Abstract
Preservation of photosynthetic activities (photophosphorylation, electron transport, fluorescence induction, 0.3-second delayed light emission) of isolated broken (class C) chloroplasts by low temperature storage was investigated under a wide range of conditions in order to optimize long time activity retention.
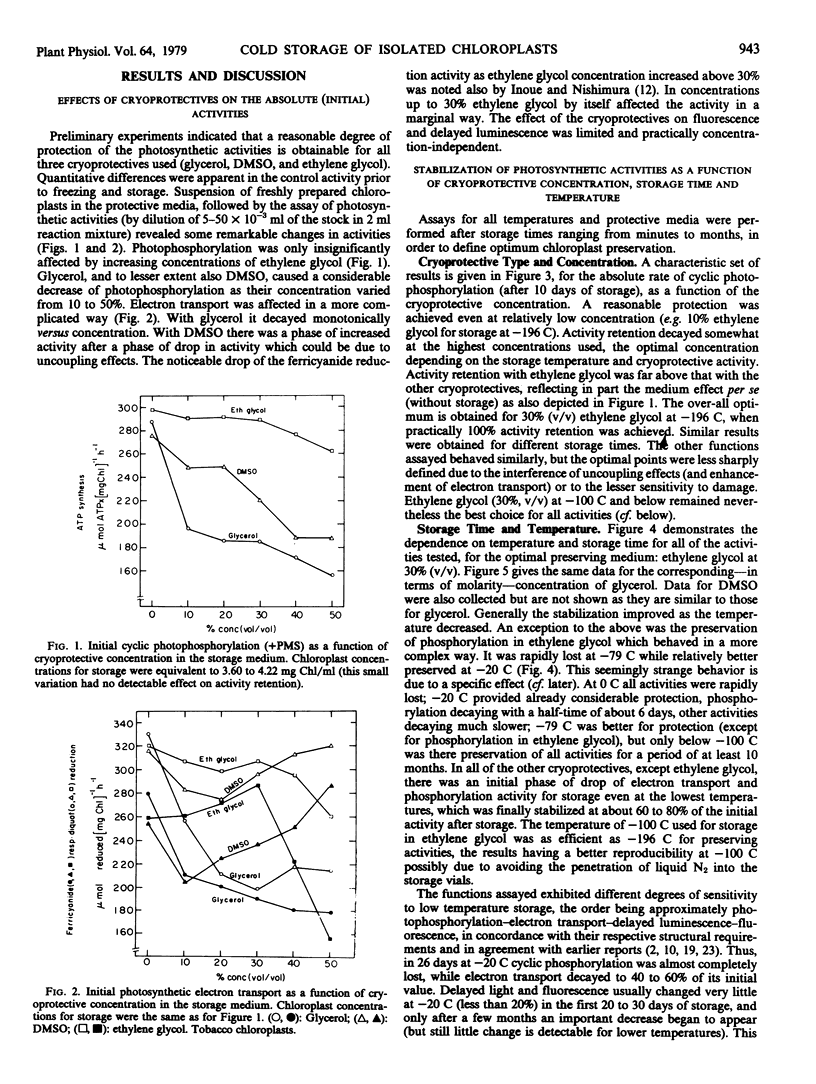
The more labile functions (photophosphorylation and electron transport) required very low temperatures (below −79 C) and relatively high (above 20%, v/v) concentrations of cryoprotectives for satisfactory stabilization. Fluorescence induction and delayed light emission were less sensitive, especially during the 1st month of storage.
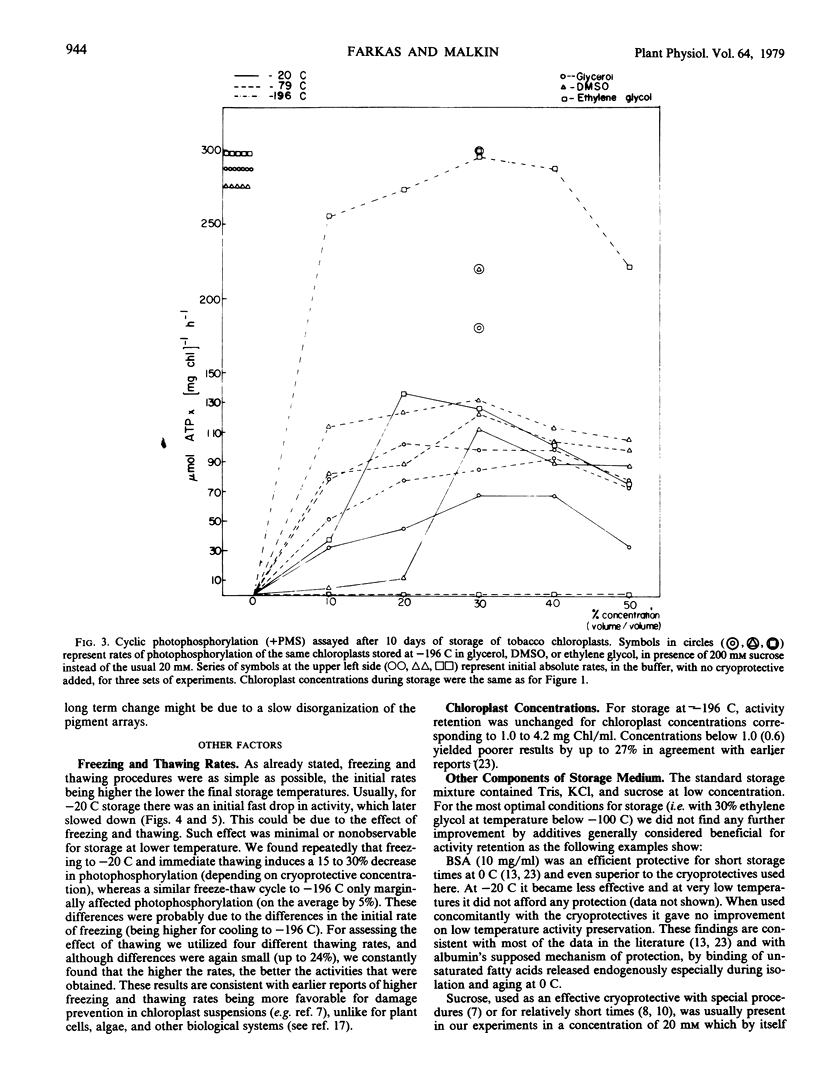
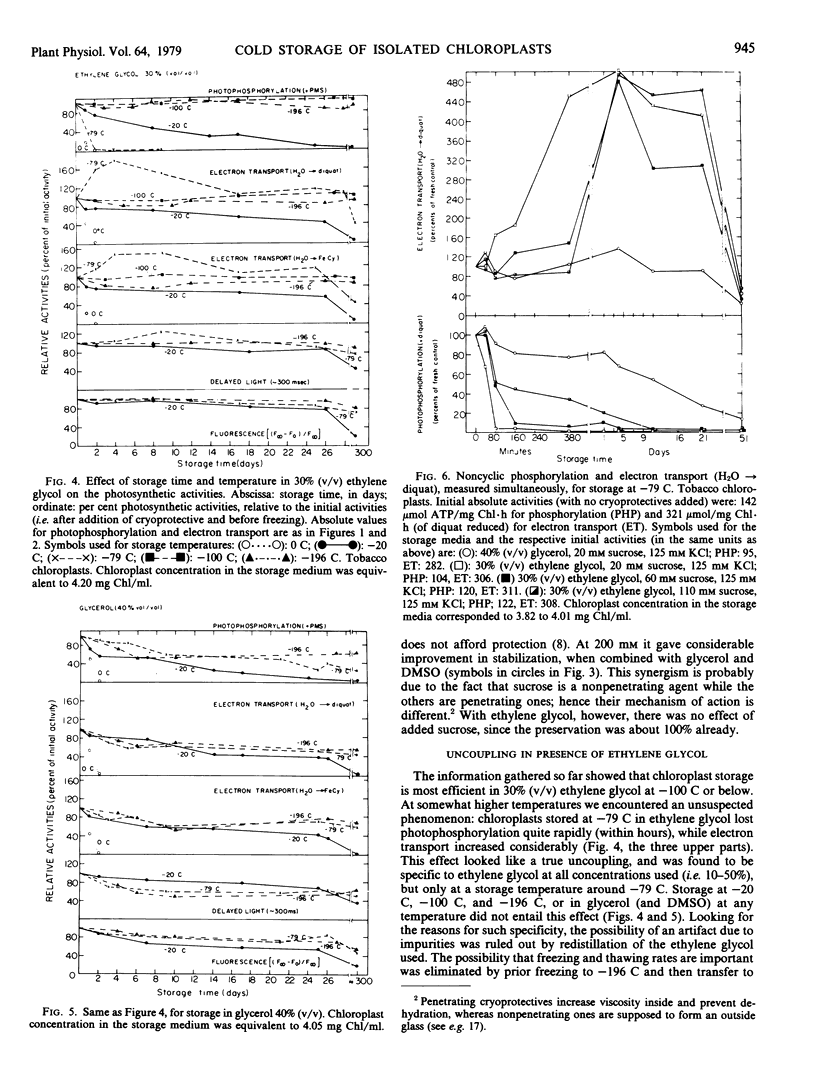
Taking into account the effect of cryoprotectives on absolute activities prior to freezing, optimum activity retention was observed with a medium containing ethylene glycol (30%, v/v) and a storage temperature of −100 C or below. In this case, given fast thawing and high chloroplast concentration, practically 100% preservation of all of the photosynthetic activities investigated was obtained for at least 10 months, even with very simple freezing and storage procedures.
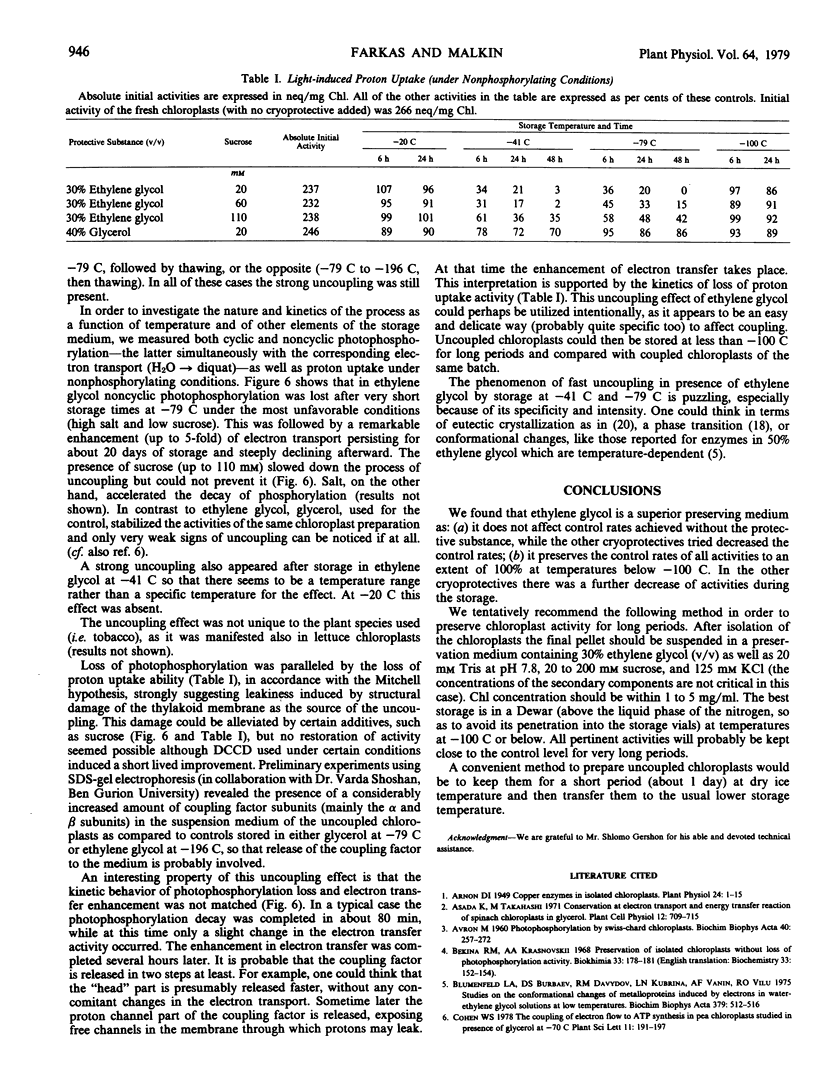
The same optimal medium at somewhat higher temperatures (−79 C and to a lesser extent at −41 C) caused a dramatic uncoupling effect: photophosphorylation was inhibited in a few hours, while electron transport increased 3- to 5-fold. The enhanced electron transport was stable for almost a month and then declined sharply. This uncoupling effect was specific only to ethylene glycol.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld L. A., Burbaev D. S., Davydov R. M., Kubrina L. N., Vanin A. F., Vilu R. O. Studies on the conformational changes of metalloproteins induced by electrons in water-ethylene glycol solutions at low temperatures. III. Adrenal ferredoxin. Biochim Biophys Acta. 1975 Feb 27;379(2):512–516. doi: 10.1016/0005-2795(75)90158-0. [DOI] [PubMed] [Google Scholar]
- Garber M. P., Steponkus P. L. Alterations in Chloroplast Thylakoids during an in Vitro Freeze-Thaw Cycle. Plant Physiol. 1976 May;57(5):673–680. doi: 10.1104/pp.57.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. Freezing injury and uncoupling of phosphorylation from electron transport in chloroplasts. Plant Physiol. 1967 Oct;42(10):1343–1350. doi: 10.1104/pp.42.10.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Tyankova L., Santarius K. A. Effects of freezing on biological membranes in vivo and in vitro. Biochim Biophys Acta. 1973 Jan 2;291(1):23–37. doi: 10.1016/0005-2736(73)90057-6. [DOI] [PubMed] [Google Scholar]
- Malkin S., Kok B. Fluorescence induction studies in isolated chloroplasts. I. Number of components involved in the reaction and quantum yields. Biochim Biophys Acta. 1966 Nov 8;126(3):413–432. doi: 10.1016/0926-6585(66)90001-x. [DOI] [PubMed] [Google Scholar]
- Packer L., Barnard A. C. Preservation of photophosphorylation in glycerinated chloroplasts. Biochim Biophys Acta. 1966 Nov 8;126(3):443–448. doi: 10.1016/0926-6585(66)90003-3. [DOI] [PubMed] [Google Scholar]
- Santarius K. A. Freezing. The effect of eutectic crystallization on biological membranes. Biochim Biophys Acta. 1973 Jan 2;291(1):38–50. doi: 10.1016/0005-2736(73)90058-8. [DOI] [PubMed] [Google Scholar]
- Tel-or E., Malkin S. The photochemical and fluorescence properties of whole cells, spheroplasts and spheroplast particles from the blue-green alga Phormidium luridum. Biochim Biophys Acta. 1977 Feb 7;459(2):157–174. doi: 10.1016/0005-2728(77)90019-6. [DOI] [PubMed] [Google Scholar]
- Varfolomeev S. D., Zaitsev S. V., Il'ina M. D., Berezin I. V. Kinetika i mekhanizm inaktivatsii izolirovannykh khloroplastov. Mol Biol (Mosk) 1975 Nov-Dec;9(6):893–902. [PubMed] [Google Scholar]
- Wasserman A. R., Fleischer S. The stabilization of chloroplast function. Biochim Biophys Acta. 1968 Jan 15;153(1):154–169. doi: 10.1016/0005-2728(68)90156-4. [DOI] [PubMed] [Google Scholar]


