pixels and then associated with the known distance of 1 mm according to the standard scale contained in the corresponding image, using the “set scale” command of ImageJ.
Fig. 2.
Dietary L. reuteri confers increased wound healing capacity in human subjects. (A) The wound area of human subjects consuming L. reuteri (BioGaia Protectis DSM 17938 chewable 100 million CFU) was significantly smaller compared to the placebo-treated (Nature Made chewable Vitamin C 60 mg) controls at three days after wounding. Biopsies were performed at Massachusetts Institute of Technology Institute for Medical Engineering and Science (IMES) Clinical Research Center (CRC). Numbers on the y-axis of the bar graph correspond to the mean ± SEM of wound area; N = 7 per treatment; p = 0·037; Mann-Whitney U. (B) To assess wound area, human skin wounds were photographed under standardized conditions. The wound margins were outlined and then the subscribed area was measured in each image. Results were recorded in mm2 using a standard scale originally contained in the images. (C) Representative images of skin wounds show features of size and morphology from placebo (upper row) and L. reuteri-treated (bottom row) individuals. The wounds of subjects consuming L. reuteri have a smaller size than those of the placebo group, compared here at d3 post-biopsy. Bar = 1 mm.
2.5. Experimental design of mouse model studies
2.5.1. Experiment 1
To probe the roles of a human breast and gut commensal microbe in physiology, we examined outbred stock CD-1 female mice. Females were selected to better match the human subject trial. Eight-week-old CD-1 mice were randomly subdivided into groups of ten mice per treatment [N = 10 mice per group] and received in their drinking water probiotic L. reuteri ATCC-PTA- 6475, as described above, continuously for four weeks until the end of the experiment at 12 weeks-of-age. At three weeks after the start of treatment, mice underwent the 0.2 mm skin wound procedure (as described above) at six days before necropsy, an experimental duration comprised of three weeks of feeding bacteria before biopsy plus six days of wound monitoring after biopsy. Tissue collections were performed after CO2 overdose and exsanguination. For complete blood counts of immune cells, whole blood was collected via cardiac puncture from unconscious mice. Blood plasma was processed immediately with a preservative and then frozen for future oxytocin and corticosterone analyses. Thymus weights were recorded upon necropsy. Tissues were collected for histology and immunohistochemistry.
2.5.2. Experiment 2
To test whether oxytocin is required for wound healing benefits of oral therapy with gut microbes, we next examined oxytocin-wt [ot-wt] and oxytocin-knockout (ot-ko) B6;129S-Oxttm1Wsy/J mice. Eight-week-old B6; 129S-Oxttm1Wsy/J mice were randomly subdivided into groups of eight-ten mice per treatment [N = 8–10 mice per group] and received in their drinking water L. reuteri ATCC-PTA- 6475 continuously until 12 weeks-of-age. Mice underwent the 0.2 mm skin wound procedure at six days before necropsy. Tissues were collected upon necropsy.
2.5.3. Experiment 3
To test whether physiological effects are achievable using non-viable microbe lysates alone, C57BL/6 WT mice underwent the same assays as above. Eight-week-old C57BL/6 mice were randomly subdivided into groups of eight-ten mice per treatment and received in their drinking water for four weeks a postbiotic lysed L. reuteri ATCC-PTA-6475 continuously until 12 weeks-of-age. Mice underwent the 0.2 mm skin wound procedure at six days before necropsy, with an experimental duration comprised of three weeks of feeding bacteria before biopsy plus six days of wound repair prior to necropsy. Tissues were collected upon necropsy, as above.
2.6. Production of sterile microbe lysate
L. reuteri ATCC-PTA-6475 was cultivated using methods as previously described (Poutahidis et al., 2013a; Saulnier et al., 2011), confirmed for purity with a gram strain, and then suspended in sterile 1xPBS and measured for concentration with a spectrophotometer in order to calculate final dosages. A bacteria pellet was obtained by centrifugation for 10 min at 14,000 rpm and then resuspended and incubated in a Lysozyme STET buffer for 4 h at 37 degrees Celsius. Bacteria-buffer was centrifuged for 10 min at 7500 rpm to obtain a pellet, that was subsequently washed 2X and then resuspended in 1xPBS before lysing by sonication in an ice water bath at 20 kHz and the amplitude of 30% intensity for one-minute-on–then-one-minute-off for 25 min. Lysed bacteria was then centrifuged for 15 min at 4000 rpm and passed through a 0.2um filter to remove whole bacteria and large fragments, with the soluble supernatant being collected as the final product. The supernatant was then confirmed to be sterile using growth in anaerobic enriched thioglycollate media with Vitamin K1 and hemin (Bectin, Dickinson and Company, Sparks, MD) and by the streak plate method on Sheep blood agar plates (Remel, Lanessa KS) with no growth after three days. Bacterial lysate was stored in 1 ml aliquots at −80 degrees Celsius until feeding to mice in experiments described above.
2.7. Special microbial treatments for animals
Mice were fed standard rodent chow (RMH 3000; Purina Labs, St Louis MO). Subsets of animals were supplemented orally with intact or lysed ATCC strain of L. reuteri 6475 as described elsewhere (Lakritz et al., 2014; Varian et al., 2016b), using a supply dosage of 3.5 × 105 organisms/mouse/day continuously in drinking water. For Experiment 1, Swiss mice received live intact L. reuteri organisms in drinking water. For Experiments 2 and 3, mice received L. reuteri as above, or, alternatively, controls received regular drinking water. For experiment #3, lysate confirmed to be sterile was delivered to C57BL/6 mice at the same concentration as live organisms in drinking water. Mice began drinking L. reuteri ATCC-PTA- 6475 organisms, as above, starting at 8 wks of age, and then underwent skin biopsy three weeks later, followed by postmortem analyses at 12 weeks of age. Drinking water was replaced twice weekly to minimize variability in microbial exposure levels. In all cases, control animals received regular drinking water.
2.8. Determining mass of thymus gland
Upon necropsy, intact mice were weighed in their entirety using a ScoutPro SP202 scale [Chaus Corporation, Pinebrook NJ]. Thymus tissue was removed and weighed separately.
2.9. Complete blood cell counts
Whole blood was collected by cardiac puncture from unconscious animals upon necropsy and suspended in EDTA to prevent clotting. Automated neutrophil counts were then performed using mouse parameters in a HemaVet 950FS (Drew Scientific, Oxford CT). Counts were confirmed by manual reading of blood smears. Terminal blood collections for mice were performed mid-day for all subjects in order to minimize variability due to circadian rhythms.
2.10. Measurement of plasma oxytocin and corticosterone levels
Whole blood was collected terminally by cardiac puncture under general anesthesia to obtain plasma. Whole blood was collected into pre-chilled 5 ml EDTA tubes with 250 KIU of an oxytocin preservative, aprotinin, and refrigerated immediately until preparation of plasma. Plasma was isolated by centrifugation at 1800g, 15 min, 4 °C, and then stored in aliquots at −70 °C. Plasma was then tested commercially for oxytocin and corticosterone by an outside laboratory with internal validations (AniLytics, Inc., Gaithersburg, MD). Euthanasia for mice was performed mid-day for all subjects (n = 8–10 per group) to minimize variability due to circadian rhythms.
2.11. Histopathology and histomorphometry
Formalin-fixed tissues were embedded in paraffin, cut at 4– 5 μm, and stained with hematoxylin and eosin (HE). Wound epidermal gap in histological images were measured using the ImageJ image processing and analysis program (NIH, Bethesda, MD) as previously described (Poutahidis et al., 2013a).
2.12. Brain tissue collection and immunohistochemistry for oxytocin
Mouse brains (including skulls) were removed and fixed in 10% formalin and stored at 4 °C until further processing. Next, brains were dissected from the skull and post-fixed in 4% paraformaldehyde in 0.1 M borate buffer (pH 9.5) for 48 h before cryoprotection in 30% sucrose (dissolved in basic physiologic saline; 0.9% NaCl) for 48 h. Following cryoprotection, brains were flash-frozen in cold methylbutane and stored at −45 °C. Coronal (30 μm) sections were collected using a cryostat and were stored free-floating in tris-buffered saline (TBS) overnight at 4 °C. The following day, tissue sections from each subject were immunolabeled for oxytocin (OT) (Ben-Barak et al., 1985; Franklin and Paxinos, 2008).
Oxytocin immunoreactivity (ir) was visualized using a monoclonal primary antibody provided by Dr. Harold Gainer (NINDS). This highly specific antibody was raised against mammalian oxytocin-associated neurophysins, and exhibits no cross-reactivity (Ben-Barak et al., 1985). Briefly, tissue sections were first washed in TBS, subjected to an antigen retrieval step (0.05 M sodium citrate in TBS), blocked in blocking solution (20% normal goat serum [NGS], 0.3% Triton-X, 1% H2O2 in TBS), and incubated overnight at 4 °C in mouse anti-oxytocin (PS38; 1:100, 2% NGS, 0.3% Triton-X). Tissue sections were then rinsed in TBS and incubated in biotinylated secondary antibody solution (goat antimouse [1:500; Vector, Burlingame, CA], 2% NGS, 0.3% Triton-X in TBS) for 1 h. Tissue sections were incubated in avidin-biotin complex (ABC Elite Kit; Vector) for 1 h and visualized using diaminobenzadine (DAB peroxidase substrate kit; Vector). Sections were mounted on gelatin-coated slides, rinsed in 50% ethanol, air-dried and coverslipped using Permount (Fisher Scientific, Pittsburgh, PA).
Images were acquired under 20× magnification based on various anatomical landmarks specific to the following subregions of the hypothalamic paraventricular nucleus (PVN): rostral (−0.70 mm posterior to bregma), intermediate (−0.94 mm), and caudal (−1.06 mm), using the Mouse Brain Atlas (Franklin and Paxinos, 2008) as a guide. Oxytocin-ir cell bodies were subsequently quantified from a representative tissue section in each region using the cell counter plugin in ImageJ (NIH; imagej.nih.-gov/ij). Data are reported as the mean number of oxytocin-ir cell bodies per treatment in the rostral, intermediate, and caudal portions of the PVN.
2.13. Statistical analysis
The Mann-Whitney U test was used for all statistical analyses (Graphpad Prism version 5.01 for windows, Graph-Pad software, San Diego, CA, USA). The occurrence of wound scab detachment was compared between experimental groups with the Chi-square test. For analyzing oxytocin-positive cell counts, we used a one way ANOVA with a tukey post-hoc test. Results are presented as the mean ± standard error of the mean (SEM). Effects were considered to be significant at p < 0.05.
3. Results
3.1. Oral administration of L. reuteri improves wound repair capacity
It was previously shown in C57BL/6 mice that L. reuteri ATCC PTA 6475 in drinking water enhances skin wound-healing capability to occur in half the time required for matched control animals via up-regulation of the neuropeptide hormone oxytocin (Poutahidis et al., 2013a). Ability to heal flesh wounds rapidly is the hallmark of sustained good health and longevity. For this reason, we have applied host capacity to repair tissues after surgical wound infliction as a surrogate marker for overall fitness. In support of this, dietary L. reuteri supplementation in mouse models has also been shown to impart a wide array of phenotypes including improved maternal care, lowered risk for obesity, with multigenerational effects on behavior, infertility, and cancer risks (Table 1), supporting use of this prototype probiotic microbe in further studies.
Table 1.
Positive effects of L. reuteri ATCC PTA 6475 on pathological conditions studied in vivo.
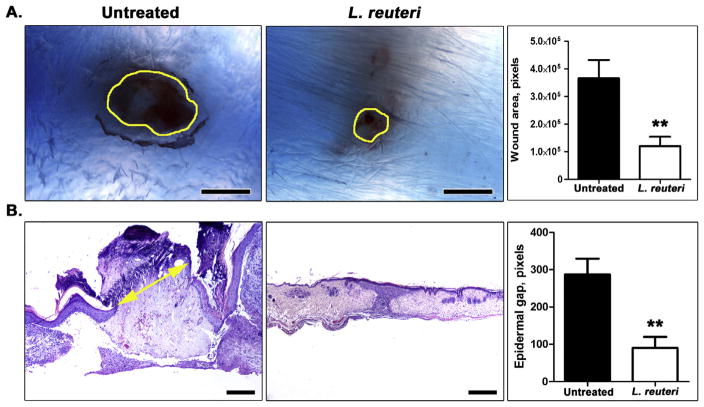
In Experiment 1, we employed a traditional skin biopsy assay in eleven-week-old outbred Swiss stock [CD-1] mice consuming L. reuteri to test wound healing capability as a surrogate marker for systemic resiliency and good health. Mice were drinking L. reuteri for three weeks prior to skin biopsy. In this case, outbred Swiss mice were selected to complement published studies in C57BL/6 mice and to overcome genetic biases imposed by inbred strains, thus broadening translational potential of resulting data. The skin wound assay applies a standardized 2.0 mm full thickness excision of dorsal skin of mice, with the wound site subsequently examined microscopically at six days after biopsy. Six days was selected as most highly significant timing based upon earlier experiments showing the rate of wound healing during the first twelve days after biopsy (Poutahidis et al., 2013a). Using this approach, we found that consuming L. reuteri (N = 10) speeds epithelial closure (p < 0.005) when compared with control mice (N = 10) drinking regular water (Fig. 1A and B). We next tested whether human subjects were similarly susceptible to benefits of consuming probiotic L. reuteri.
Fig. 1.
L. reuteri accelerates healing of skin wounds in mouse models. (A) Subgross microscopy of formalin-fixed, paraffinized wounded skin at 6 days after full thickness skin excision. The margins of wounds are outlined with yellow color. The wound area in outbred Swiss mice is significantly smaller in the L. reuteri-treated (n = 10) mouse group compared to untreated controls (n = 10). (B) Histopathology of wounds (n = 10 per group) at the same time-point reflects the more rapid wound healing rate of mice consuming L. reuteri. The representative untreated mouse wound shown here has a clear epidermal gap (note the double-headed yellow arrow pointing to epidermal edges), edematous wound bed and retained scab. By contrast, the wound of an L. reuteri-treated mouse given for side-by-side comparison is completely covered with epidermis, has mature collagen in the wound bed and lacks a scab. The L. reuteri effect on wound re-epithelization is statistically significant. Hematoxylin and Eosin (B). Scale bars: 1000 μm (a) and 500 μm (b). Numbers on the y-axis of bar graphs correspond to the mean ± SEM of the parameter assessed; **p < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Building upon this microbe-centric wound repair paradigm, we tested in a small pilot study on human subjects whether daily consumption of probiotic L. reuteri, also proven therapeutic in human gastrointestinal diseases (Preidis and Versalovic, 2009) is sufficient to improve skin wound-healing as seen in mouse models. For this experiment, fourteen healthy female volunteer subjects in a double blind placebo controlled study consumed chewable L. reuteri DSM17938 supplements (BioGaia Protectis) or placebo 60 mg vitamin C twice daily for three weeks before undergoing a full-thickness biopsy of forearm skin at MIT’s Clinical Research Center. Subjects had a mean age of 29 years (range, 19–42y) and included individuals with diverse ethnicity (Suppl. Table 1). Three days following biopsy, patients consuming L. reuteri had more rapid skin closure (N = 7 per treatment; p = 0.037) compared with placebo (Fig. 2A). Standardized macroscopic photography (Fig. 2B) revealed smaller wound sizes and more advanced healing in individuals after treatment with L. reuteri (N = 7) when compared with placebo-treated controls (N = 7) (Fig. 2C).
3.2. Drinking of L. reuteri leads to higher blood levels of oxytocin in mice
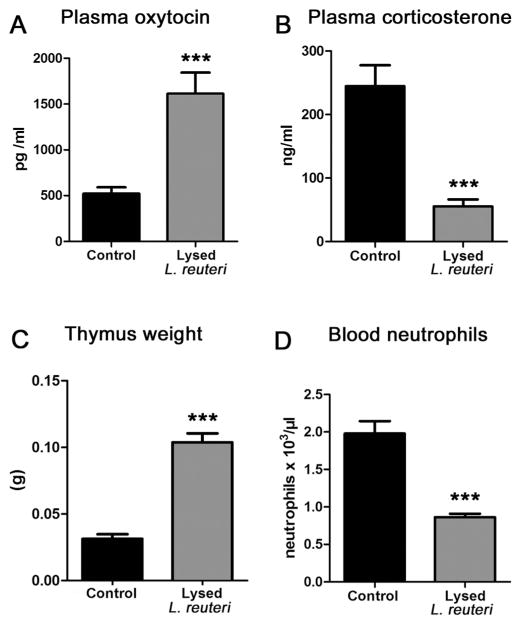
Oxytocin is pivotal in normal mammalian wound healing processes (Gavrilenko et al., 2003; Gouin et al., 2010; Poutahidis et al., 2013a; Vitalo et al., 2009), and may serve to bridge bacteria-triggered behaviors and stress responses with physical fitness. Thus, we tested oxytocin levels in blood plasma of Swiss mice in Experiment 1, and found significant systemic elevation of this hormone in animals drinking L. reuteri daily (N = 10) when compared with matched untreated controls (N = 10) [Fig. 3A]. The microbe-enhanced skin wound repair capacity in mice was found to rely upon oxytocin when tested in a second experiment using oxytocin-knockout (ot-ko) B6; 129S-Oxttm1Wsy/J mice. By comparing wound sizes (measured microscopically in pixels) it was found that ot-wt mice consuming L reuteri (N = 8) at 6 days-post- biopsy had smaller wounds (68,464 ± 13,997 pixels; mean ± SE) vs those seen in ot-ko mice consuming L. reuteri (N = 8) (225,937 ± 27,539 pixels, p = 0.0003), matching earlier findings of oxytocin-dependency in mouse models (Poutahidis et al., 2013a).
Fig. 3.
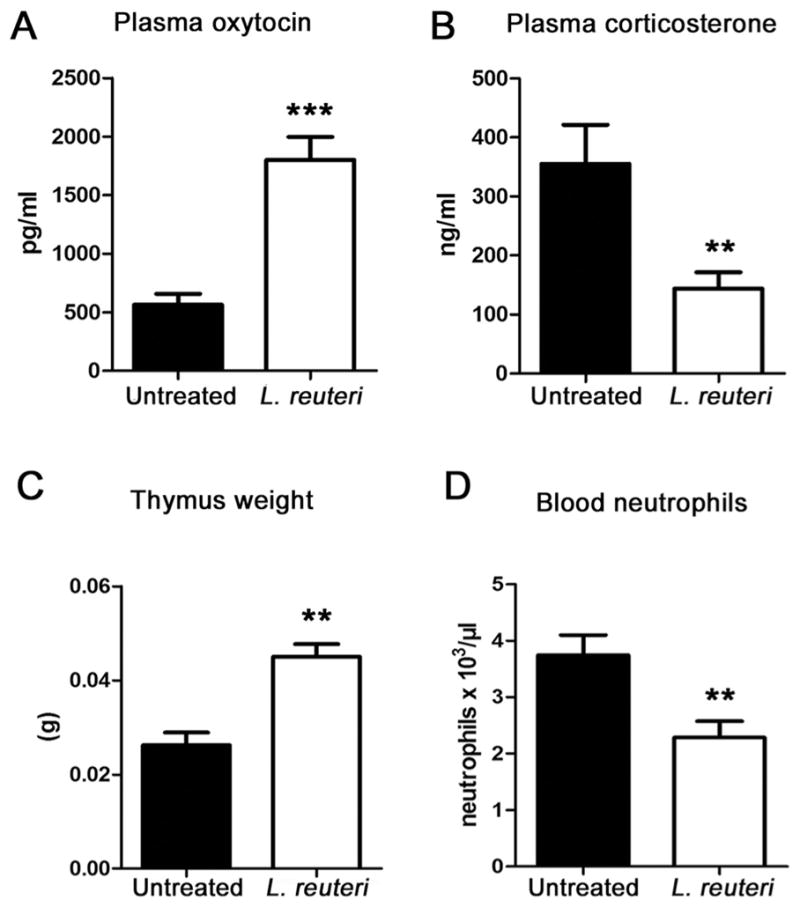
L. reuteri effects on hormone levels and thymus size of Swiss mice. L. reuteri consumption leads to statistically significant changes including (A) increased blood levels of oxytocin (Untreated N = 10, L. reuteri N = 10), (B) decreased levels of circulating corticosterone (N = 10 per group), (C) increased thymus weight, (N = 10 per group) and (D) lower circulating neutrophil counts (N = 10 per group). Numbers on the y-axis of bar graphs correspond to the mean ± SEM of the parameter assessed; **p < 0.001, ***p < 0.0001.
3.3. Oral L. reuteri down-regulates blood levels of stress hormone corticosterone
An inverse relationship exists, in general, between systemic levels of oxytocin and stress-related hormones cortisol and corticosterone (Burkett et al., 2016; Cohen et al., 2010; Smith et al., 2016a; Stanic et al., 2016; Vilela et al., 2013; Wang et al., 2012). Finding that oral L. reuteri therapy increased circulating levels of oxytocin in our animal model, we theorized that L. reuteri associated with better maternal care and nursing behavior may also down-regulate stress levels in host animals. Indeed, it was previously shown that favorable mood rises after consuming other Lactobacillus sps (Bravo et al., 2011). To examine this possibility further, we examined levels of stress biomarker hormone corticosterone in mice (N = 10 per group), and found lower stress hormone levels in Swiss mice drinking L. reuteri (p < 0.01) (Fig. 3B). Increased corticosterone levels in rodent models of stress have been linked with a decrease of thymus gland weight (Listowska et al., 2015; Monteiro et al., 2015; Rosa et al., 2014; Zivkovic et al., 2005), and premature thymic involution, leading to host animal immune dysregulation.
3.4. Thymus gland size is increased after oral dosing with L. reuteri
Accumulated data from animal models and human subjects shows that premature thymic involution results in T-lymphocyte deficiency and produces a wide array of detrimental outcomes linked with systemic immunodeficiency (Taub and Longo, 2005; Ventevogel and Sempowski, 2013). Knowing that oxytocin has been implicated in improved immune health (Barnard et al., 2008), and that feeding of L. reuteri ATCC PTA 6475 increases thymus gland size in mice (Varian et al., 2016a), we examined postmortem thymus gland weights in the same Swiss mice consuming L. reuteri in drinking water. We found that the thymus gland weight is significantly increased (p < 0.01) in the probiotic-treated mice (N = 10) when compared to age-matched control animals (N = 10) (Fig. 3C).
3.5. Circulating neutrophil counts are decreased in mice consuming L. reuteri
In addition, in previous studies it was shown that oral L. reuteri treatment also is associated with a subclinical reduction of circulating neutrophils (Varian et al., 2016a,b). Therefore, we reason that blood neutrophils counts are a candidate biomarker for influences of L. reuteri on systemic immune status. We tested this possibility in the present experiment using outbred Swiss mice (N = 10 per group) and find that probiotic treatment reduced (p < 0.01) the numbers of circulating (blood) neutrophils (Fig. 3D). Lowered neutrophil counts in mice was found to rely upon oxytocin when tested in a second experiment using oxytocin-knockout (ot-ko) B6;129S-Oxttm1Wsy/J mice. By comparing neutrophil counts (measured as cell/ul in a CBC) it was found that ot-wt mice consuming L reuteri (N = 8) at 6 days- post-biopsy had fewer (p < 0.01) neutrophils (1.586 ± 0.122) vs those seen in ot-ko mice drinking L. reuteri (N = 8) (3.252 ± 0.289), matching earlier findings of oxytocin-dependency in mouse models (Poutahidis et al., 2013a).
3.6. Killed sterile L. reuteri lysate is sufficient to elevate plasma oxytocin levels
Recognizing that infection with L. reuteri leads to higher levels of oxytocin in mice (Buffington et al., 2016; Poutahidis et al., 2013a; Varian et al., 2016b), raises many questions about probiotic organism viability requirements and interactions with other host microbes. It was earlier shown that oxytocin-mediated slenderizing effects of L. reuteri were achievable when using a lysed sterile L. reuteri preparation alone (Varian et al., 2016b). We next tested in Experiment 3 whether oral administration of L. reuteri ATCC 6475 sterile lysate alone was sufficient to raise endogenous oxytocin levels in our mouse models. A soluble extract of sterile microbial lysate was prepared using sonication with repeated centrifugation, resulting in a sterile soluble fraction delivered to mice in their drinking water. Plasma oxytocin levels were then examined in twelve-week-old wild type C57BL/6 mice consuming the equivalent of 3.5 × 105 L. reuteri ATCC 6475 organisms per day added in the form of lysate to their drinking water. We find that mice drinking sterile lysate (N = 10) had higher circulating levels of oxytocin measurable in blood plasma by comparison with control mice (N = 9) (p < 0.001) (Fig. 4A). We also found that mice consuming lysate had lower blood levels of stress hormone corticosterone (Lysed L. reuteri N = 10; Control N = 9) (Fig. 4B), larger thymus glands (Fig. 4C) (N = 8–10 mice per group) and fewer circulating neutrophils (Lysed LR N = 10; Control N = 9) (Fig. 4D) when compared with untreated controls getting regular water. These data matched earlier findings using viable L. reuteri.
Fig. 4.
Lysed L. reuteri products alter blood hormone levels and thymus gland size of C57BL/6 mice. Edible sterile, lysed L. reuteri is sufficient to (A) upregulate blood levels of oxytocin (Untreated N = 9; Lysed L. reuteri N = 10), (B) downregulate blood levels of corticosterone (Control N = 9; Lysed L. reuteri N = 10), (C) bestow increased thymus weight (N = 8–10 per treatment group) at statistically significant levels, and (D) lower circulating neutrophil counts (Control N = 9; Lysed L. reuteri N = 10). Numbers on the y-axis of bar graphs correspond to the mean ± SEM of the parameter assessed; ***p < 0.0001.
3.7. Sterile L. reuteri lysate increases oxytocin-positive cell counts in the paraventricular nucleus [PVN] of hypothalamus
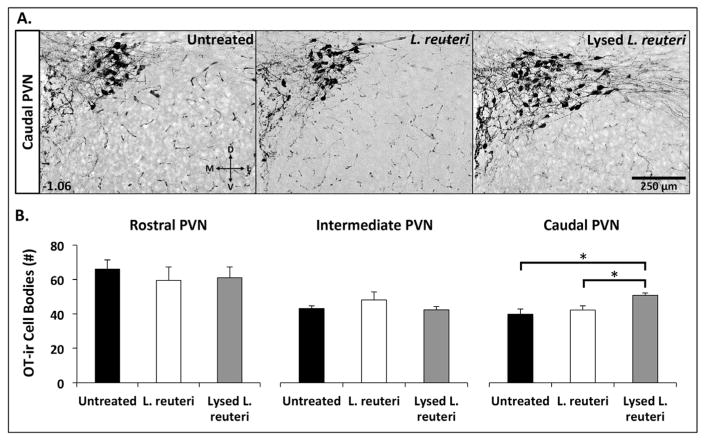
To determine the source of systemic oxytocin elevations, and in particular whether microbe treatment had an effect on the number of oxytocin-ir neurons in the PVN, mice treated with either lysed L. reuteri, live L. reuteri, or normal drinking water (untreated controls) were euthanized, and their brains were immunolabeled for oxytocin-associated neurophysin (Fig. 5A; Ben-Barak et al., 1985). Lysed L. reuteri treated mice had more oxytocin-ir neurons in the caudal PVN compared to mice treated with live L. reuteri or normal drinking water (treatment effect: F(2,22) = 7.61; p = 0.003; Fig. 5B). No microbe treatment effects were observed on the number of oxytocin-ir neurons in the rostral (F(2,22) = 0.25; p = 0.78) or intermediate portions of the PVN (F(2,22) = 1.26; p = 0.30; Fig. 5B). Thus, microbe lysate consumption stimulated oxytocin-producing neurons in the hypothalamus, which coincides with increased levels of plasma oxytocin in mice.
Fig. 5.
Mice treated with lysed L. reuteri have more oxytocin-immunoreactive (OT-ir) neurons in the caudal PVN. (A) Representative photomicrographs depicting oxytocin-ir neurons in the caudal PVN of mice treated with normal drinking water (Untreated N = 9), or live L. reuteri (N = 8), or lysed L. reuteri (N = 10). The numerical value in the bottom right corner of top plates represents the distance (in millimeters) posterior to bregma for the rostral, intermediate and caudal PVN. (B) Average number of oxytocin-ir cells observed in the rostral, intermediate, and caudal PVN of mice treated with normal drinking water (Untreated), live L. reuteri, or lysed L. reuteri. *p < 0.05 (Tukey post hoc tests following one-way ANOVA).
3.8. Ingestion of microbe lysate conveys increased wound healing capacity
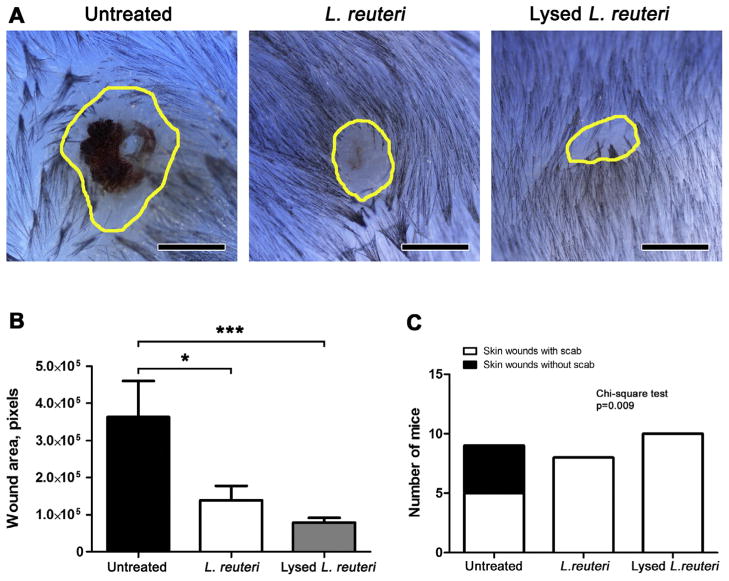
Finally, to test the efficacy of lysed L. reuteri upon epithelial wound healing in the C57BL/6 mouse model, mice receiving for four weeks either live (N = 8) or lysed (N = 10) L. reuteri ATCC 6475 underwent the skin wound assay and were compared with untreated mice serving as controls (N = 9). Examining wound areas at the 6th day after skin excision, we find that both viable and lysed L. reuteri treatments led to significant acceleration of wound closure (Fig. 6A and B) compared to untreated control mice. Faster wound healing rates of pro- or postbiotic-treated mice were also characterized by more frequent detachment of wound scabs at 6 days after experimental wound induction (Fig. 6C). In summary, these data led us to propose a host stress paradigm whereby microbiota and their products modulate stress hormone levels leading to immune modulation subsequently influencing homeostasis and wound healing processes throughout the body (Fig. 7).
Fig. 6.
L. reuteri viability is not required for skin wound healing benefits. (A) Standard wound healing assay results in C57BL/6 mice confirm that sonication-killed L. reuteri consumption is associated with accelerated wound closure. Comparing the size of yellow color-outlined wounds at 6 days post infliction, which are placed side-by-side according to treatment, reveals (B) the beneficial effect of lysed L. reuteri reaches particularly high levels of statistical significance (C) The increased occurrence of early wound scab detachment at 6 days post-biopsy reflects the faster healing rate conferred by both probiotic and postbiotic L. reuteri treatments (Control N = 9, L. reuteri N = 8, Lysed L. reuteri N = 10) Scale bars: 1000 μm (a). Numbers on the y-axis of bar graphs correspond to the mean ± SEM of the parameter assessed; *p < 0.05, ***p < 0.0001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 7.
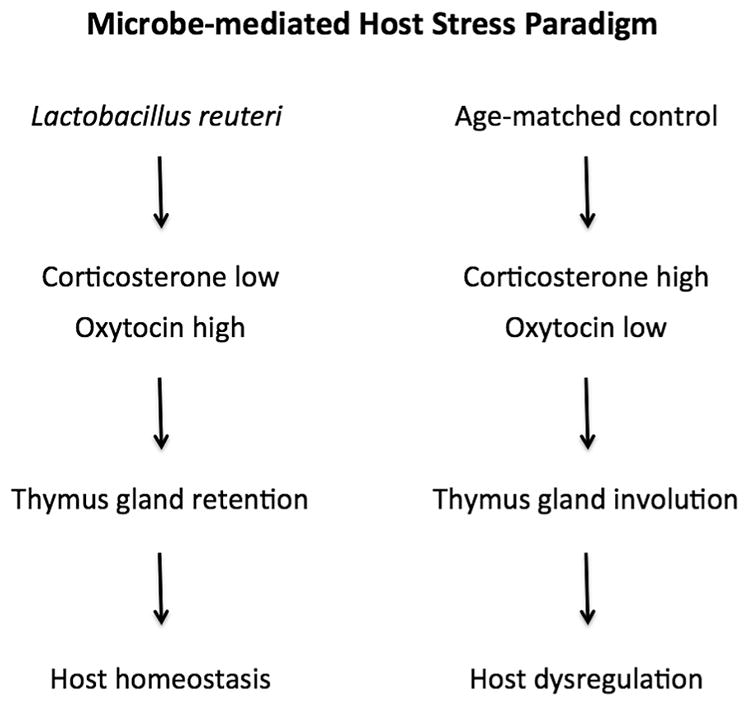
L. reuteri modulates host animal stress hormones and immune homeostasis. Consumption of L. reuteri is sufficient to up-regulate oxytocin and down-regulate corticosterone levels, resulting in larger thymus gland size and immune homeostasis with improved host wound repair capacity.
4. Discussion
Here we provide evidence that postbiotic (non-viable) preparations of L. reuteri are sufficient to elevate blood levels of oxytocin and increase the number of oxytocin-positive cells in the PVN of mice. Microbe-triggered increases in blood and brain oxytocin were associated with improved wound healing capacity, lowered blood levels of stress hormone corticosterone, plus a larger thymus gland and fewer pro-inflammatory blood neutrophils in mice consuming L. reuteri. Microbe-induced oxytocin was necessary for more proficient wound repair. Human subject findings linking L. reuteri consumption with improved wound repair capacity reinforce translational potential of the findings in mice. Finally, sterile preparations of L. reuteri lysate were sufficient for achieving health benefits in mice, suggesting that these phenomena are triggered by a bacterial component, rather than by activities or interactions of live bacteria.
We found that mice treated with L. reuteri lysate had more oxytocin immunoreactive neurons in the caudal PVN. Oxytocin produced in the PVN can be released in the brain via axonal projections, somato-dendritic release or volume transmission (Knobloch et al., 2012; Landgraf and Neumann, 2004; Ludwig and Leng, 2006) and act as a neuromodulator. Alternatively, oxytocin can be released in the periphery via axonal projections to the posterior pituitary, where it functions as a hormone. Oxytocin exerts its actions through binding to the oxytocin receptor, which is widely expressed in the brain and periphery (Gimpl and Fahrenholz, 2001; Jard et al., 1987; Smith et al., 2016a,b). This allows oxytocin to modulate a large array of physiological processes, including immune-related processes. Oxytocin-positive PVN neurons can be parvocellular or magnocellular, and both phenotypes have been observed in the caudal PVN (Eliava et al., 2016; Herman et al., 2002a,b). Parvocellular oxytocin-positive neurons extend axonal projections throughout the brain, whereas magnocellular oxytocin-positive neurons project primarily to the periphery, but also possess collateral branches projecting to forebrain regions (Knobloch et al., 2012). This may indicate that increased oxytocin production in L. reuteri extract-treated mice could contribute to improving the observed wound healing capacity via both central and peripheral pathways.
Although speculative, an increase in oxytocin-positive PVN neurons in L. reuteri lysate-treated mice could reflect oxytocin-negative PVN neurons that are recruited to express oxytocin in response to a higher demand for oxytocin synthesis. This higher demand for oxytocin synthesis is likely a direct result of increased levels of plasma oxytocin, as was observed in L. reuteri-treated mice. It should be noted that, in addition to the PVN, oxytocin synthesized in the supraoptic nucleus (SON) might also have been affected by microbe treatment. Unfortunately, oxytocin immunoreactive cells in the SON were too dense to distinguish individually in order to quantify cell numbers. Thus, we cannot exclude the possibility that oxytocin neurons in the SON, in addition to the PVN, contributed to the increase in plasma oxytocin levels observed in mice treated with L. reuteri. This could be addressed in future studies by quantifying oxytocin mRNA expression in both the PVN and SON, which may provide additional information regarding the need for higher oxytocin synthesis in microbe lysate-treated mice.
The observation that lysed L. reuteri ATCC 6475 cells were comparable with their viable counterparts in conferring typical L. reuteri- induced health benefits in mice is important. Indeed, the ingestion of the killed form of the probiotic served to up-regulate oxytocin, down-regulate corticosterone, increase thymic mass, decrease circulating neutrophils, and accelerate skin wound healing. These findings are in accordance with the results of another recent study using a different L. reuteri isolated from pet dogs (Varian et al., 2016b). In that study, lysed extract of canine L. reuteri strain 2546 counteracted obesity and decreased blood neutrophils of mice, recapitulating earlier findings for viable L. reuteri ATCC PTA 6475 (Poutahidis et al., 2013b; Varian et al., 2016a,b, 2014). Using oxytocin-deficient mice in that earlier study showed that the slenderizing effects of L. reuteri 2546 lysate depended on the hormone oxytocin (Varian et al., 2016b).
Specific bacterial components with health-promoting effects are termed ‘postbiotics’ and hold promise as a more precise, controlled and effective therapeutic approach compared to live probiotic cell consumption (Adams, 2010; Caselli et al., 2011; Kataria et al., 2009; Ruiz et al., 2014; Sanchez et al., 2010). The retaining of beneficial health effects and immunomodulatory properties has been described for many different postbiotic forms of Lactobacillus spp. and other beneficial bacteria (Adams, 2010; Caselli et al., 2011; Kataria et al., 2009; Ruiz et al., 2014; Sanchez et al., 2010). Based on accumulated studies the increased value of using non-viable bacterial cells is now emerging (Adams, 2010; Kataria et al., 2009). Killed forms are considered safer than live bacteria for many reasons (Adams, 2010; Kataria et al., 2009; Ruiz et al., 2014). The identification of the biologically-active ingredients of killed bacteria is considered as a step forward in microbe-based research.
Postbiotic bacterial components with beneficial properties are enticing but remain largely uncharacterized (Adams, 2010; Caselli et al., 2011; Kataria et al., 2009; Ruiz et al., 2014; Sanchez et al., 2010). In humans, spray-dried non-viable cells of L. reuteri DSMZ17648 were potent enough to decrease Helicobacter pylori load in the stomach (Mehling and Busjahn, 2013). In addition, the capsular polysaccharide A of Bacteroides fragilis has been shown to have desirable immunomodulatory effects in intestinal and neuronal tissue (Surana and Kasper, 2012). Along the same lines, bacterial DNA, exopolysaccharides, bacteriocins, lipoteichoic acids and other microbial cell wall components possess promising postbiotic attributes (Adams, 2010; Caselli et al., 2011; Kataria et al., 2009; Ruiz et al., 2014; Sanchez et al., 2010; Surana and Kasper, 2012). Thus far postbiotic compounds identified for L. reuteri include histamine and the bifunctional dihydrofolate synthase/ folylpolyglutamate synthase type 2 (folC2)-mediated folate metabolism products essential for anti-inflammatory properties (Gao et al., 2015; Thomas et al., 2016). Taken together, this suggests that L. reuteri possesses powerful bioactive postbiotic molecules that are worthy of further exploration and testing for pharmaceutical applications.
In the present study, lysed L. reuteri ATCC 6475 was not heat-treated. In other studies, heat-killed non-viable forms of L. reuteri GMNL-263 were potent enough to prevent weight gain in a diet-induced obesity rat model (Hsieh et al., 2016). In high-fat diet fed hamsters, the same heat- treated material reduced liver fibrosis, blood LDL-cholesterol and plasma malondialdehyde and myocardial cell apoptosis (Ting et al., 2015a,b). A heat-killed L. reuteri ATCC 23272 also reduced pro-inflammatory cytokines, but was less potent than viable forms in reducing eosinophil influx and airway damage (Forsythe et al., 2007); however, killed bacteria were able to reduce visceral pain (Kamiya et al., 2006). Importantly, Buffington et al. (2016) found that heat-killed L. reuteri ATCC 6475 failed to up-regulate oxytocin without behavioral benefits in mice (Buffington et al., 2016). This aspect remains to be investigated further, but indirectly points to a biologically active microbial protein in modulating host oxytocin levels in the present studies.
An interesting aspect of edible bacteria-derived compounds is their multi-dimensional beneficial activities. Microbe-based therapies may promote overall health by awakening complex and multidimensional systematic pathways for good health that otherwise remain latent due to modernized urban life-style (Erdman and Poutahidis, 2010; Rook, 2013; Walter et al., 2011). Preliminary evidence from the present and prior studies suggests that L. reuteri activates diverse homeostatic pathways at the whole organism level (Erdman and Poutahidis, 2014; Ibrahim et al., 2014; Lakritz et al., 2014; Levkovich et al., 2013; Poutahidis et al., 2013a,b, 2014, 2015; Varian et al., 2016a,b, 2014). These involve inter-related gut, immune, endocrine, and brain functions that confer longevity and counteract senility-associated imbalances of host inflammatory responses (Erdman and Poutahidis, 2014; Ibrahim et al., 2014; Lakritz et al., 2014; Levkovich et al., 2013; Poutahidis et al., 2013a,b, 2014, 2015; Varian et al., 2016a,b, 2014). In short, these microbes restore whole body homeostasis.
Along these lines, testing the effects of bacterial products on the thymus gland, an elementary immune system organ, is very informative. Firstly, because thymus is profoundly affected by normal aging progression (Taub and Longo, 2005). Secondly, thymus gland function is clearly influenced by neuroendocrine signaling networks. Finally, there is extensive evidence in both animal models and human subjects that premature thymic involution results in T-lymphocyte deficiency and produces a wide array of detrimental outcomes linked with systemic immunodeficiency (Taub and Longo, 2005; Ventevogel and Sempowski, 2013). In the light of these facts, the finding that mice consuming lysed L. reuteri extract have a significantly larger thymus gland compared to their age-matched controls may be particularly important. The youthfully-sized thymus after postbiotic consumption coincided with increased oxytocin levels in CNS and blood and decreased circulating corticosterone levels.
Connections between oxytocin and normal thymus function are indeed well-documented. Oxytocin has been shown to be essential for thymic lymphocyte differentiation and selection (Hansenne et al., 2005). Furthermore, the autoimmune regulator gene/protein (Aire) – important for natural T regulatory (Treg) cell differentiation in the thymus gland (Nomura and Sakaguchi, 2007) – is induced by oxytocin in thymic epithelial cells (Hansenne et al., 2009). Oxytocin cross-talk with immune system cells happens in part via membrane oxytocin receptors that promote peripheral mononuclear cell proliferation and suppress pro-inflammatory cytokines (Wang et al., 2015). Based on both preclinical models and human studies, exogenous oxytocin treatment emerges as a novel therapy for uncontrolled inflammation and immune-mediated tissue damage (Al-Amran and Shahkolahi, 2013; Biyikli et al., 2006; Clodi et al., 2008; Iseri et al., 2005a; Petersson et al., 2001; Wang et al., 2015). Specific mechanisms in the present model, whether immune or neuronal in origin, remain to be determined.
In the present mouse model studies, live L. reuteri organism consumption leads to increased levels of oxytocin coincident with decreased levels of the stress-related hormone corticosterone. The present study also shows that this inverse correlation of oxytocin and corticosterone emerges in mice consuming lysed L. reuteri. This finding is in line with the known role of oxytocin in improving social and non-social behaviors, and dampening anxiety, stress and depression (Baribeau and Anagnostou, 2015; Carter, 2014; Feldman et al., 2016). It is also consistent with previous reports describing the oxytocin-corticosterone interplay in rodent models of both social (Burkett et al., 2016; Wang et al., 2012) and non-social- related stress (Cohen et al., 2010; Smith et al., 2016a; Stanic et al., 2016; Vilela et al., 2013). It will be interesting to investigate roles for L. reuteri in stress-induced corticosterone levels and animal behaviors.
Interestingly, increased corticosterone levels in rodent models of stress have been shown to correlate with a decrease of thymus gland weight (Listowska et al., 2015; Monteiro et al., 2015; Rosa et al., 2014; Zivkovic et al., 2005), although some studies suggest that this effect depends on the type of stressor and strain of mouse used (Cruz et al., 2012; Savignac et al., 2011). By contrast, stimulation of social behavior or improving sense of well-being by enrichment of caging decreased corticosterone levels while counteracting thymus shrinkage in both mice and rats (Abou-Ismail and Mahboub, 2011; Seetharaman et al., 2016; Van Loo et al., 2004). A study in genetically engineered mice has provided direct evidence linking thymus gland size and function with corticosterone (Youn et al., 2011). Specifically, Youn et al. (2011) have used mice that were deficient in Bag3, a multifunctional molecule involved in cell survival, migration, chaperone regulation, and cellular protein metabolism. This mouse model has highly elevated levels of corticosterone due to adrenal gland zona reticularis hyperplasia coexisting with severe thymus gland atrophy. Remarkably, the premature thymic involution in this model has been shown to be due to the increased production of adrenal gland corticosterone and not due to a direct effect of Bag3 depletion in the thymus or an impaired CRH and ACTH hypothalamic-pituitary gland negative feedback signaling (Youn et al., 2011).
In previous research (Poutahidis et al., 2013a; Varian et al., 2016a,b) and the present study we find circulating neutrophils offer an important immune cell target of the gut-immune-endocrine interactive axis that is activated by L. reuteri consumption. Presently we show that drinking nonviable L. reuteri cells is as potent as drinking live probiotics in down-regulating circulating neutrophils. Previously we have ascribed this down-regulation to potency of regulatory T cells, the peripheral induction of which is highly upregulated after L. reuteri consumption(Erdman and Poutahidis, 2014; Lakritz et al., 2014; Poutahidis et al., 2013a; Varian et al., 2016a). Our accumulated data (Poutahidis et al., 2013a; Varian et al., 2016a,b) together with present findings, however, suggest that hormones such as oxytocin and corticosterone may also contribute directly to this immune-mediated phenomenon. Oxytocin’s reported actions upon inflammatory processes appear to involve neutrophil homeostasis as one of its most characteristic actions (Al-Amran and Shahkolahi, 2013; Biyikli et al., 2006; Clodi et al., 2008; Iseri et al., 2005a; Petersson et al., 2001; Wang et al., 2015). Indeed, we have found that otherwise untreated oxytocin-deficient mice, which are clinically healthy and show no evidence of inflammatory disease, have a significant subclinical elevation of blood neutrophils compared to their wild-type controls [data not shown]. Lower corticosterone levels after L. reuteri treatment may also contribute to the downregulation of neutrophils. Increased corticosterone in mice subjected to maternal separation or social stress connects with significantly increased circulating neutrophils (Avitsur et al., 2002; Kinsey et al., 2008; Pinheiro et al., 2011; Zimecki et al., 2009). Zimecki et al. (2009) have also shown that the lactoferrin-induced myelopoiesis leads to increased neutrophils in the blood of mice, and depends upon the lactoferrin-associated increase of serum corticosterone (Zimecki et al., 2009).
Although effective neutrophil-mediated responses are required for fighting infections, they are also key mediators of obesity-associated disorders, including cardiovascular disease and diabetes (Fc, 2016; Manda-Handzlik and Demkow, 2015; Mayadas et al., 2014). The role of neutrophils in carcinogenesis and tumor evolution is also emerging, and a therapeutic approach of targeting tumor-associated neutrophils has been recently introduced (Coffelt, 2016; Gregory and Houghton, 2011; Manda-Handzlik and Demkow, 2015; Rao et al., 2007, 2006; Lakritz et al., 2015). According to a recent report the microbiota drives neutrophil aging via Toll-like receptor and myeloid differentiation factor 88, making aged neutrophils particularly effective as disease-promoting agents (Zhang et al., 2015a). In the light of this evidence, lowering the chronic systematic neutrophilic inflammatory tone without compromising ability of neutrophils to counteract pathogens may be important for human health. A previous study showing acceleration of skin wound healing using edible L. reuteri supplementation exemplifies such a therapeutic strategy (Poutahidis et al., 2013a). Using this mouse model, it was shown that L. reuteri did not compromise the beneficial acute stages of neutrophilic infiltration in the wound. Instead, it accelerated closure of the wound bed earlier in the healing process. Therefore L. reuteri treatment enforced the physiological balance of immune cells and orchestration of wound healing process and led to faster wound healing without compromising beneficial functions of specific immune cells including neutrophils (Poutahidis et al., 2013a).
Wound healing is an elementary biological process that includes the timely implementation of several different basic physiological phenomena (hemostasis, inflammation, extracellular matrix and connective tissue formation, angiogenesis, tissue remodeling). The ability to heal wounds effectively and swiftly reflects good health and fitness, and connects with youthfulness and longevity (Eming et al., 2014; Gurtner et al., 2008; Yanai et al., 2011). Therefore, the mouse skin wound healing model is an attractive platform to test the systemic health promoting effects of edible probiotic and postbiotic extract products. In the present study we show that lysed L. reuteri is as effective as the viable bacteria in accelerating skin wound healing in mice.
Going one step further, in the present studies we find that human subject outcomes support benefit of L. reuteri with improved wound repair capacity, thus reinforcing the translational potential of findings in mice (Poutahidis et al., 2013a). Significant improvement in wound healing in just 14 patients with diverse backgrounds is exciting, and raises the question whether benefits will translate to individuals plagued with various other ailments including diabetes and heart disease. As ongoing trials begin to address these clinical implications, emerging data suggest health is indeed due largely to microbial factors that can be readily modified (Hsieh et al., 2016), that when harnessed impart resiliency typical of much younger subjects (Varian et al., 2014). Given the safety of food-grade microbes consumed in fermented beverages for thousands of years, edible bacteria and their products may offer a low-risk/high-impact remedy for trauma, elective procedures and poorly healing chronic wounds that affect millions of patients in the burgeoning health care and economic crisis.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants P30-ES002109 (pilot project award to S.E.E), RO1CA108854 (to S.E. E), and U01 CA164337 (to S.E.E.).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bbi.2016.11.002.
Footnotes
Conflict of interest
The authors have declared that no competing interests exist.
References
- Abou-Ismail UA, Mahboub HD. The effects of enriching laboratory cages using various physical structures on multiple measures of welfare in singly-housed rats. Lab Anim. 2011;45:145–153. doi: 10.1258/la.2011.010149. [DOI] [PubMed] [Google Scholar]
- Adams CA. The probiotic paradox: live and dead cells are biological response modifiers. Nutr Res Rev. 2010;23:37–46. doi: 10.1017/S0954422410000090. [DOI] [PubMed] [Google Scholar]
- Al-Amran F, Shahkolahi M. Oxytocin ameliorates the immediate myocardial injury in rat heart transplant through downregulation of neutrophil-dependent myocardial apoptosis. Transplant Proc. 2013;45:2506–2512. doi: 10.1016/j.transproceed.2013.03.022. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Dhabhar FS, Sheridan JF. Social stress alters splenocyte phenotype and function. J Neuroimmunol. 2002;132:66–71. doi: 10.1016/s0165-5728(02)00310-7. [DOI] [PubMed] [Google Scholar]
- Barengolts E. Oxytocin – An emerging treatment for obesity and dysglycemia: review of randomized controlled trials and cohort studies. Endocr Pract. 2016 doi: 10.4158/EP151192.RA. [DOI] [PubMed] [Google Scholar]
- Baribeau DA, Anagnostou E. Oxytocin and vasopressin: linking pituitary neuropeptides and their receptors to social neurocircuits. Front Neurosci. 2015;9:335. doi: 10.3389/fnins.2015.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard A, Layton D, Hince M, Sakkal S, Bernard C, Chidgey A, Boyd R. Impact of the neuroendocrine system on thymus and bone marrow function. Neuroimmunomodulation. 2008;15:7–18. doi: 10.1159/000135619. [DOI] [PubMed] [Google Scholar]
- Bartz J, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, Vicens V, Hollander E. Oxytocin can hinder trust and cooperation in borderline personality disorder. Soc Cogn Affect Neurosci. 2011;6:556–563. doi: 10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Barak Y, Russell JT, Whitnall MH, Ozato K, Gainer H. Neurophysin in the hypothalamo-neurohypophysial system. I Production and characterization of monoclonal antibodies. J Neurosci. 1985;5:81–97. doi: 10.1523/JNEUROSCI.05-01-00081.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biyikli NK, Tugtepe H, Sener G, Velioglu-Ogunc A, Cetinel S, Midillioglu S, Gedik N, Yegen BC. Oxytocin alleviates oxidative renal injury in pyelonephritic rats via a neutrophil-dependent mechanism. Peptides. 2006;27:2249– 2257. doi: 10.1016/j.peptides.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Baskin DG. Translational and therapeutic potential of oxytocin as an anti-obesity strategy: Insights from rodents, nonhuman primates and humans. Physiol Behav. 2015;152:438–449. doi: 10.1016/j.physbeh.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, Parameswaran N, McCabe LR. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 2014;229:1822–1830. doi: 10.1002/jcp.24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. 2016;165:1762–1775. doi: 10.1016/j.cell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB, Young LJ. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351:375–378. doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS. Oxytocin pathways and the evolution of human behavior. Annu Rev Psychol. 2014;65:17–39. doi: 10.1146/annurev-psych-010213-115110. [DOI] [PubMed] [Google Scholar]
- Caselli M, Vaira G, Calo G, Papini F, Holton J, Vaira D. Structural bacterial molecules as potential candidates for an evolution of the classical concept of probiotics. Adv Nutr. 2011;2:372–376. doi: 10.3945/an.111.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clodi M, Vila G, Geyeregger R, Riedl M, Stulnig TM, Struck J, Luger TA, Luger A. Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. Am J Physiol Endocrinol Metab. 2008;295:E686–E691. doi: 10.1152/ajpendo.90263.2008. [DOI] [PubMed] [Google Scholar]
- Coffelt SB, Wellenstein Max D, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Kozlovsky N, Gidron Y, Matar MA, Zohar J. Hippocampal microinfusion of oxytocin attenuates the behavioural response to stress by means of dynamic interplay with the glucocorticoid-catecholamine responses. J Neuroendocrinol. 2010;22:889–904. doi: 10.1111/j.1365-2826.2010.02003.x. [DOI] [PubMed] [Google Scholar]
- Colaianni G, Sun L, Zaidi M, Zallone A. Oxytocin and bone. Am J Physiol Regul Integr Comp Physiol. 2014;307:R970–R977. doi: 10.1152/ajpregu.00040.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FL, Irwin R, Bierhalter H, Schepper J, Britton RA, Parameswaran N, McCabe LR. Lactobacillus reuteri 6475 increases bone density in intact females only under an inflammatory setting. PLoS One. 2016;11:e0153180. doi: 10.1371/journal.pone.0153180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Rossi E, Scicchitano BM, Coletti D, Moresi V, Adamo S. Neurohypophyseal hormones: novel actors of striated muscle development and homeostasis. Eur J Transl Myol. 2014;24:3790. doi: 10.4081/ejtm.2014.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruchet S, Furnes R, Maruy A, Hebel E, Palacios J, Medina F, Ramirez N, Orsi M, Rondon L, Sdepanian V, Xochihua L, Ybarra M, Zablah RA. The use of probiotics in pediatric gastroenterology: a review of the literature and recommendations by Latin-American experts. Paediatr Drugs. 2015;17:199–216. doi: 10.1007/s40272-015-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Marin MT, Leao RM, Planeta CS. Behavioral and neuroendocrine effects of the exposure to chronic restraint or variable stress in early adolescent rats. Int J Dev Neurosci. 2012;30:19–23. doi: 10.1016/j.ijdevneu.2011.10.005. [DOI] [PubMed] [Google Scholar]
- De Dreu CK, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJ. Oxytocin promotes human ethnocentrism. Proc Natl Acad Sci USA. 2011;108:1262–1266. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74:720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Eaton KA, Honkala A, Auchtung TA, Britton RA. Probiotic Lactobacillus reuteri ameliorates disease due to enterohemorrhagic Escherichia coli in germfree mice. Infect Immun. 2011;79(1):185–191. doi: 10.1128/IAI.00880-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elabd C, Cousin W, Upadhyayula P, Chen RY, Chooljian MS, Li J, Kung S, Jiang KP, Conboy IM. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun. 2014;5:4082. doi: 10.1038/ncomms5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliava M, Melchior M, Knobloch-Bollmann HS, Wahis J, da Silva Gouveia M, Tang Y, Ciobanu AC, Triana del Rio R, Roth LC, Althammer F, Chavant V, Goumon Y, Gruber T, Petit-Demouliere N, Busnelli M, Chini B, Tan LL, Mitre M, Froemke RC, Chao MV, Giese G, Sprengel R, Kuner R, Poisbeau P, Seeburg PH, Stoop R, Charlet A, Grinevich V. A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron. 2016;89:1291–1304. doi: 10.1016/j.neuron.2016.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265sr266. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdman SE, Poutahidis T. Cancer inflammation and regulatory T cells. Int J Cancer. 2010;127:768–779. doi: 10.1002/ijc.25430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdman SE, Poutahidis T. Probiotic ‘glow of health’: it’s more than skin deep. Benef Microbes. 2014;5:109–119. doi: 10.3920/BM2013.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fak F, Backhed F. Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe−/− Mice. PLoS One. 2012;7:e46837. doi: 10.1371/journal.pone.0046837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fc P. The role of inflammation in cardiovascular diseases: the predictive value of neutrophil-lymphocyte ratio as a marker in peripheral arterial disease. J Ther Clin Risk Manag. 2016;12:10. doi: 10.2147/TCRM.S107635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Monakhov M, Pratt M, Ebstein RP. Oxytocin pathway genes: evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biol Psychiatry. 2016;79:174–184. doi: 10.1016/j.biopsych.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Forsythe P, Inman MD, Bienenstock J. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. Am J Respir Crit Care Med. 2007;175:561–569. doi: 10.1164/rccm.200606-821OC. [DOI] [PubMed] [Google Scholar]
- Francavilla R, Polimeno L, Demichina A, Maurogiovanni G, Principi B, Scaccianoce G, Ierardi E, Russo F, Riezzo G, Di Leo A, Cavallo L, Francavilla A, Versalovic J. Lactobacillus reuteri strain combination in Helicobacter pylori infection: a randomized, double-blind, placebo-controlled study. J Clin Gastroenterol. 2014;48(5):407–413. doi: 10.1097/MCG.0000000000000007. [DOI] [PubMed] [Google Scholar]
- Franklin Keith BJ, Paxinos George. The Mouse Brain in Stereotaxic Coordinates. Gulf Professional Publishing; San Diego, CA: 2008. [Google Scholar]
- Gao C, Major A, Rendon D, Lugo M, Jackson V, Shi Z, Mori-Akiyama Y, Versalovic J. Histamine H2 receptor-mediated suppression of intestinal inflammation by probiotic Lactobacillus reuteri. mBio. 2015;6:e01358–e01415. doi: 10.1128/mBio.01358-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilenko VG, Esipov VK, Sivozhelezov KG. Morphological characteristic of wound healing process in patients with diabetic purulent-necrotic foot lesion treated with oxytocin. Morfologiia. 2003;124:24–27. [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Gouin JP, Carter CS, Pournajafi-Nazarloo H, Glaser R, Malarkey WB, Loving TJ, Stowell J, Kiecolt-Glaser JK. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology. 2010;35:1082–1090. doi: 10.1016/j.psyneuen.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411–2416. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Hansenne I, Louis C, Martens H, Dorban G, Charlet-Renard C, Peterson P, Geenen V. Aire and Foxp3 expression in a particular microenvironment for T cell differentiation. Neuroimmunomodulation. 2009;16:35–44. doi: 10.1159/000179665. [DOI] [PubMed] [Google Scholar]
- Hansenne I, Rasier G, Pequeux C, Brilot F, Renard C, Breton C, Greimers R, Legros JJ, Geenen V, Martens HJ. Ontogenesis and functional aspects of oxytocin and vasopressin gene expression in the thymus network. J Neuroimmunol. 2005;158:67–75. doi: 10.1016/j.jneuroim.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci. 2002a;16:381–385. doi: 10.1046/j.1460-9568.2002.02133.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacol Biochem Behav. 2002b;71:457–468. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- Hemarajata P, Gao C, Pflughoeft KJ, Thomas CM, Saulnier DM, Spinler JK, Versalovic J. Lactobacillus reuteri-specific immunoregulatory gene rsiR modulates histamine production and immunomodulation by Lactobacillus reuteri. J Bacteriol. 2013;195(24):5567–5576. doi: 10.1128/JB.00261-13. http://dx.doi.org/10.1128/JB.00261-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh FC, Lan CC, Huang TY, Chen KW, Chai CY, Chen WT, Fang AH, Chen YH, Wu CS. Heat-killed and live Lactobacillus reuteri GMNL-263 exhibit similar effects on improving metabolic functions in high-fat diet-induced obese rats. Food Funct. 2016;7:2374–2388. doi: 10.1039/c5fo01396h. [DOI] [PubMed] [Google Scholar]
- Ibrahim YM, Kearney SM, Levkovich T, Springer A, Mirabal S, Poutahidis T, Varian BJ, Lakritz JR, Alm EJ, Erdman SE. Maternal gut microbes control offspring sex and survival. J Probiotics Health. 2014;2:6. [Google Scholar]
- Iniesta M, Herrera D, Montero E, Zurbriggen M, Matos AR, Marin MJ, Sanchez-Beltran MC, Llama-Palacio A, Sanz M. Probiotic effects of orally administered Lactobacillus reuteri-containing tablets on the subgingival and salivary microbiota in patients with gingivitis. A randomized clinical trial. J Clin Periodontol. 2012;39:736–744. doi: 10.1111/j.1600-051X.2012.01914.x. [DOI] [PubMed] [Google Scholar]
- Iseri SO, Sener G, Saglam B, Gedik N, Ercan F, Yegen BC. Oxytocin ameliorates oxidative colonic inflammation by a neutrophil-dependent mechanism. Peptides. 2005a;26:483–491. doi: 10.1016/j.peptides.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Iseri SO, Sener G, Saglam B, Gedik N, Ercan F, Yegen BC. Oxytocin protects against sepsis-induced multiple organ damage: role of neutrophils. J Surg Res. 2005b;126:73–81. doi: 10.1016/j.jss.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Jard S, Barberis C, Audigier S, Tribollet E. Neurohypophyseal hormone receptor systems in brain and periphery. Prog Brain Res. 1987;72:173–187. doi: 10.1016/s0079-6123(08)60206-x. [DOI] [PubMed] [Google Scholar]
- Kamiya T, Wang L, Forsythe P, Goettsche G, Mao Y, Wang Y, Tougas G, Bienenstock J. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55:191–196. doi: 10.1136/gut.2005.070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataria J, Li N, Wynn JL, Neu J. Probiotic microbes: do they need to be alive to be beneficial? Nutr Rev. 2009;67:546–550. doi: 10.1111/j.1753-4887.2009.00226.x. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbiota axis: challenges for translation in psychiatry. Ann Epidemiol. 2016;26:366–372. doi: 10.1016/j.annepidem.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Bailey MT, Sheridan JF, Padgett DA. The inflammatory response to social defeat is increased in older mice. Physiol Behav. 2008;93:628– 636. doi: 10.1016/j.physbeh.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Lakritz JR, Poutahidis T, Levkovich T, Varian BJ, Ibrahim YM, Chatzigiagkos A, Mirabal S, Alm EJ, Erdman SE. Beneficial bacteria stimulate host immune cells to counteract dietary and genetic predisposition to mammary cancer in mice. Int J Cancer. 2014;135:529–540. doi: 10.1002/ijc.28702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakritz JR, Poutahidis T, Mirabal S, Varian BJ, Levkovich T, Ibrahim YM, Ward JM, Teng EC, Fisher B, Parry N, Lesage S, Alberg N, Gourishetti S, Fox JG, Ge Z, Erdman SE. Gut bacteria require neutrophils to promote mammary tumorigenesis. Oncotarget. 2015;6:9387–9396. doi: 10.18632/oncotarget.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lee J, Yang W, Hostetler A, Schultz N, Suckow MA, Stewart KL, Kim DD, Kim HS. Characterization of the anti-inflammatory Lactobacillus reuteri BM36301 and its probiotic benefits on aged mice. BMC Microbiol. 2016;16:69. doi: 10.1186/s12866-016-0686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre A, Sirigu A. The two fold role of oxytocin in social developmental disorders: A cause and a remedy? Neurosci Biobehav Rev. 2016;63:168–176. doi: 10.1016/j.neubiorev.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Leng G, Ludwig M. Intranasal oxytocin: myths and delusions. Biol Psychiatry. 2016;79:243–250. doi: 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Levkovich T, Poutahidis T, Smillie C, Varian BJ, Ibrahim YM, Lakritz JR, Alm EJ, Erdman SE. Probiotic bacteria induce a ‘glow of health’. PLoS One. 2013;8:e53867. doi: 10.1371/journal.pone.0053867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listowska M, Glac W, Grembecka B, Grzybowska M, Wrona D. Changes in blood CD4+T and CD8+T lymphocytes in stressed rats pretreated chronically with desipramine are more pronounced after chronic open field stress challenge. J Neuroimmunol. 2015;282:54–62. doi: 10.1016/j.jneuroim.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fatheree NY, Mangalat N, Rhoads JM. Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299(5):G1087–96. doi: 10.1152/ajpgi.00124.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fatheree NY, Mangalat N, Rhoads JM. Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NF-kappaB signaling in the intestine. Am J Physiol Gastrointest Liver Physiol. 2012;302:G608–G617. doi: 10.1152/ajpgi.00266.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston M, Loach D, Wilson M, Tannock GW, Baird M. Gut commensal Lactobacillus reuteri 100–23 stimulates an immunoregulatory response. Immunol Cell Biol. 2010;88:99–102. doi: 10.1038/icb.2009.71. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Manda-Handzlik A, Demkow U. Neutrophils: the role of oxidative and nitrosative stress in health and disease. Adv Exp Med Biol. 2015;857:51–60. doi: 10.1007/5584_2015_117. [DOI] [PubMed] [Google Scholar]
- Martin-Cabezas R, Davideau JL, Tenenbaum H, Huck O. Clinical efficacy of probiotics as an adjunctive therapy to non-surgical periodontal treatment of chronic periodontitis: a systematic review and meta-analysis. J Clin Periodontol. 2016;43:520–530. doi: 10.1111/jcpe.12545. [DOI] [PubMed] [Google Scholar]
- Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe LR, Irwin R, Schaefer L, Britton RA. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol. 2013;228(8):1793–1798. doi: 10.1002/jcp.24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling H, Busjahn A. Non-viable Lactobacillus reuteri DSMZ 17648 (Pylopass) as a new approach to Helicobacter pylori control in humans. Nutrients. 2013;5:3062–3073. doi: 10.3390/nu5083062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro S, Roque S, de Sa-Calcada D, Sousa N, Correia-Neves M, Cerqueira JJ. An efficient chronic unpredictable stress protocol to induce stress-related responses in C57BL/6 mice. Front Psychiatry. 2015;6:6. doi: 10.3389/fpsyt.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehls M, Pfeifer D, Schorpp M, Hedrich H, Boehm T. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature. 1994;372:103–107. doi: 10.1038/372103a0. [DOI] [PubMed] [Google Scholar]
- Nomura T, Sakaguchi S. Foxp3 and Aire in thymus-generated Treg cells: a link in self-tolerance. Nat Immunol. 2007;8:333–334. doi: 10.1038/ni0407-333. [DOI] [PubMed] [Google Scholar]
- Petersson M, Wiberg U, Lundeberg T, Uvnas-Moberg K. Oxytocin decreases carrageenan induced inflammation in rats. Peptides. 2001;22:1479–1484. doi: 10.1016/s0196-9781(01)00469-7. [DOI] [PubMed] [Google Scholar]
- Pinheiro ML, Ferraz-de-Paula V, Ribeiro A, Sakai M, Bernardi MM, Palermo-Neto J. Long-term maternal separation differentially alters serum corticosterone levels and blood neutrophil activity in A/J and C57BL/6 mouse offspring. Neuroimmunomodulation. 2011;18:184–190. doi: 10.1159/000323516. [DOI] [PubMed] [Google Scholar]
- Poutahidis T, Kearney SM, Levkovich T, Qi P, Varian BJ, Lakritz JR, Ibrahim YM, Chatzigiagkos A, Alm EJ, Erdman SE. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS One. 2013a;8:e78898. doi: 10.1371/journal.pone.0078898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutahidis T, Kleinewietfeld M, Smillie C, Levkovich T, Perrotta A, Bhela S, Varian BJ, Ibrahim YM, Lakritz JR, Kearney SM, Chatzigiagkos A, Hafler DA, Alm EJ, Erdman SE. Microbial reprogramming inhibits Western diet-associated obesity. PLoS One. 2013b;8:e68596. doi: 10.1371/journal.pone.0068596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutahidis T, Springer A, Levkovich T, Qi P, Varian BJ, Lakritz JR, Ibrahim YM, Chatzigiagkos A, Alm EJ, Erdman SE. Probiotic microbes sustain youthful serum testosterone levels and testicular size in aging mice. PLoS One. 2014;9:e84877. doi: 10.1371/journal.pone.0084877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutahidis T, Varian BJ, Levkovich T, Lakritz JR, Mirabal S, Kwok C, Ibrahim YM, Kearney SM, Chatzigiagkos A, Alm EJ, Erdman SE. Dietary microbes modulate transgenerational cancer risk. Cancer Res. 2015;75:1197–1204. doi: 10.1158/0008-5472.CAN-14-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preidis GA, Versalovic J. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era. Gastroenterology. 2009;136:2015–2031. doi: 10.1053/j.gastro.2009.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preidis GA, Saulnier DM, Blutt SE, Mistretta TA, Riehle KP, Major AM, Venable SF, Barrish JP, Finegold MJ, Petrosino JF, Guerrant RL, Conner ME, Versalovic J. Host response to probiotics determined by nutritional status of rotavirus-infected neonatal mice. J Pediatr Gastroenterol Nutr. 2012;55(3):299–307. doi: 10.1097/MPG.0b013e31824d2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y, Sun J, Xia S, Li L, Li Y, Wang P, Shi Y, Le G. Effects of different Lactobacillus reuteri on inflammatory and fat storage in high-fat diet-induced obesity mice model. J Funct Foods. 2015;14:424–434. [Google Scholar]
- Rao VP, Poutahidis T, Fox JG, Erdman SE. Breast cancer: should gastrointestinal bacteria be on our radar screen? Cancer Res. 2007;67:847–850. doi: 10.1158/0008-5472.CAN-06-3468. [DOI] [PubMed] [Google Scholar]
- Rao VP, Poutahidis T, Ge Z, Nambiar PR, Horwitz BH, Fox JG, Erdman SE. Proinflammatory CD4+ CD45RB(hi) lymphocytes promote mammary and intestinal carcinogenesis in Apc(Min/+) mice. Cancer Res. 2006;66:57–61. doi: 10.1158/0008-5472.CAN-05-3445. [DOI] [PubMed] [Google Scholar]
- Romano R, Palamaro L, Fusco A, Giardino G, Gallo V, Del Vecchio L, Pignata C. FOXN1: a master regulator gene of thymic epithelial development program. Front Immunol. 2013;4:187. doi: 10.3389/fimmu.2013.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GA. Regulation of the immune system by biodiversity from the natural environment: an ecosystem service essential to health. Proc Natl Acad Sci US A. 2013;110:18360–18367. doi: 10.1073/pnas.1313731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa EF, Alves GA, Luz J, Silva SM, Suchecki D, Pesquero JB, Aboulafia J, Nouailhetas VL. Activation of HPA axis and remodeling of body chemical composition in response to an intense and exhaustive exercise in C57BL/6 mice. Physiol Res. 2014;63:605–613. doi: 10.33549/physiolres.932562. [DOI] [PubMed] [Google Scholar]
- Ruiz L, Hevia A, Bernardo D, Margolles A, Sanchez B. Extracellular molecular effectors mediating probiotic attributes. FEMS Microbiol Lett. 2014;359:1–11. doi: 10.1111/1574-6968.12576. [DOI] [PubMed] [Google Scholar]
- Sanchez B, Urdaci MC, Margolles A. Extracellular proteins secreted by probiotic bacteria as mediators of effects that promote mucosa-bacteria interactions. Microbiology. 2010;156:3232–3242. doi: 10.1099/mic.0.044057-0. [DOI] [PubMed] [Google Scholar]
- Saulnier DM, Santos F, Roos S, Mistretta TA, Spinler JK, Molenaar D, Teusink B, Versalovic J. Exploring metabolic pathway reconstruction and genome-wide expression profiling in Lactobacillus reuteri to define functional probiotic features. PLoS One. 2011;6:e18783. doi: 10.1371/journal.pone.0018783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savignac HM, Finger BC, Pizzo RC, O’Leary OF, Dinan TG, Cryan JF. Increased sensitivity to the effects of chronic social defeat stress in an innately anxious mouse strain. Neuroscience. 2011;192:524–536. doi: 10.1016/j.neuroscience.2011.04.054. [DOI] [PubMed] [Google Scholar]
- Schreiber O, Petersson J, Phillipson M, Perry M, Roos S, Holm L. Lactobacillus reuteri prevents colitis by reducing P-selectin-associated leukocyte- and platelet-endothelial cell interactions. Am J Physiol Gastrointest Liver Physiol. 2009;296:G534–G542. doi: 10.1152/ajpgi.90470.2008. [DOI] [PubMed] [Google Scholar]
- Seetharaman S, Fleshner M, Park CR, Diamond DM. Influence of daily social stimulation on behavioral and physiological outcomes in an animal model of PTSD. Brain Behav. 2016;6:e00458. doi: 10.1002/brb3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seijkens T, Kusters P, Chatzigeorgiou A, Chavakis T, Lutgens E. Immune cell crosstalk in obesity: a key role for costimulation? Diabetes. 2014;63:3982–3991. doi: 10.2337/db14-0272. [DOI] [PubMed] [Google Scholar]
- Sherwin E, Rea K, Dinan TG, Cryan JF. A gut (microbiome) feeling about the brain. Curr Opin Gastroenterol. 2016;32:96–102. doi: 10.1097/MOG.0000000000000244. [DOI] [PubMed] [Google Scholar]
- Simon MC, Strassburger K, Nowotny B, Kolb H, Nowotny P, Burkart V, Zivehe F, Hwang JH, Stehle P, Pacini G, Hartmann B, Holst JJ, MacKenzie C, Bindels LB, Martinez I, Walter J, Henrich B, Schloot NC, Roden M. Intake of Lactobacillus reuteri improves incretin and insulin secretion in glucose-tolerant humans: a proof of concept. Diabetes Care. 2015;38:1827–1834. doi: 10.2337/dc14-2690. [DOI] [PubMed] [Google Scholar]
- Smith AS, Tabbaa M, Lei K, Eastham P, Butler MJ, Linton L, Altshuler R, Liu Y, Wang Z. Local oxytocin tempers anxiety by activating GABAA receptors in the hypothalamic paraventricular nucleus. Psychoneuroendocrinology. 2016a;63:50–58. doi: 10.1016/j.psyneuen.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Poehlmann ML, Li S, Ratnaseelan AM, Bredewold R, Veenema AH. Age and sex differences in oxytocin and vasopressin V1a receptor binding densities in the rat brain: focus on the social decision-making network. Brain Struct Funct. 2016b doi: 10.1007/s00429-016-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanic D, Plecas-Solarovic B, Petrovic J, Bogavac-Stanojevic N, Sopic M, Kotur-Stevuljevic J, Ignjatovic S, Pesic V. Hydrogen peroxide-induced oxidative damage in peripheral blood lymphocytes from rats chronically treated with corticosterone: The protective effect of oxytocin treatment. Chem Biol Interact. 2016;256:134–141. doi: 10.1016/j.cbi.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Sun L, Tamma R, Yuen T, Colaianni G, Ji Y, Cuscito C, Bailey J, Dhawan S, Lu P, Calvano CD, Zhu LL, Zambonin CG, Di Benedetto A, Stachnik A, Liu P, Grano M, Colucci S, Davies TF, New MI, Zallone A, Zaidi M. Functions of vasopressin and oxytocin in bone mass regulation. Proc Natl Acad Sci USA. 2016;113:164–169. doi: 10.1073/pnas.1523762113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surana NK, Kasper DL. The yin yang of bacterial polysaccharides: lessons learned from B. fragilis PSA. Immunol Rev. 2012;245:13–26. doi: 10.1111/j.1600-065X.2011.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamma R, Colaianni G, Zhu LL, DiBenedetto A, Greco G, Montemurro G, Patano N, Strippoli M, Vergari R, Mancini L, Colucci S, Grano M, Faccio R, Liu X, Li J, Usmani S, Bachar M, Bab I, Nishimori K, Young LJ, Buettner C, Iqbal J, Sun L, Zaidi M, Zallone A. Oxytocin is an anabolic bone hormone. Proc Natl Acad Sci USA. 2009;106:7149–7154. doi: 10.1073/pnas.0901890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- Thomas CM, Saulnier DM, Spinler JK, Hemarajata P, Gao C, Jones SE, Grimm A, Balderas MA, Burstein MD, Morra C, Roeth D, Kalkum M, Versalovic J. FolC2-mediated folate metabolism contributes to suppression of inflammation by probiotic Lactobacillus reuteri. Microbiologyopen. 2016 doi: 10.1002/mbo3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting WJ, Kuo WW, Hsieh DJ, Yeh YL, Day CH, Chen YH, Chen RJ, Padma VV, Chen YH, Huang CY. Heat killed Lactobacillus reuteri GMNL-263 reduces fibrosis effects on the liver and heart in high fat diet-hamsters via TGF-beta suppression. Int J Mol Sci. 2015a;16:25881–25896. doi: 10.3390/ijms161025881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting WJ, Kuo WW, Kuo CH, Yeh YL, Shen CY, Chen YH, Ho TJ, Viswanadha VP, Huang CY. Supplementary heat-killed Lactobacillus reuteri GMNL-263 ameliorates hyperlipidaemic and cardiac apoptosis in high-fat diet-fed hamsters to maintain cardiovascular function. Br J Nutr. 2015b;114:706–712. doi: 10.1017/S0007114515002469. [DOI] [PubMed] [Google Scholar]
- Van Loo PL, Van der Meer E, Kruitwagen CL, Koolhaas JM, Van Zutphen LF, Baumans V. Long-term effects of husbandry procedures on stress-related parameters in male mice of two strains. Lab Anim. 2004;38:169–177. doi: 10.1258/002367704322968858. [DOI] [PubMed] [Google Scholar]
- Varian BJ, Goureshetti S, Poutahidis T, Lakritz JR, Levkovich T, Kwok C, Teliousis K, Ibrahim YM, Mirabal S, Erdman SE. Beneficial bacteria inhibit cachexia. Oncotarget. 2016a;7:11803–11816. doi: 10.18632/oncotarget.7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varian BJ, Levkovich T, Poutahidis T, Ibrahim YM, Perrotta A, Alm EJ, Erdman SE. Beneficial dog bacteria up-regulate oxytocin and lower risk of obesity. J Probiotics Health. 2016b;4:1–9. [Google Scholar]
- Varian BJ, Poutahidis T, Levkovich T, Ibrahim YM, Lakritz JR, Chatzigiagkos A, Scherer-Hoock A, Alm EJ, Erdman SE. Beneficial bacteria stimulate youthful thyroid gland activity. J Obesity Weight Loss Ther. 2014;4:1–8. [Google Scholar]
- Ventevogel MS, Sempowski GD. Thymic rejuvenation and aging. Curr Opin Immunol. 2013;25:516–522. doi: 10.1016/j.coi.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilela FC, Antunes-Rodrigues J, Elias LL, Giusti-Paiva A. Corticosterone synthesis inhibitor metyrapone preserves changes in maternal behavior and neuroendocrine responses during immunological challenge in lactating rats. Neuroendocrinology. 2013;97:322–330. doi: 10.1159/000346354. [DOI] [PubMed] [Google Scholar]
- Vitalo A, Fricchione J, Casali M, Berdichevsky Y, Hoge EA, Rauch SL, Berthiaume F, Yarmush ML, Benson H, Fricchione GL, Levine JB. Nest making and oxytocin comparably promote wound healing in isolation reared rats. PLoS One. 2009;4:e5523. doi: 10.1371/journal.pone.0005523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J, Britton RA, Roos S. Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4645–4652. doi: 10.1073/pnas.1000099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tai F, Yan X, Yu P. Paternal deprivation alters play-fighting, serum corticosterone and the expression of hypothalamic vasopressin and oxytocin in juvenile male mandarin voles. J Comp Physiol A Neuroethol Sens Neural. 2012;198:787–796. doi: 10.1007/s00359-012-0748-8. [DOI] [PubMed] [Google Scholar]
- Wang P, Yang HP, Tian S, Wang L, Wang SC, Zhang F, Wang YF. Oxytocin-secreting system: A major part of the neuroendocrine center regulating immunologic activity. J Neuroimmunol. 2015;289:152–161. doi: 10.1016/j.jneuroim.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Yanai H, Budovsky A, Tacutu R, Fraifeld VE. Is rate of skin wound healing associated with aging or longevity phenotype? Biogerontology. 2011;12:591–597. doi: 10.1007/s10522-011-9343-6. [DOI] [PubMed] [Google Scholar]
- Youn DY, Yoon JS, Kim YK, Yeum CE, Lee SB, Youn HJ, Tsujimoto Y, Lee JH. Deletion of the bis gene results in a marked increase in the production of corticosterone that is associated with thymic atrophy in mice. Am J Physiol Endocrinol Metab. 2011;301:E223–E231. doi: 10.1152/ajpendo.00604.2010. [DOI] [PubMed] [Google Scholar]
- Zhang D, Chen G, Manwani D, Mortha A, Xu C, Faith JJ, Burk RD, Kunisaki Y, Jang JE, Scheiermann C, Merad M, Frenette PS. Neutrophil ageing is regulated by the microbiome. Nature. 2015a;525:528–532. doi: 10.1038/nature15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Motyl KJ, Irwin R, MacDougald OA, Britton RA, McCabe LR. Loss of bone and Wnt10b expression in male type 1 diabetic mice is blocked by the probiotic Lactobacillus reuteri. Endocrinology. 2015b;156:3169–3182. doi: 10.1210/EN.2015-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Burnley P, Coder B, Su DM. Insights on FoxN1 biological significance and usages of the “nude” mouse in studies of T-lymphopoiesis. Int J Biol Sci. 2012;8:1156–1167. doi: 10.7150/ijbs.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimecki M, Artym J, Kocieba M. Endogenous steroids are responsible for lactoferrin-induced myelopoiesis in mice. Pharmacol Rep. 2009;61:705–710. doi: 10.1016/s1734-1140(09)70123-9. [DOI] [PubMed] [Google Scholar]
- Zivkovic IP, Rakin AK, Petrovic-Djergovic DM, Kosec DJ, Micic MV. Exposure to forced swim stress alters morphofunctional characteristics of the rat thymus. J Neuroimmunol. 2005;160:77–86. doi: 10.1016/j.jneuroim.2004.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.