Abstract
Angiotensin II receptor blockers (ARBs) are anti-hypertensive drugs that competitively inhibit the binding of angiotensin II to its receptor, resulting in blood vessel dilation and the reduction of blood pressure. These antagonists are also known as sartans, and are a group of pharmaceuticals that possess tetrazole or imidazole groups. In the present study, the anticancer and antimetastatic effects of the ARBs fimasartan, losartan, eprosartan and valsartan on the human prostate cancer PC-3, DU-145 and LNCap-LN3 cell lines were investigated in vitro. The proliferation of the prostate cancer cells was inhibited following treatment with 100 µM ARB. In particular, treatment with fimasartan resulted in marked anti-proliferative activity compared with the other ARBs. With respect to the molecular mechanism of the growth inhibition exhibited by the ARBs, 3-methyladenin (3-MA), an autophagy inhibitor, was revealed to increase the survival rate of PC-3 cells when cell death inhibitors were pretreated with fimasartan. In addition, the ARBs induced autophagy with increased expression levels of autophagy protein (Atg) 5–12, Atg 16-like-1, beclin-1 and microtubule-associated protein 1A/1B-light chain 3 (LC3). Notably, the enhanced expression of LC3-II (a 6.7-fold increase at 72 h) was observed in PC3 cells treated with fimasartan. This was supported by the observation of the time-dependent accumulation of LC3-positive foci in PC-3. In addition, a migration assay indicated that the ARBs induced anti-metastatic effects in PC-3 and DU-145 cells. The aforementioned results suggest that ARBs may induce autophagy-associated cell death and anti-metastatic activity in prostate cancer cells. Thus, ARBs may be a potential medication for patients with prostate cancer and hypertension.
Keywords: angiotensin II receptor blockers, prostate cancer, autophagy, anti-cancer, anti-metastatic
Introduction
As the average life span has increased with the advancement of medicine, there has been an increase in the proportion of elderly people in the population worldwide (1). Despite advances in medical technology, the number of elderly people with cancer has increased, and this population is difficult to treat (2). In addition, elderly people exhibit an elevated incidence of high blood pressure (3), and numerous studies have reported that hypertension is associated with cancer (4–8). For anticancer therapy in this population, angiotensin receptor II blockers (ARBs) are currently being investigated (9–11).
Angiotensin II is the activated form of the protein angiotensin, resulting from the cleavage of angiotensin I by angiotensin-converting enzyme, and promotes the reabsorption of water and sodium ions and the contraction of blood vessels, thereby reducing blood pressure (12). Therefore, the blood pressure of patients with hypertension can be reduced by targeting angiotensin receptor II (13). Angiotensin II receptor antagonists, also known as sartans, are a group of pharmaceuticals that possess tetrazole or imidazole groups, which function as anti-hypertensive drugs (14). Previous studies have indicated that angiotensin II promotes the proliferation and metastasis of tumors (15,16), and that ARBs exhibit antiproliferative and antimetastatic effects on tumors (17–20). In addition, it has been reported that ARBs inhibit the growth of prostate cancer cell lines via suppression of the mitogen-activated protein kinase (MAPK) or signal transducer and activator of transcription 3 (STAT3) phosphorylation (21) and exhibited an antitumor effect on patients with prostate cancer (22–24).
The ultimate aim of cancer therapy is the death of cancer cells, which may be induced by apoptotic or necrotic pathways (25,26). However, cancer cells are able to evade cell death mechanisms (27), therefore a novel approach to target anti-apoptotic mechanisms in cancer is required. Previous reports have indicated that the induction of autophagic signals led to the death of cancer cells, despite autophagy being used as a survival strategy in cells experiencing insufficient supply of nutrients under hypoxic conditions (28–30). The phenomenon is termed autophagy-induced cell death, and is an alternative therapeutic approach to apoptosis-resistant cancer cells.
In the present study, the dose- and time-dependent anticancer effects of commercially available ARBs, and whether the ARBs were able to induce autophagy-induced cell death, were investigated in prostate cancer cells. Furthermore, the inhibitory effect of the ARBs with respect to the migration and proliferation of tumor cells was investigated.
Materials and methods
Reagents
Fimasartan was obtained from Boryung Pharmaceutical Co., Ltd. (Seoul, Korea). Losartan potassium (Merck Sharp & Dohme, Hoddesdon, UK), eprosartan mesylate (Solvay Pharmaceuticals, Weesp, Netherlands) and valsartan (Novartis AG, Basel, Switzerland) were used in the present study. All ARBs were used at concentrations of 100, 200 and 400 µM. The inhibitors 3-methyladenine (3-MA; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), Z-VAD-FMK (Calbiochem, Billerica, MA, USA), a pan-caspase inhibitor, and Necrostatin-1 (Sigma-Aldrich, St Louis, MO, USA) were used to treat the human cancer cell lines to inhibit each type of cell death.
Cell lines and cell culture
The human prostate cancer cell lines PC3 [Korean Cell Line Bank (KCLB) no. 21435], DU145 (KCLB no. 30081) and LNCap-LN3 (KCLB no. 80018) were obtained from the KCLB (Seoul, Korea). A total of 3×105 of each type of prostate cancer cell was plated in 60-mm cell culture dishes (SPL Life Sciences, Pocheon, Korea). Cells were grown in RPMI-1640 medium (GE Healthcare Life Sciences, Logan, UT, USA) and supplemented with 10% heat inactivated fetal bovine serum (FBS; Lonza Group Ltd., Walkersville, MD, USA) and antibiotics (1,000 U/ml penicillin and 1,000 µg/ml streptomycin; both GE Healthcare Life Sciences). The FBS was heat inactivated for 1 h at 56°C. The prostate cancer cells were cultured at 37°C, in a humidified atmosphere containing 5% CO2. To verify the mechanisms of cell death with treatment of ARBs, PC-3 cells were pretreated with 10 mM 3-MA, 50 µM Z-VAD-FMK or 50 µM Necrostatin-1 or 3 h at 37°C prior to fimasartan treatment at a concentration of 200 µM.
Protein extraction and western blotting
The prostate cancer cell lines (3×105 cells per cell line) were treated with 200 µM ARBs for 0, 24, 48, 72 and 96 h at 37°C under 5% CO2. The cells were then harvested and the proteins were extracted, using radioimmunoprecipitation assay buffer containing 10 mM Tris-HCl, 1 mM EDTA, 140 mM NaCl, 0.1% deoxycholate, 0.1% SDS, 100% Triton X-100 and 100X Protease Inhibitor Cocktail Set I (EMD Millipore, Billerica, MA, USA). The protein samples of the cell lysates were quantified using a Bradford assay. In total, 30 µg of each protein samples loaded onto the gel. Each sample was separated by 10–15% SDS-PAGE and transferred onto a 0.45 µm polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA) for 2 h at 100 constant voltage on ice. The membranes were blocked with 5% skimmed milk in TBS-T (TBS and Tween-20) buffer for 1 h at room temperature. The primary antibodies used were anti-LC3B (cat. no. 2775; Cell Signaling Technology, Inc., Danvers, MA, USA), anti-Atg12 (cat. no. 4180; Cell Signaling Technology, Inc.), anti-Atg16L1 (cat. no. 8089; Cell Signaling Technology, Inc.), anti-Beclin-1 (cat. no. 3738; Cell Signaling Technology, Inc.) and anti-β-actin (cat. no. sc-130656; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Anti-rabbit IgG (cat. no. 7074; Cell Signaling Technology, Boston, MA, USA) was used as the secondary antibody. All antibodies were diluted 1,000-fold with blocking buffer. Membranes were incubated with primary antibodies for 3.5 h and with secondary antibody for 2.5 h at room temperature. The membranes were washed 3 times in TBS-T buffer for 10 min. The membranes were developed using an enhanced chemiluminescence western blotting detection system (BD Biosciences, San Hose, CA, USA) according to the protocol of the manufacturer, and exposed to X-ray sheets (Agfa-Gevaert N.V., Mortsel, Belgium). The western blotting results were quantified using densitometry (ImageJ; version 1.48; National Institutes of Health, Bethesda, MD, USA).
Cell proliferation assay
Cell viability in the presence or absence of the ARBs was determined using a Premix WST-1 Cell Proliferation Assay System (Takara Bio, Inc., Otsu, Japan). In total, 1×103 cells (100 µl/well) were incubated in 96-well plates for 24 h at 37°C under 5% CO2. Subsequently, 100, 200 and 400 µM ARBs in RPMI-1640 were added to the wells and cultured at 37°C for 48 and 72 h for PC3 and DU145 cells, and 60 and 120 h for LNCap-LN3 cells. A total of 10 µl WST-1 solution was added to each well, and the plates were incubated for 30 min at 37°C, following which the absorbance at 450 and 690 nm was measured using a microplate reader.
Confocal microscopy
The PC3 human prostate cancer cells were seeded onto glass coverslips (Paul Marienfeld GmbH & Co., KG, Lauda-Königshofen, Germany) at a density of 1×105 cells/well in 6-well plates for 24 h, and were treated with fimasartan (200 µM) for 24, 48 and 72 h at 37°C under 5% CO2. Following all treatment and incubation, the cells were fixed with 4% formaldehyde at room temperature for 15 min. The cells were permeabilized with 0.2% Triton X-100 in PBS for 15–20 min, then blocked with 1% bovine serum albumin (BSA; Sigma-Aldrich; Merck KGaA) in PBS solution for 1 h at room temperature. On the basis of a previous study (30), PC3 cells was incubated with anti-LC3 antibody (cat. no. PM036; Medical & Biological Laboratories Co., Ltd., Nagoya, Japan; 1:500 dilution in 1% BSA and 0.05% Triton X-100 in PBS) for 2 h at room temperature. Subsequent to washing with PBS, cells were treated with anti-rabbit-fluorescein isothiocyanate (cat. no. 111-095-003; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA; 1:500 dilution in 1% BSA, 0.05% Triton X-100 in PBS) for 2 h at room temperature. Each well was washed twice with 0.02% Tween-20 and 1% BSA in PBS. The coverslips were mounted and the cells were examined using confocal microscopy (FV1000; Olympus Corporation, Tokyo, Japan). The results were quantified by densitometry.
Migration assay
A Transwell (6.5 µm pore; Corning Incorporated, Corning, NY, USA) migration assay was performed. Top chambers were seeded with 100 µl RPMI-1640 containing PC3 and DU145 cells (1×104 cells per well) and the bottom chambers were filled with 600 µl RPMI-1640, and the chambers were incubated for 24 h at 37°C under 5% CO2. After 24 h, the bottom chamber was washed with 1xPBS and was filled with 600 µl RPMI-1640 with or without 200 µM fimasartan for 6 h at 37°C. Migrated cells on the bottom side of the membrane were fixed with 4% paraformaldehyde for 30 min at room temperature and washed with PBS twice. Cell migration was evaluated through hematoxylin and eosin staining. The results were measured as the mean number of migrated cells counted using a Motic AE31 optical microscope (Motic, Xiamen, China). Data are expressed as the mean ± standard deviation from 3 independent experiments.
Statistical analysis
All data are presented as the mean ± the standard deviation. Statistical analysis was performed with one-way analysis of variance followed by a Tukey-Kramer multiple comparison test by GraphPad Prism (version 5.0; GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference. Experiments were repeated a minimum of 3 times for each condition.
Results
Reduced cell viability in ARB-treated prostate cancer cell lines
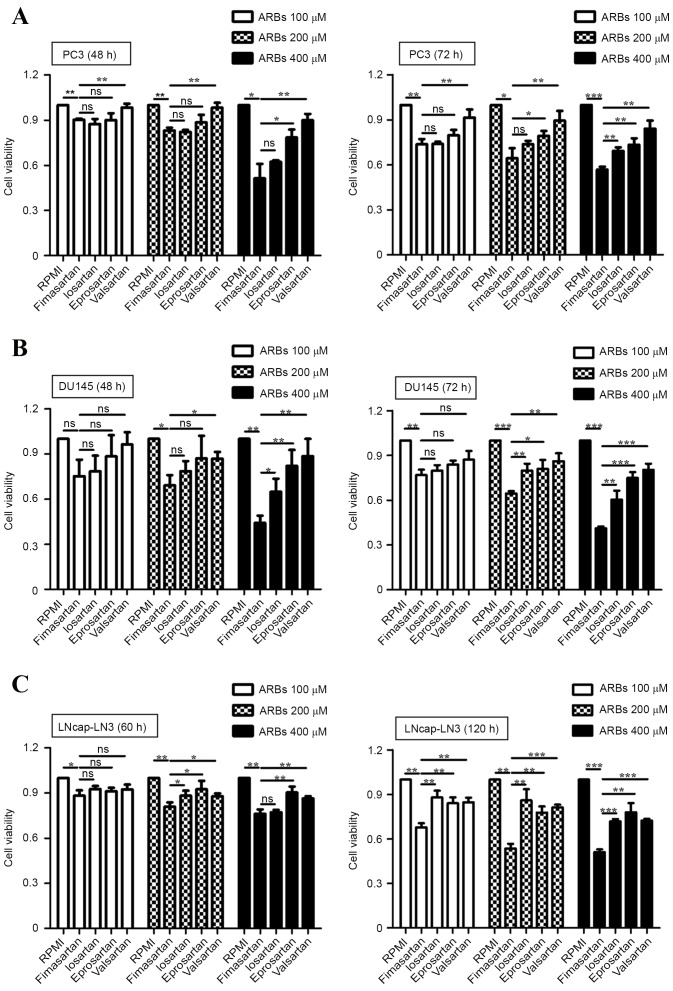
The expression of angiotensin II receptor has been reported in the human prostate cancer cell lines PC3, DU145 and LNCap-LN3 (31). Therefore, the anti-proliferative effects of the ARBs fimasartan, losartar, eprosartan and valsartan were investigated in human prostate cancer cells in the present study (Fig. 1). The ARBs at concentrations 100, 200 and 400 µM were applied to PC3, DU145 and LNCap-LN3 cells, and the cytotoxicity of the ARBs was evaluated by a WST-1 assay. Considering the difference in the doubling time of the cells (PC3, 35 h; DU145, 29 h; and LNCap-LN3, 60 h), cell proliferation was measured at 48 and 72 h in the PC3 and DU145 cells, and at 60 and 120 h in the LNCap-LN3 cells. Compared with the control group, the ARB-treated cells showed reduced cell viability. At 400 µM all the ARBs exerted anti-proliferative effects on prostate cancer cells at each time point, but fimasartan exhibited the greatest cytotoxicity (Fig. 1). Valsartan demonstrated the lowest anti-proliferative activity compared with other ARBs in the prostate cancer cells. LNCap-LN3 had the longest doubling time of the cells investigated, and demonstrated similar cytotoxic effects in response to ARBs to the other cells (Fig. 1C) The present results are consistent with previous studies in which ARBs were demonstrated to inhibit the growth of bladder, breast and gastric cancer (17–20), and fimasartan was observed to exert the greatest anti-proliferative effect on prostate cancer cells.
Figure 1.
Analysis of cell proliferation following treatment of prostate cancer cell lines with ARBs. A WST-1 assay was conducted to analyze cell viability. Cell viability was measured at 48 and 72 h in (A) PC3 and (B) DU145 cells or at 60 and 120 h in (C) LNCap-LN3 cells. Data are expressed as the mean ± standard deviation from 3 experiments with 3 replicates each. *P<0.05, **P<0.01, ***P<0.001. ARB, angiotensin II receptor blocker.
Autophagy-induced cell death in ARB-treated PC3 cells
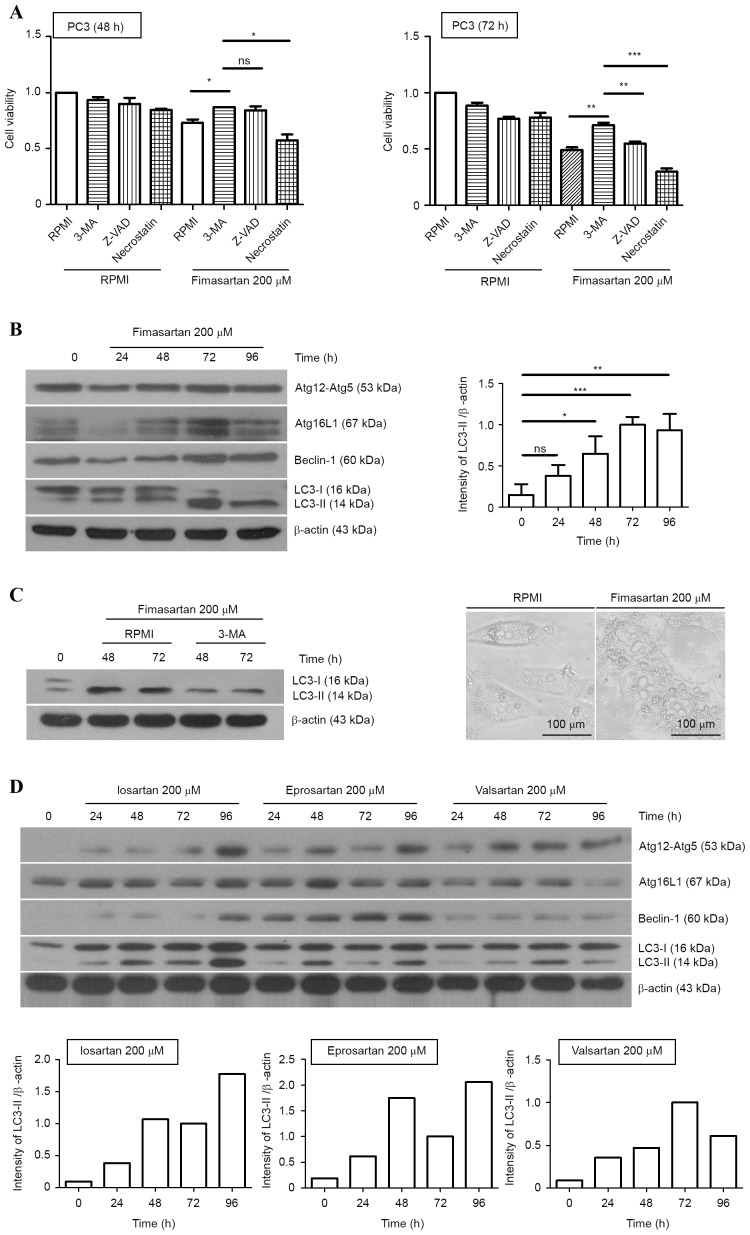
To verify the type of cell death mechanism involved in prostate cancer cells following ARB treatment, specific inhibitors that block each type of cell death were administered to PC-3 cells with fimasartan. Although pan-caspase inhibitor, an apoptosis blocker, slightly reduced ARB-induced cell death at 48 h, the cell survival fraction was restored significantly when the cells were treated with 3-MA, an autophagy inhibitor, at 72 h. Necrostatin-1, a necrosis inhibitor, did not significantly increase the cell viability in fimasartan-treated PC-3 cells. Autophagy-induced cell death, also known as type II programmed cell death, was proposed by Schweichel and Merker (32); it is a cellular suicide process accompanied by the appearance of a giant cytoplasmic vacuole known as the autophagosome. This process may be induced in cancer cells that are resistant to apoptosis due to a deficiency in apoptosis-associated proteins, including B-cell lymphoma-2-like protein 4 and B-cell lymphoma-2 homologous antagonist/killer. Additionally, the inhibition of caspases, which are key apoptosis-associated proteins, is able to prevent cell death (33). Therefore, the induction of autophagic cell death is an alternative death mechanism in apoptosis-resistant cells. LC3, the mammalian homologue of yeast Atg8, is a marker of autophagosome formation. When autophagic signals are activated, a phagophore is formed and LC3-I is conjugated to phosphatidylethanolamine, forming LC3-II, which, with the Atg12-Atg5-Atg16 complex, serves an important role in phagophore elongation. Therefore, the detection of LC3, the Atg12-5 complex, Atg16 and Beclin-1 using western blotting provides suitable markers by which to measure the signaling initiation of autophagy. The PC3 cells were treated with 200 µM fimasartan, and harvested at 0, 24, 48, 72 and 96 h, following which western blotting was performed. This indicated changes in the expression levels of autophagy-associated proteins, with the peak level of LC3-II expression induced at 72 h (6.7-fold increase; Fig. 2B). The inhibitor 3-MA reduced the expression level of converted LC3-II in fimasartan-treated cells (Fig. 2C). In addition, numerous vacuolar compartments were observed in the PC-3 cells subsequent to treatment with fimasartan at 72 h (Fig. 2C). Therefore, fimasartan may be regarded as an autophagy inducer in PC3 human prostate cancer cells. Additionally, treatment with 200 µM losartan, eprosartan and valsartan resulted in alterations in the expression of autophagy-associated proteins in PC3 cells, similar to the result following fimasartan treatment (Fig. 2D). Losartan and eprosartan induced peak levels of LC3-II at 96 h (18.8- and 11.1-fold increases, respectively), and valsartan treatment resulted in the largest increase in the expression of LC3-II at 72 h (11.2-fold increase), measured using densitometry.
Figure 2.
Autophagy-induced cell death in the angiotensin II receptor blocker-treated PC3 cells. (A) PC3 cells were pretreated with 3-MA (10 mM), Z-VAD-FMK (50 µM) or Necrostatin-1 (50 µM) for 3 h. Each group of cells was stimulated by fimasartan (200 µM) and cell viability was analyzed for 48 and 72 h via WST-1 assay. (B) PC3 cells were treated with 200 µM fimasartan, and changes in the expression levels of Atg-12-Atg5, Atg16L1, Beclin-1 and LC-3 were measured using western blot analysis at each time point. (C) The expression levels of LC3-II were significantly reduced with 3-MA pretreatment (left panel). PC3 cells were observed at 72 h subsequent to 200 µM fimasartan treatment under a microscope. (D) PC3 cells were treated with 200 µM losartan, eprosartan or valsartan, and western blot analysis was performed to detect alterations in the expression of autophagy-associated proteins. Densitometry results are presented as the mean ± standard deviation of triplicate experiments. β-actin was used as a loading control. ns, non-significant; *P<0.05, **P<0.01, ***P<0.001. 3-MA, 3-methyladenine; Atg, autophagy protein; LC3, microtubule-associated protein 1A/1B-light chain 3.
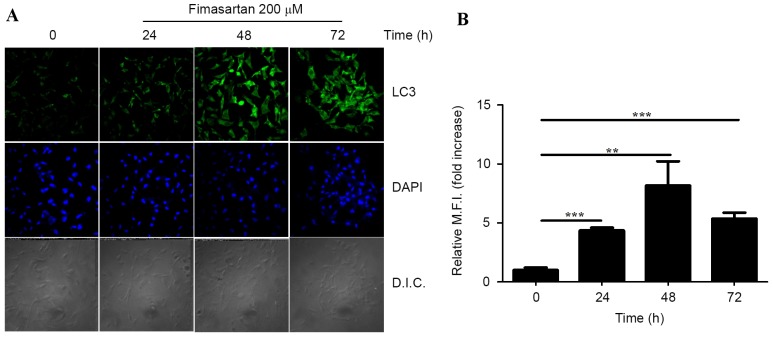
To evaluate whether autophagy was occurring in the PC3 cells following treatment with fimasartan, confocal imaging of immunofluorescent staining was performed. LC3-positive foci were observed scattered throughout the cytoplasm following fimasartan treatment (Fig. 3A). Furthermore, the number of LC3-positive foci increased and the intensity of fluorescein isothiocyanate-fluorescence was enhanced in a time-dependent manner. The results were quantified by measuring the number of cells containing LC3-positive foci compared with the total number of cells (Fig. 3B). Therefore, it appears that ARBs are able to induce autophagy and initiate growth inhibition in human prostate cancer cell lines.
Figure 3.
Formation of LC3-positive foci in the fimasartan-treated PC3 cells. (A) The PC3 cells were treated with 200 µM fimasartan for 24, 48 or 72 h, and endogenous LC3 was detected using an immunofluorescence assay. Cells were stained with DAPI to visualize the nuclei (blue), and immunolabeled with the anti-LC3 antibody, with the addition of fluorescein isothiocyanate-conjugated IgG (green). (B) The quantification for the immunofluorescence images of 3 independent replicates. Values are presented as the mean ± standard deviation. DAPI, 4,6′-diamidino-2-phenylindole; LC3, microtubule-associated protein 1A/1B-light chain 3; D.I.C, disseminated intravascular coagulation; M.F.I, mean fluorescent intensity; **P<0.01, ***P<0.001.
Anti-migratory effect of ARBs
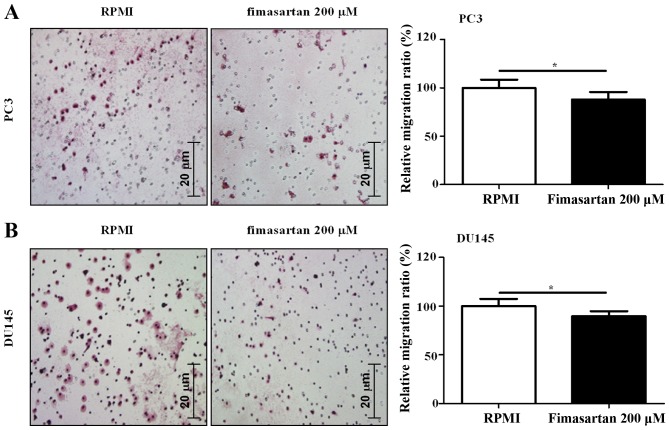
A measurement of the potential of a compound to be used as an anti-cancer agent is whether it exerts anti-migratory effects on cancer cells. Therefore, an experiment was designed to verify anti-migratory activity in PC3 and DU145 cells, using a Transwell assay, to investigate whether fimasartan exerts a suppressive effect on tumor metastasis. The results indicated that treatment with 200 µM fimasartan induced anti-migratory activity in PC3 and DU145 cells (0.88- and 0.90-fold increase, respectively; Fig. 4). Thus, fimasartan may possess the potential to be used as an anti-cancer agent for hypertensive patients with prostate cancer.
Figure 4.
Results of the migration assay. A total of 1×104 (A) PC3 and (B) DU145 cells were seeded in 6-Transwell cell culture plates and treated with 200 µM fimasartan. Following 6 h treatment, a migration assay was performed in addition to hematoxylin and eosin staining. Data are expressed as the mean ± standard deviation from 3 independent experiments. *P<0.05.
Discussion
Multiple signaling pathways are activated in cells stimulated by angiotensin II, including the Janus kinase/signal transducer and activator of transcription Jak2/STAT, MAPK, phosphoinositide 3 kinase/protein kinase B Akt and extracellular growth factor signaling pathways, which may result in cell proliferation, migration and tubulogenesis (34). This suggests that the treatment of cancer cells with ARBs may inhibit the proliferation and migration of tumor cells. The present study indicated that ARBs induce autophagy in prostate cancer cells and may therefore be potential anti-cancer agents. In addition, the present study suggests that ARBs may be used as a therapeutic agent for patients with prostate cancer and hypertension. However, the present study was unable to ascertain whether autophagy is associated with the antiproliferation and antimigration effects of ARBs on prostate cancer cells. The induction of autophagy and inhibition of proliferation by a 5′-adenosine monophospate-activated protein kinase inhibitor, compound C, in human colorectal cancer cells has been reported (35). In addition, compound C has been reported to inhibit DNA-damage regulated autophagy modulator 1 and p62, autophagy-associated factors, and to regulate cell migration and invasion in glioblastoma (36). Furthermore, autophagy was induced in mice injected with mitoxantrone leading to an anticancer immune response (37). The aforementioned studies suggest that ARBs may be potential antitumoral agents via alterations in the autophagic process. The results of the present study indicated that ARBs increased autophagy in prostate cancer cell lines, supported by the increased expression levels of autophagy-associated genes and observation of LC3-positive foci by confocal microscopy. In addition, fimasartan exhibited superior antitumor activity compared with losartan, eprosartan and valsartan. Low concentrations (0.1, 1 and 10 µM) of ARBs did not exhibit antitumor effects in prostate cancer cells in a preliminary study (data not shown), therefore higher doses (100, 200 and 400 µM) of ARBs were used in the present study. These concentrations were suitable for investigation, as previous studies have not reported side effects in patients with hypertension prescribed similar high doses of ARBs (38,39).
Considering the presence of other cells in the tumor microenvironment in addition to the tumor cells, the degree of influence of ARB treatment on cancer accessory cells, including immune cells, should be considered. During tumor formation, circulating monocytes infiltrate tumor tissue and polarize into tumor-associated macrophages that express beneficial factors for the tumor, including vascular endothelial growth factor and arginase 1 (40). Therefore, it is important to consider the effect of ARBs on the macrophages present within the tumor tissue. Angiotensin II is a key mediator of fibrosis in cardiac fibrosis, and enhances the infiltration of macrophages into tissues and the subsequent polarization of the cells into the M2 phenotype via serum-glucocorticoid-regulated kinase 1 (41). As ARBs exhibited potential in terms of weakening prostate cancer in clinical trials (22–24), it may be suggested that the polarizing of tumor-associated macrophages from monocytes may be inhibited by ARB treatment. Therefore, the results of this study indicate that ARBs possess the potential to be a novel therapeutic agent for patients with prostate cancer and high blood pressure, since ARBs induce autophagy and lead to anticancer effects in prostate cancer cells.
Acknowledgements
The present study was supported by a National Research Foundation of Korea grant funded by the Ministry of Education, Science and Technology (grant no. 2012R1A1A2006349) and Ministry of Science, ICT and Future Planning (grant nos. 2014M2B2A9030381 and 2015M2B2A6028602). Funding was also provided by a 2015 (C1011746-01-01) and 2016 Research Grant from Kangwon National University.
References
- 1.Daniels N. Global aging and the allocation of health care across the life span. Am J Bioeth. 2013;13:1–2. doi: 10.1080/15265161.2013.807187. [DOI] [PubMed] [Google Scholar]
- 2.Orom H, Penner LA, West BT, Downs TM, Rayford W, Underwood W. Personality predicts prostate cancer treatment decision-making difficulty and satisfaction. Psychooncology. 2009;18:290–299. doi: 10.1002/pon.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guxens M, Fitó M, Martínez-González MA, Salas-Salvadó J, Estruch R, Vinyoles E, Fiol M, Corella D, Arós F, Gómez-Gracia E, et al. Hypertensive status and lipoprotein oxidation in an elderly population at high cardiovascular risk. Am J Hypertens. 2009;22:68–73. doi: 10.1038/ajh.2008.313. [DOI] [PubMed] [Google Scholar]
- 4.Lin GM, Liu PY, Wu CF, Wang WB, Han CL. Carvedilol use and specific cancer risk in the population with hypertension. Int J Cardiol. 2015;186:52–53. doi: 10.1016/j.ijcard.2015.03.266. [DOI] [PubMed] [Google Scholar]
- 5.Mouhayar E, Salahudeen A. Hypertension in cancer patients. Tex Heart Inst J. 2011;38:263–265. [PMC free article] [PubMed] [Google Scholar]
- 6.Valcamonico F, Arcangeli G, Consoli F, Nonnis D, Grisanti S, Gatti E, Berruti A, Ferrari V. Idiopathic intracranial hypertension: A possible complication in the natural history of advanced prostate cancer. Int J Urol. 2014;21:335–337. doi: 10.1111/iju.12273. [DOI] [PubMed] [Google Scholar]
- 7.Tahover E, Uziely B, Salah A, Temper M, Peretz T, Hubert A. Hypertension as a predictive biomarker in bevacizumab treatment for colorectal cancer patients. Med Oncol. 2013;30:327. doi: 10.1007/s12032-012-0327-4. [DOI] [PubMed] [Google Scholar]
- 8.Pant S, Martin LK, Geyer S, Wei L, Van Loon K, Sommovilla N, Zalupski M, Iyer R, Fogelman D, Ko AH, Bekaii-Saab T. Treatment-related hypertension as a pharmacodynamic biomarker for the efficacy of bevacizumab in advanced pancreas cancer: A pooled analysis of 4 prospective trials of gemcitabine-based therapy with bevacizumab. Am J Clin Oncol. 2016;39:614–618. doi: 10.1097/COC.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Liu J, Chen J, Li X, Wu Y, Chen H, Wu W, Zhang K, Gu L. Angiotensin receptor blockers (ARBs) reduce the risk of lung cancer: A systematic review and meta-analysis. Int J Clin Exp Med. 2015;8:12656–12660. [PMC free article] [PubMed] [Google Scholar]
- 10.Chae YK, Valsecchi ME, Kim J, Bianchi AL, Khemasuwan D, Desai A, Tester W. Reduced risk of breast cancer recurrence in patients using ACE inhibitors, ARBs, and/or statins. Cancer Invest. 2011;29:585–593. doi: 10.3109/07357907.2011.616252. [DOI] [PubMed] [Google Scholar]
- 11.Mann SJ, Christos PJ. ACE inhibitors and ARBs: Do they reduce the risk of cancer? J Clin Hypertens (Greenwich) 2014;16:6–7. doi: 10.1111/jch.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigmund CD. Structural biology: On stress and pressure. Nature. 2010;468:46–47. doi: 10.1038/468046a. [DOI] [PubMed] [Google Scholar]
- 13.Asmar R. Targeting effective blood pressure control with angiotensin receptor blockers. Int J Clin Pract. 2006;60:315–320. doi: 10.1111/j.1368-5031.2006.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Zwieten PA. Angiotensin II receptor antagonists (AT1-blockers, ARBs, sartans): Similarities and differences. Neth Heart J. 2006;14:381–387. [PMC free article] [PubMed] [Google Scholar]
- 15.Imai N, Hashimoto T, Kihara M, Yoshida S, Kawana I, Yazawa T, Kitamura H, Umemura S. Roles for host and tumor angiotensin II type 1 receptor in tumor growth and tumor-associated angiogenesis. Lab Invest. 2007;87:189–198. doi: 10.1038/labinvest.3700504. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues-Ferreira S, Abdelkarim M, Dillenburg-Pilla P, Luissint AC, di-Tommaso A, Deshayes F, Pontes CL, Molina A, Cagnard N, Letourneur F, et al. Angiotensin II facilitates breast cancer cell migration and metastasis. PLoS One. 2012;7:e35667. doi: 10.1371/journal.pone.0035667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosugi M, Miyajima A, Kikuchi E, Horiguchi Y, Murai M. Angiotensin II type 1 receptor antagonist candesartan as an angiogenic inhibitor in a xenograft model of bladder cancer. Clin Cancer Res. 2006;12:2888–2893. doi: 10.1158/1078-0432.CCR-05-2213. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues-Ferreira S, Morel M, Reis RI, Cormier F, Baud V, Costa-Neto CM, Nahmias C. A novel cellular model to study angiotensin II AT2 receptor function in breast cancer cells. Int J Pept. 2012;2012:745027. doi: 10.1155/2012/745027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carl-McGrath S, Ebert MP, Lendeckel U, Röcken C. Expression of the local angiotensin II system in gastric cancer may facilitate lymphatic invasion and nodal spread. Cancer Biol Ther. 2007;6:1218–1226. doi: 10.4161/cbt.6.8.4412. [DOI] [PubMed] [Google Scholar]
- 20.Matsuyama M, Funao K, Kuratsukuri K, Tanaka T, Kawahito Y, Sano H, Chargui J, Touraine JL, Yoshimura N, Yoshimura R. Telmisartan inhibits human urological cancer cell growth through early apoptosis. Exp Ther Med. 2010;1:301–306. doi: 10.3892/etm_00000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uemura H, Ishiguro H, Nakaigawa N, Nagashima Y, Miyoshi Y, Fujinami K, Sakaguchi A, Kubota Y. Angiotensin II receptor blocker shows antiproliferative activity in prostate cancer cells: A possibility of tyrosine kinase inhibitor of growth factor. Mol Cancer Ther. 2003;2:1139–1147. [PubMed] [Google Scholar]
- 22.Uemura H, Hoshino K, Kubota Y. Role of renin-angiotensin system and antitumor effect of ARB in prostate cancer. Nihon Rinsho. 2011;69:S155–S159. (Suppl 5) (In Japanese) [PubMed] [Google Scholar]
- 23.Uemura H, Kubota Y. Application of angiotensin II receptor blocker in prostate cancer. Nihon Rinsho. 2009;67:807–811. (In Japanese) [PubMed] [Google Scholar]
- 24.Funao K, Matsuyama M, Kawahito Y, Sano H, Chargui J, Touraine JL, Nakatani T, Yoshimura R. Telmisartan is a potent target for prevention and treatment in human prostate cancer. Oncol Rep. 2008;20:295–300. [PubMed] [Google Scholar]
- 25.Burikhanov R, Zhao Y, Goswami A, Qiu S, Schwarze SR, Rangnekar VM. The tumor suppressor Par-4 activates an extrinsic pathway for apoptosis. Cell. 2009;138:377–388. doi: 10.1016/j.cell.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer. 2004;4:592–603. doi: 10.1038/nrc1412. [DOI] [PubMed] [Google Scholar]
- 27.Kelly GL, Strasser A. The essential role of evasion from cell death in cancer. Adv Cancer Res. 2011;111:39–96. doi: 10.1016/B978-0-12-385524-4.00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 29.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang SJ, Tak JH, Cho JH, Lee HJ, Jung YJ. Stimulation of the endosomal TLR pathway enhances autophagy-induced cell death in radiotherapy of breast cancer. Genes & Genomics. 2010;32:599–606. doi: 10.1007/s13258-010-0139-x. [DOI] [Google Scholar]
- 31.Bose SK, Gibson W, Giri S, Nath N, Donald CD. Angiotensin II up-regulates PAX2 oncogene expression and activity in prostate cancer via the angiotensin II type I receptor. Prostate. 2009;69:1334–1342. doi: 10.1002/pros.20980. [DOI] [PubMed] [Google Scholar]
- 32.Schweichel JU, Merker HJ. The morphology of various types of cell death in prenatal tissues. Teratology. 1973;7:253–266. doi: 10.1002/tera.1420070306. [DOI] [PubMed] [Google Scholar]
- 33.Kroemer G, Levine B. Autophagic cell death: The story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yosypiv IV, Schroeder M, El-Dahr SS. Angiotensin II type 1 receptor-EGF receptor cross-talk regulates ureteric bud branching morphogenesis. J Am Soc Nephrol. 2006;17:1005–1014. doi: 10.1681/ASN.2005080803. [DOI] [PubMed] [Google Scholar]
- 35.Yang WL, Perillo W, Liou D, Marambaud P, Wang P. AMPK inhibitor compound C suppresses cell proliferation by induction of apoptosis and autophagy in human colorectal cancer cells. J Surg Oncol. 2012;106:680–688. doi: 10.1002/jso.23184. [DOI] [PubMed] [Google Scholar]
- 36.Galavotti S, Bartesaghi S, Faccenda D, Shaked-Rabi M, Sanzone S, McEvoy A, Dinsdale D, Condorelli F, Brandner S, Campanella M, et al. The autophagy-associated factors DRAM1 and p62 regulate cell migration and invasion in glioblastoma stem cells. Oncogene. 2013;32:699–712. doi: 10.1038/onc.2012.111. [DOI] [PubMed] [Google Scholar]
- 37.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 38.Konstam MA, Neaton JD, Dickstein K, Drexler H, Komajda M, Martinez FA, Riegger GA, Malbecq W, Smith RD, Guptha S, et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): A randomised, double-blind trial. Lancet. 2009;374:1840–1848. doi: 10.1016/S0140-6736(09)61913-9. [DOI] [PubMed] [Google Scholar]
- 39.Abe M, Okada K, Maruyama T, Matsumoto S, Matsumoto K. Blood pressure-lowering and antiproteinuric effect of switching from high-dose angiotensin receptor blockers to normal-dose telmisartan and low-dose hydrochlorothiazide in hypertensive patients with chronic kidney disease. Int J Clin Pharmacol Ther. 2010;48:206–213. doi: 10.5414/CPP48206. [DOI] [PubMed] [Google Scholar]
- 40.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang M, Zheng J, Miao Y, Wang Y, Cui W, Guo J, Qiu S, Han Y, Jia L, Li H, et al. Serum-glucocorticoid regulated kinase 1 regulates alternatively activated macrophage polarization contributing to angiotensin II-induced inflammation and cardiac fibrosis. Arterioscler Thromb Vasc Biol. 2012;32:1675–1686. doi: 10.1161/ATVBAHA.112.248732. [DOI] [PubMed] [Google Scholar]