Abstract
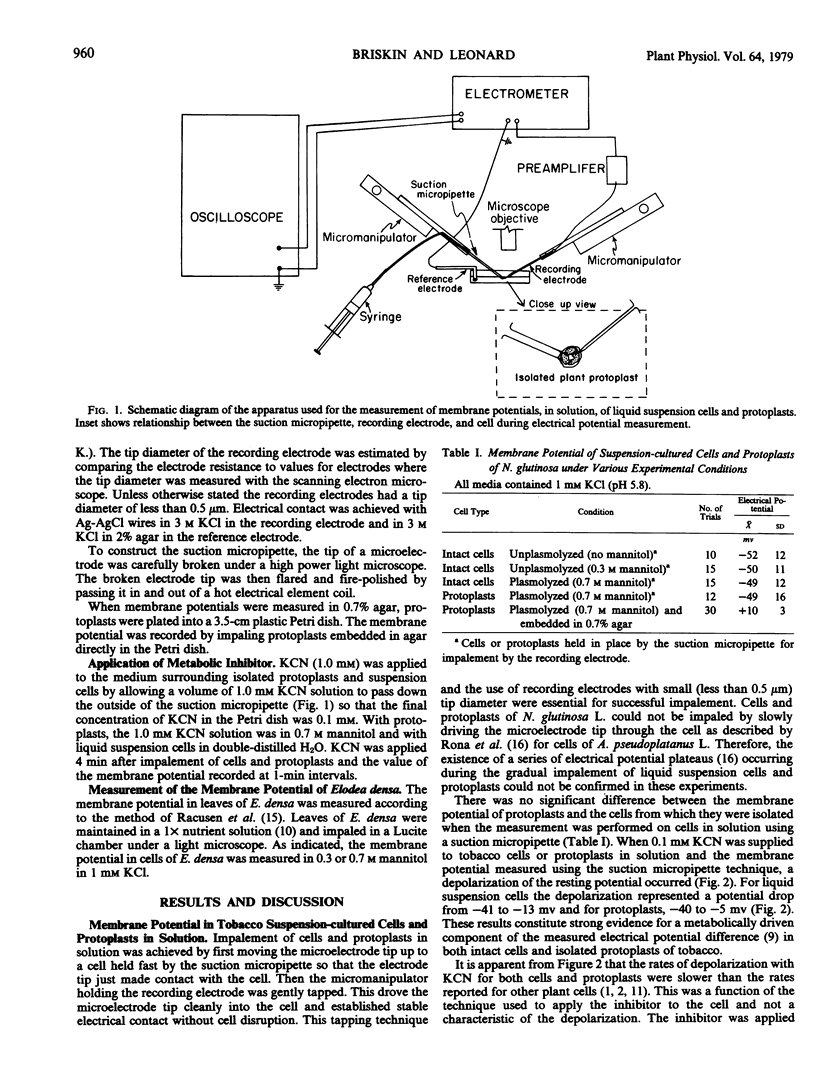
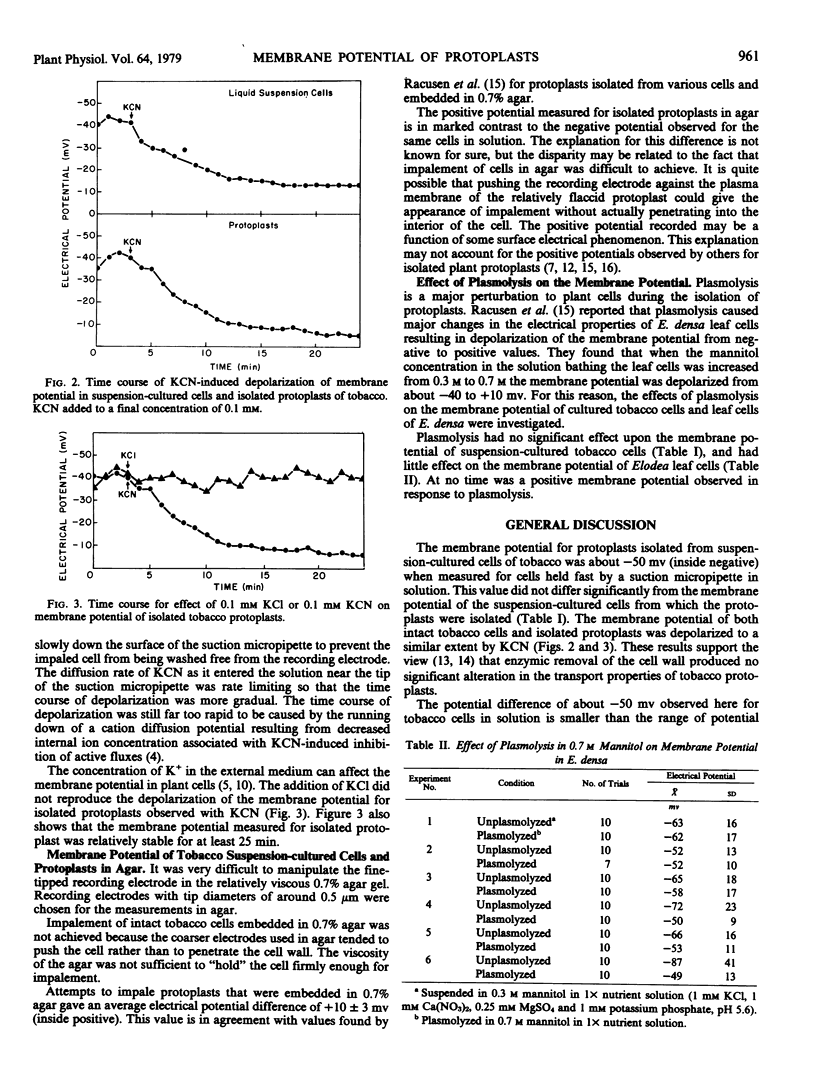
The membrane electrical potential difference was measured in cultured cells and isolated protoplasts of tobacco (Nicotiana glutinosa L.) by inserting a microelectrode into cells held fast by a suction micropipette. The potential difference (± standard deviation) for unplasmolyzed tobacco cells was −52 ± 12 millivolts, for cells in 0.3 molar mannitol, −50 ± 11 millivolts; and for cells plasmolyzed in 0.7 molar mannitol, −49 ± 12 millivolts all inside negative. The potential difference for isolated protoplasts in 0.7 molar mannitol was −49 ± 16 millivolts, inside negative. In both cultured cells and protoplasts, the addition of 0.1 millimolar KCN caused a depolarization of the membrane potential. It was concluded that plasmolysis and enzymic release of the protoplast had no significant effect on the membrane potential of cultured tobacco cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. P., Hendrix D. L., Higinbotham N. The effect of cyanide and carbon monoxide on the electrical potential and resistance of cell membranes. Plant Physiol. 1974 Nov;54(5):712–716. doi: 10.1104/pp.54.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber J. Measurement of the membrane potential and evidence for active transport of ions in Chlorella pyrenoidosa. Biochim Biophys Acta. 1968 Jun 11;150(4):618–625. doi: 10.1016/0005-2736(68)90051-5. [DOI] [PubMed] [Google Scholar]
- Contardi P. J., Davis R. F. Membrane Potential in Phaeoceros laevis: Effects of Anoxia, External Ions, Light, and Inhibitors. Plant Physiol. 1978 Feb;61(2):164–169. doi: 10.1104/pp.61.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ETHERTON B., HIGINBOTHAM N. Transmembrane potential measurements of cells of higher plants as related to salt uptake. Science. 1960 Feb 12;131(3398):409–410. doi: 10.1126/science.131.3398.409. [DOI] [PubMed] [Google Scholar]
- Higinbotham N., Etherton B., Foster R. J. Effect of External K, NH(4), Na, Ca, Mg, and H Ions on the Cell Transmembrane Electropotential of Avena Coleoptile. Plant Physiol. 1964 Mar;39(2):196–203. doi: 10.1104/pp.39.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler I. J., Leonard R. T. Ion Transport in Isolated Protoplasts from Tobacco Suspension Cells: II. Selectivity and Kinetics. Plant Physiol. 1979 Jan;63(1):191–194. doi: 10.1104/pp.63.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler I. J., Leonard R. T. Ion transport in isolated protoplasts from tobacco suspension cells: I. General characteristics. Plant Physiol. 1979 Jan;63(1):183–190. doi: 10.1104/pp.63.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racusen R. H., Kinnersley A. M., Galston A. W. Osmotically induced changes in electrical properties of plant protoplast membranes. Science. 1977 Oct 28;198(4315):405–407. doi: 10.1126/science.198.4315.405. [DOI] [PubMed] [Google Scholar]


