Abstract
Treatment of intracranial aneurysms (IAs) has been revolutionized by the advent of endovascular Flow Diverters (FDs), which disrupt blood flow within the aneurysm to induce pro-thrombotic conditions, and serves as a scaffold for endothelial ingrowth and arterial remodeling. Despite good clinical success of FDs, complications like incomplete occlusion and post-treatment rupture leading to subarachnoid hemorrhage have been reported. In silico computational fluid dynamic analysis of the pre- and post-treated geometries of IA patients can shed light on the contrasting blood hemodynamics associated with different clinical outcomes. In this study, we analyzed hemodynamic modifications in 15 IA patients treated using a single FD; 10 IAs were completely occluded (successful) and 5 were partially occluded (unsuccessful) at 12-month follow-up. An in-house virtual stenting workflow was used to recapitulate the clinical intervention on these cases, followed by CFD to obtain pre- and post-treatment hemodynamics. Bulk hemodynamic parameters showed comparable reductions in both groups with average inflow rate and aneurysmal velocity reduction of 40.3% and 52.4% in successful cases, and 34.4% and 49.2% in unsuccessful cases. There was a substantial reduction in localized parameter like vortex coreline length and Energy Loss for successful cases, 38.2% and 42.9% compared to 10.1% and 10.5% for unsuccessful cases. This suggest that for successfully treated IAs, the localized complex blood flow is disrupted more prominently by the FD as compared to unsuccessful cases. These localized hemodynamic parameters can be potentially used in prediction of treatment outcome, thus aiding the clinicians in a priori assessment of different treatment strategies.
Keywords: intracranial aneurysm, computational fluid dynamics, flow diverter, virtual stenting, device modeling, virtual stenting, endovascular intervention, patient-specific
1. INTRODUCTION
Flow diverters (FDs) - densely woven stent-meshes with high metal coverage and low porosity - have recently emerged as the preferred treatment modality for intracranial aneurysms (IAs), especially for traditionally challenging wide-necked and fusiform IAs. The implantation of a FD redirects the blood flow away from the aneurysm, inducing stagnation of the flow inside the IA sac resulting in thrombosis in the IA. The dense-mesh of the FD also provides a scaffold for facilitating endoluminal reconstruction of the parent artery across the neck of the IA. Despite the success of FDs in treating IAs,[1] undesirable FD related treatment outcomes like delayed occlusion of the IA, late bleeding after FD-implantation, and post-treatment rupture leading to subarachnoid hemorrhage have been reported in the literature.[2–5] Blood flow modifications induced by the implantation of a FD plays a pivotal role in creating the pro-thrombotic environment in the IA, and in the eventual outcome of the treatment. For patients with failed outcomes, we believe that the flow diversion induced by a FD could not enable pro-thrombotic environments in the aneurysmal sac. In silico modeling of blood flow modifications induced by the FD can help gain insights on the different flow mechanisms relating to different treatment outcomes.
Image-based computational fluid dynamic (CFD) analysis on patient-specific IAs can provide detailed understanding of the hemodynamics in IAs. However, in order to obtain post-treatment hemodynamics, the deployment of FDs in patient-specific IAs need to be simulated. We have recently developed an efficient virtual stenting workflow (VSW), [6–8] that can simulate the clinical intervention of FDs in IA geometries. This virtual intervention followed by CFD can provide insights on contrasting blood flow modifications in different clinical outcomes, and identify hemodynamic modification that are associated with pro-thrombotic conditions.
In this study, we used VSW to virtually recapitulate the clinical intervention of patients treated using FDs at our center. CFD simulations were performed to obtain pre- and post-treatment hemodynamics. The pre- and post-treatment hemodynamic parameters were then correlated with the real clinical outcome of these FD-treated IAs.
2. METHODS
2.1 Clinical Data
Patient-specific DICOM images and clinical information was retrospectively collected from the Gates Vascular Institute (Buffalo, NY) for patients that were treated using the commercial FD: Pipeline Embolization Device (PED, Covidien, Irvine, CA). The data collection for this study was approved by the Institutional Review Board of the University at Buffalo. The inclusion criteria for this study was:
IAs treated using a single PED with no prior treatment
Good quality 3D digital subtraction angiographic (DSA) image available for image reconstruction
2D image available for identifying the landing zone of the device
12-month clinical follow-up outcome available.
Based on the above selection criteria, 15 IAs in 13 patients were selected for this study. Ten IAs were completely occluded (successful) and 5 were partially occluded (unsuccessful) at 12-month follow-up.
2.2 Patient-specific Virtual Stenting
3D vessel segmentation of the DSA images was performed in vascular modeling toolkit (vmtk) to obtain the surface of the IA models. We then used the previously mentioned VSW [6–8] to recapitulate the process of FD deployment in each IA geometry. The workflow consists of three steps: (1) Preprocessing: isolation of the parent vessel and generation of a generic simplex mesh structure along the vessel centerline using vessel-specific initialization. (2) Expansion: Radial expansion of the simplex mesh structure using mathematical forces until the mesh apposes with the parent vessel wall. (3) Post-processing: Mapping of the FD pattern on the deployed simplex mesh and sweeping the wires into 3D structures. Details of the expansion algorithm used in VSW can be found in Paliwal et al.[7]
2.3 CFD Simulations
CFD analysis, including volumetric meshing of the flow domain, on the untreated and virtually treated IA geometries was performed using the commercial CFD package STAR-CCM+ v10.02 (CD-adapco, Melville, NY). Polyhedral cells were used to discretize the flow domain into volumetric elements. The base size for the mesh discretization was 0.1 mm, with a resolution of 0.01 mm near the wires of the FD. The Navier–Stokes equations were solved assuming laminar, incompressible, Newtonian blood flow, with a density of 1056 kg/m3 and dynamic viscosity as 0.0035 Pa-s. The vessel walls were assumed rigid, with no-slip conditions. A pulsatile velocity profile obtained by transcranial Doppler in a normal subject was applied for the inflow boundary condition. The simulations were run for 3 cardiac cycle for numerical stability, and the results from the last cardiac cycle were obtained and used as the result of the CFD simulations.
For flow visualization, we used the volume rendering of the time-averaged velocity magnitude in the IA geometry. We also used the vortex corelines and the associated streamlines to capture the presence and strength of the vortex structure inside the IA sac. Quantitatively, we analyzed the following time-averaged hemodynamic parameters before and after FD-placement for each case: (1) Inflow rate (IR): the rate of flow entering the IA from the neck plane. (2) Aneurysm averaged velocity (AAV): the spatial average of velocity magnitude in the IA sac. (3) Vortex coreline length: the sum of lengths of all the vortex corelines in the IA sac. (4) Energy loss (EL) by the FD: the loss in viscous dissipation due to the presence of the FD.
3. RESULTS
3.1 Virtual Deployment of FDs
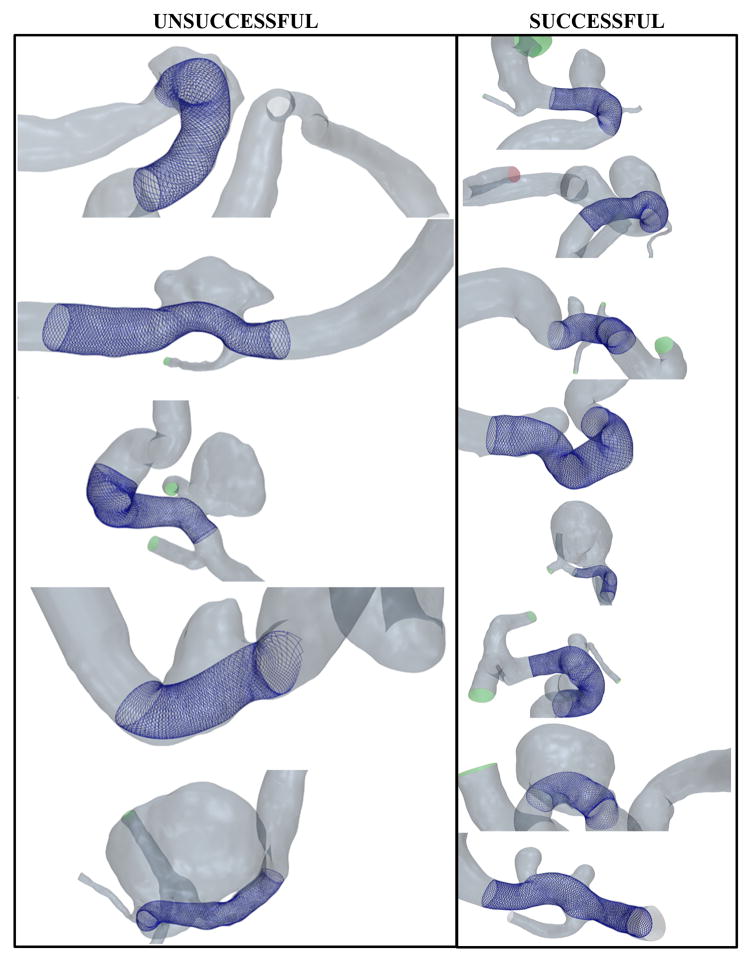
Figure 1 shows the FD deployment results on the unsuccessful and successful clinical IA geometries. The results show a good overall apposition of the FDs along the parent vessel wall in all the cases. For each case, the execution time for VSW was less than a minute.
Figure 1.
Virtually treated IA geometries for the clinical cases with unsuccessful (partially occluded, left) and successful (completely occluded, right) clinical outcomes at 12-month follow-up after FD-treatment.
3.2 Hemodynamic Modifications Induced by the FD
To understand the local hemodynamic changes induced by the FD, we visualized the time-averaged 3D rendering of the velocity magnitude, and vortex corelines (representing the core of the vortex structure) with streamlines around them (representing the strength of the vortex). Figures 2 and 3 show the local hemodynamics before and after FD-treatment in two representative successful and unsuccessful IA cases, along with the clinical images before treatment and at 12-month follow-up.
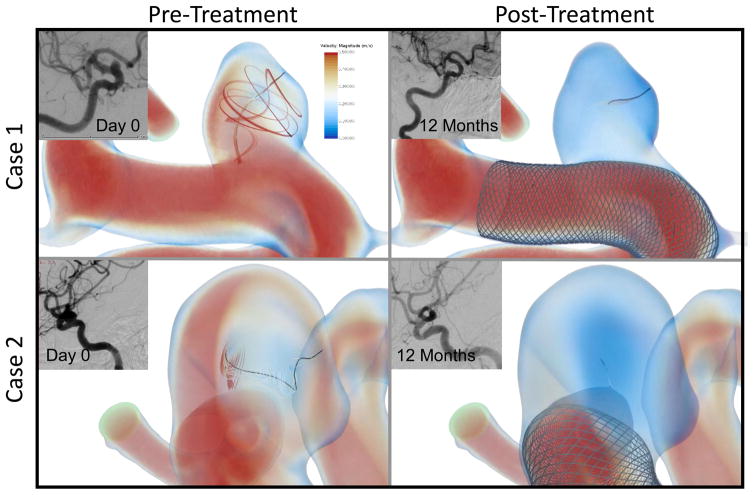
Figure 2.
Pre- and post-treatment velocity magnitude rendering and vortex corelines (black) with associated velocity streamlines for two representative aneurysms with successful outcome. Also shown is the angiographic image of the aneurysms before treatment and at 12-month follow-up showing complete obliteration of the aneurysm at follow-up. The complex vortex structures present in both the aneurysms in the pre-treatment CFD (left), which are diminished after implantation of the FD (right).
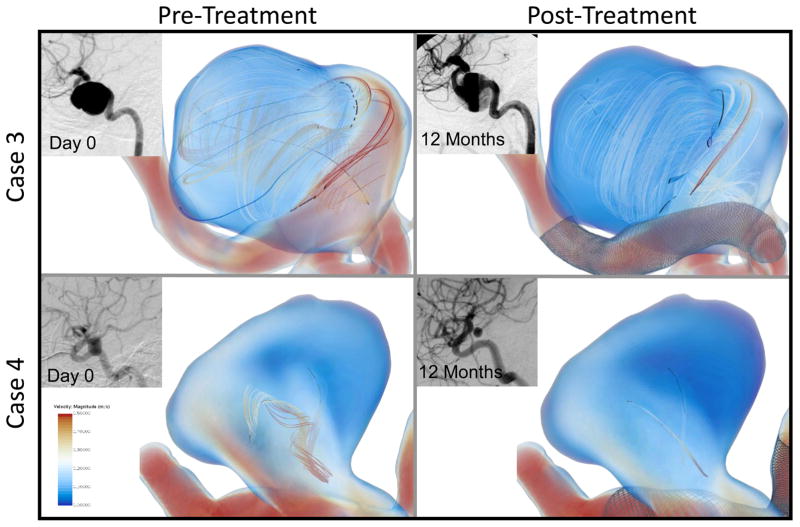
Figure 3.
Pre- and post-treatment velocity magnitude rendering and vortex corelines (black) with associated velocity streamlines for two representative aneurysms with unsuccessful outcome, as seen in the clinical follow up images. For these unsuccessful clinical cases, persisting vortex corelines and the swirling streamlines around them even after the FD placement (right) suggest that the FD was unable to disrupt the complex flow inside the aneurysm, and hence the aneurysm did not occlude.
As seen in Figure 2, the complex flow represented by the vortex corelines was diminished after the placement of a FD. Changes in the volumetric color rendering in the IA sac also shows that the flow was considerable slowed down after the implantation of a FD. The angiographic images show the clinical outcome of patients, showing that the aneurysm was completely obliterated for these two IAs at 12-month follow-up.
Interestingly, for the unsuccessfully treated IAs the FD was unable to reduce the complex flow inside the IA, as shown in Figure 3. The vortex corelines were reduced, and overall flow was lower in the treated IAs, but the vortex corelines were still persistent in both the representative cases. In these cases, as shown in the radiographic images, there was persistent flow in the IAs at 12-month clinical follow-up.
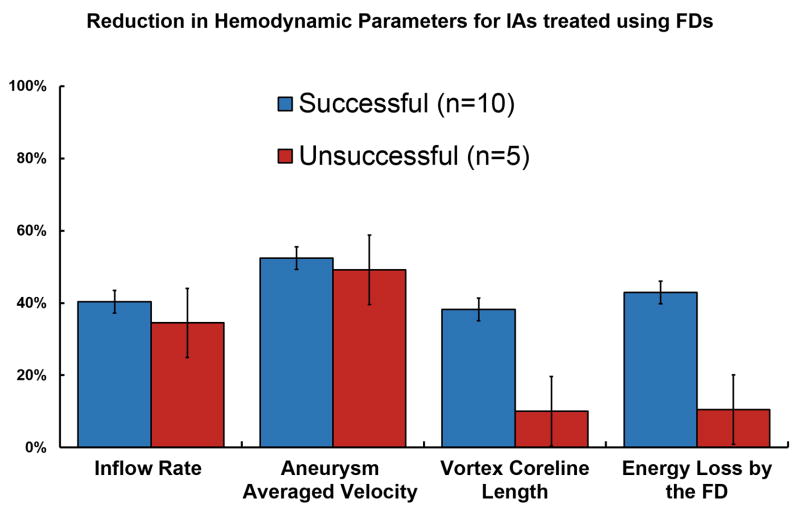
Quantification of the hemodynamic parameters (Figure 4) revealed average reduction in the inflow rate to be 40.3% in successful cases and 34.4% in unsuccessful cases. Aneurysmal average velocity reduced by 52.4% in successful cases, whereas the reduction in the unsuccessful cases was 49.2%.
Figure 4.
Flow reduction in hemodynamic parameters induced by a FD in IAs that occluded within 12 months (blue) versus IAs with incomplete occlusion after 12-month clinical follow-up (red).
There was a substantially larger relative reduction in vortex coreline length and EL for successful cases with reductions of 38.2% and 42.9% respectively as compared to 10.1% and 10.5% reduction in unsuccessful cases.
4. DISCUSSION
4.1 Flow Alterations by the FD and Its Association with Clinical Outcome
The implantation of a FD reduces the blood flow in the aneurysm, which is evident from the reduction in all the hemodynamic parameters after the deployment of a FD (Figure 4). However, the reduction in average bulk hemodynamic parameters like inflow rate and aneurysm average velocity was similar in successful and unsuccessful cases. This suggests that bulk flow parameters cannot differentiate between the differences in the hemodynamics between the two groups. However, a noticeably higher reduction of vortex corelines and EL for successfully treated IAs as compared to the unsuccessful ones suggest that the localized complex flow patterns were substantially dispersed for completely occluded cases, whereas in patent cases the FD was unable to reduce the complex flow inside the aneurysmal sac (e.g. see Figures 2 & 3). The results also suggest that simplifying complex flow, as demonstrated by dispersed vortex corelines in Figure 2 and EL reduction in Figure 4, might have a correlation with the clinical outcome.
This study presented preliminary results of an ongoing study that aims at prospectively predicting outcome of different treatment strategies using flow diverters. We plan to increase the number of patients in our current study and perform statistical analysis to identify hemodynamic parameters that can predict the outcome of IAs treated using FDs. This translational research can potentially aid the clinicians to prospectively test different treatment strategies (using FDs) and perform treatment optimization before actual intervention.
4.2 Potential Clinical Utility of VSW
This study demonstrates first application of VSW on clinical IA cases that were treated using the commercial PED flow diverter. We have validated the deployment and post-implantation results of VSW in a patient-specific IA geometry treated using the neurovascular Enterprise stent.[8] We are currently working towards validating the FD deployment results obtained from VSW against experimental deployment results in IA silicone phantoms, and post-treatment hemodynamic predictions after FD implantation against experimental velocity measurements using particle image velocimetry.
Despite the ongoing validation of VSW, recent studies have extensively used VSW on clinical cases that were treated using traditional neurovascular stents and FDs.[9–16] The broad use of VSW in large number of clinical studies suggests that owing to its high efficiency, VSW can rapidly provide deployed stents and FDs in patient-specific IA geometries, which can provide detailed flow dynamic predictions after stent-implantation in real clinical cases. These hemodynamic predictions can help analyzing and correlating the flow modifications due to the stent-implant with clinical outcome of the patients. We hope that VSW can be integrated in the clinical setting in the future, and provide real-time virtual deployment of various stents and FDs, thus aiding in potential clinical treatment optimization.
5. CONCLUSION
This study presents in silico modeling of the clinical intervention on 15 patients that were treated using a FD to obtain pre- and post-treatment hemodynamics. The results showed that the successfully treated IA cases had higher reductions in hemodynamic parameters; especially in the vortex coreline length and the EL by the FD. The large reductions in these parameters for successful cases suggest that the clinical outcome might be related to the extent of reductions in these local flow parameters. Given a larger number of patient database in future studies, these parameters can be potentially used predict the outcome of IA patients treated using FD, and aid the clinicians in a priori evaluation of different treatment strategies using FDs.
Acknowledgments
This work was supported by the National Institutes of Health (grant number: R01 NS 091075). The authors would like to thank Vincent Tutino for assistance in preparation of figures. We also acknowledge the Center for Computational Research at the University at Buffalo for providing the computational support for CFD simulations.
References
- 1.Becske T, Kallmes DF, Saatci I, Mcdougall CG, Szikora I, Lanzino G, Moran CJ, Woo HH, Lopes DK, Berez AL. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology. 2013;267(3):858–868. doi: 10.1148/radiol.13120099. [DOI] [PubMed] [Google Scholar]
- 2.Cebral JR, Mut F, Raschi M, Scrivano E, Ceratto R, Lylyk P, Putman CM. Aneurysm Rupture Following Treatment with Flow-Diverting Stents: Computational Hemodynamics Analysis of Treatment. American Journal of Neuroradiology. 2011;32(1):27–33. doi: 10.3174/ajnr.A2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siddiqui AH, Abla AA, Kan P, Dumont TM, Jahshan S, Britz GW, Hopkins LN, Levy EI. Panacea or problem: flow diverters in the treatment of symptomatic large or giant fusiform vertebrobasilar aneurysms. Journal of Neurosurgery. 2012;116(6):1258–1266. doi: 10.3171/2012.2.JNS111942. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui AH, Kan P, Abla AA, Hopkins LN, Levy EI. Complications after treatment with pipeline embolization for giant distal intracranial aneurysms with or without coil embolization. Neurosurgery. 2012;71(2):E509–E513. doi: 10.1227/NEU.0b013e318258e1f8. [DOI] [PubMed] [Google Scholar]
- 5.Kulcsár Z, Houdart E, Bonafe A, Parker G, Millar J, Goddard A, Renowden S, Gal G, Turowski B, Mitchell K. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. American Journal of Neuroradiology. 2011;32(1):20–25. doi: 10.3174/ajnr.A2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paliwal N, Yu H, Damiano R, Xiang J, Yang X, Siddiqui A, Li H, Meng H. Fast Virtual Stenting With Vessel-Specific Initialization and Collision Detection. V003T12A014-V003T12A014. [Google Scholar]
- 7.Paliwal N, Yu H, Xu J, Xiang J, Siddiqui AH, Yang X, Li H, Meng H. Virtual stenting workflow with vessel-specific initialization and adaptive expansion for neurovascular stents and flow diverters. Comput Methods Biomech Biomed Engin. 2016:1–9. doi: 10.1080/10255842.2016.1149573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q, Meng Z, Zhang Y, Yao K, Liu J, Zhang Y, Jing L, Yang X, Paliwal N, Meng H. Phantom-based experimental validation of fast virtual deployment of self-expandable stents for cerebral aneurysms. BioMedical Engineering OnLine. 2016;15(2):431. doi: 10.1186/s12938-016-0250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Jing L, Wang C, Paliwal N, Wang S, Zhang Y, Xiang J, Siddiqui AH, Meng H, Yang X. Effect of hemodynamics on outcome of subtotally occluded paraclinoid aneurysms after stent-assisted coil embolization. Journal of neurointerventional surgery, neurintsurg-2015-012050. 2015 doi: 10.1136/neurintsurg-2015-012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Tian Z, Liu J, Jing L, Paliwal N, Wang S, Zhang Y, Xiang J, Siddiqui AH, Meng H. Hemodynamic alterations after stent implantation in 15 cases of intracranial aneurysm. Acta neurochirurgica. 2016:1–9. doi: 10.1007/s00701-015-2696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Tian Z, Liu J, Jing L, Paliwal N, Zhang Y, Xiang J, Siddiqui AH, Meng H, Wang S, Yang X. Hemodynamic Alterations for Various Stent Configurations in Idealized Wide-neck Basilar Tip Aneurysm. Journal of Medical and Biological Engineering. 2016:1–7. [Google Scholar]
- 12.Jing L, Zhong J, Liu J, Yang X, Paliwal N, Meng H, Wang S, Zhang Y. Hemodynamic Effect of Flow Diverter and Coils in Treatment of Large and Giant Intracranial Aneurysms. World neurosurgery. 2016;89:199–207. doi: 10.1016/j.wneu.2016.01.079. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Jing L, Zhang Y, Song Y, Wang Y, Li C, Wang Y, Mu S, Paliwal N, Meng H. Successful Retreatment of Recurrent Intracranial Vertebral Artery Dissecting Aneurysms After Stent-Assisted Coil Embolization: A Self-Controlled Hemodynamic Analysis. World Neurosurgery. 2017;97:344–350. doi: 10.1016/j.wneu.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jing L, Liu J, Zhang Y, Paliwal N, Meng H, Wang S, Yang X. Analysis of Multiple Intracranial Aneurysms with Different Outcomes in the Same Patient After Endovascular Treatment. World neurosurgery. 2016;91:399–408. doi: 10.1016/j.wneu.2016.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C, Tian Z, Liu J, Jing L, Paliwal N, Wang S, Zhang Y, Xiang J, Siddiqui AH, Meng H. Flow diverter effect of LVIS stent on cerebral aneurysm hemodynamics: a comparison with Enterprise stents and the Pipeline device. Journal of Translational Medicine. 2016;14(1):199. doi: 10.1186/s12967-016-0959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Jing L, Zhang Y, Song Y, Wang Y, Li C, Mu S, Paliwal N, Meng H, Linfante I. O-017 Recurrent Intracranial Vertebral Artery Dissecting Aneurysms After Stent-assisted Coil Embolization-A Computational Fluid Dynamic Analysis. Journal of NeuroInterventional Surgery. 2016;8(Suppl 1):A11–A12. [Google Scholar]