Abstract
Introduction:
The aim of this study was to evaluate the antimicrobial effect of Eucalyptus galbie and Myrtus communis L. methanolic extracts, chlorhexidine (CHX) and sodium hypochlorite (NaOCl) on Enterococcus faecalis (E. faecalis) as the predominant species isolated from infected root canals.
Methods and Materials:
One hundred twenty mandibular premolars were randomly divided into 8 groups: Eucalyptus galbie (E. galbie) 12.5 mg/mL, Myrtus communis L. (M. communis L.) 6.25 mg/mL, 0.2% CHX, %2 CHX, 2.5% NaOCl, 5.25% NaOCl, positive and negative control group. Sampling was performed using paper points (from the root canal space lumen) and Gates-Glidden drills (from the dentinal tubules); then colony forming units (CFU) were counted and analyzed using the Kruskal-Wallis test, followed by Mann Whitney U test. The level of significance was set at 0.05.
Results:
All irrigants reduced more than 99% of bacteria in root canal. In the presence of M. communis L. and E. galbie, the bacterial count in dentin were significantly more than CHX and NaOCl groups (P<0.05) except 0.2% CHX in 200 µm and 400 µm depths (P>0.05).
Conclusion:
Although 5.25% NaOCl was the most effective irrigant, all agents exerted acceptable antimicrobial activity against E. faecalis.
Key Words: Antibacterial Agent, Eucalyptus, Myrtus, Root Canal Therapy
Introduction
Bacteria and their byproducts have key role in the initiation and progression of pulpal and periapical diseases [1]. Root canal morphology is a complex system with fins, anastomoses and lateral or accessory canals which harbor microorganisms. These regions are difficult to clean mechanically and not easily accessible to antibacterial solutions. Microorganisms are also present within dentinal tubules. The infected dentin might potentially contribute in the development of persistent endodontic infections [2]. Enterococcus faecalis (E. faecalis) which is a gram-positive, facultative, anaerobic cocci, is the major etiology of periradicular lesions after root canal treatment (RCT). E. faecalis can survive starvation due to physicochemical characteristics, including formation of biofilm, innate antibacterial resistance and capacity to invade into dentinal tubules [3-5]. However mechanical instrumentation can eliminate most bacteria in root canals, antibacterial irrigants are essential for successful root canal therapy [6].
Several irrigants have been suggested for use in combination with mechanical preparation. Sodium hypochlorite (NaOCl) and chlorhexidine (CHX) are two popular intracanal irrigants with good antibacterial activity [6]. NaOCl is the most common used root canal irrigant. The bactericidal ability of NaOCl is due to the creation of hypochlorous acid (HOCl) while contacting with organic matter [7, 8]. However NaOCl has some drawbacks such as being corrosiveness to devices, reduction in elastic modulus and flexural strength of dentin [9], implementing cytotoxic effects on surrounding tissues and unpleasant taste [10]. CHX is a broad-spectrum antimicrobial agent which has antibacterial efficacy comparable to that of NaOCl and less toxic effects [11]. The cationic component of this agent connects with the anionic component of bacterial surface leading to disruption of its integrity [12]. CHX may also exhibit cytotoxicity on corneal and endothelial cells, as well as neurotoxicity [13, 14].
Due to unwanted chemical reactions induced by commercial intracanal medicaments and increasing antibiotic resistance strains, new intracanal medicaments should be considered. Recently, use of herbal products as root canal disinfectants has been widely examined in endodontics because of their efficiency, safety and availability [15]. Eucalyptus galbie (E. galbie) is a miscellaneous genus of trees, from the family of Myrtaceae [16]. Myrtus communis L. (M. communis L.) which is an ever green small tree, is also one of the members of the Myrtaceae family. A large number of studies have demonstrated the antimicrobial, antifungal, analgesic and anti-inflammatory effects of Eucalyptus and M. communis L. species [16-18].
Raoof et al. [19] evaluated the antibacterial effect of methanolic extracts of ten plants against endodontic pathogens including E. faecalis, Porphyromonas gingivalis, Fusobacterium nucleatum. Assessing the minimal inhibition concentration (MIC) of extracts showed that Eucalyptus galbie and Myrtus communis L. had the highest antibacterial effect in all concentrations. In the subsequent study, Raoof et al. [20] compared the antibacterial efficacy of Eucalyptus galbie and Myrtus communis L. with calcium hydroxide against E. faecalis. It was defined that the highest antibacterial agent in 30 days was for M. communis L. 6.25 mg/mL and in 7 days was for E. galbie 12.5 mg/mL. The literature shows no study comparing the antimicrobial effects of E. galbie, M. communis L., CHX and NaOCl in endodontics. Therefore, this in vitro study was proposed to assess the antibacterial effect of E. galbie, M. communis L., CHX and NaOCl against E. faecalis.
Materials and Methods
Preparation of specimens
One hundred and twenty caries-free human mandibular premolars were selected for this study. All teeth had single root canal without any signs of crack, groove, resorption and root canal calcification. The external surfaces of teeth were cleaned with periodontal curettes. Then they were placed in 2.5% NaOCl solution (Golrang, Pakshoo Co. Tehran, Iran) for disinfection and stored in saline solution until beginning of the experiment. The crowns were cut and roots were standardized to a length of 15 mm. The root canals were prepared with K-files up to #20 (Dentsply-Maillefer, Ballaigues, Switzerland), under irrigation with tap water. The smear layer was removed in an ultrasonic bath with 17% EDTA (Aria Dent, Asia ChemiTeb, Tehran, Iran) for 10 min, followed by 5.25% NaOCl irrigation for 10 min and tap water for 1 h to remove chemicals. Then external surfaces and root apices of samples were covered with nail polish and resin (respectively), for prevention of bacterial leakage [21, 22]. The teeth were placed into glass tubes of brain heart infusion (BHI) broth medium (Merck, Darmstadt, Germany) and autoclaved at 121°C, for 15 min and stored in an incubator at 37°C for 48 h.
E. faecalis (ATCC 29212) was obtained from Iranian Research Organization for Science and Technology, was grown overnight in BHI to get turbidity of 0.5 McFarland standard (1.5×108 CFU/mL). The glass tubes containing teeth were opened and 2 mL of sterile BHI were removed and replaced with 2 mL of the bacterial inoculum. The flasks were kept at 37ºC for 21 days [2]. The medium was refreshed every 2 days to confirm the growth of bacteria. The purity of infection was checked by gram staining and colony morphology on BHI blood agar and streptococcus faecalis broth and bile-esculin tests after 21 days. If any contaminants would have been observed, the teeth would be excluded.
At the end of day 21, the specimens were irrigated with sterile saline and dried by sterile gauze then randomly divided into 8 groups (n=15), according to the intracanal irrigant, as follows: Group 1, 5.25% NaOCl; group 2, 2.5% NaOCl; group 3, 2% CHX (FGM, Joinville, Brazil); group 4, 0.2% CHX; group 5, Eucalyptus Galbie 12.5 mg/mL; group 6, Myrtus communis L. 6.25 mg/mL; group 7, saline (Positive control) and group 8, negative control.
Preparation of plant extracts
The fresh leaves of M. communis L. and E. galbie plants were collected from the southern regions of Iran, around Kerman. After washing with distilled water, plants were air-dried at room temperature and powdered. Two hundred grams of each powdered plant were dissolved in methanol and extractions were prepared by maceration technique and dried using rotary vapor.
Study design
Teeth were instrumented using RaCe rotary system (FKG Dentaire, La Chaux-de-Fonds, Switzerland) with a single-length technique according to the manufacturer. Before using a new instrument, the canal was irrigated with 2 mL of each irrigant (5.25% NaOCl, 2.5% NaOCl, 2% CHX, 0.2% CHX, E. galbie 12.5 mg/mL, M. communis L. 6.25 mg/mL, saline) using 29 gauge needle. Following the use of each instrument, the canal was irrigated with 4 mL of saline solution so final volume of irrigation in each sample was 30 mL.
Bacterial sampling
Sampling of each canal (before and after instrumentation) was done using three sterile paper points (Meta Dental Co., Seoul, Korea). After preparation, 5% sodium thiosulphate solution and 0.5% Tween 80 + 0.07% lecithin were used to neutralize NaOCl and CHX, respectively. Paper points were transferred to tubes containing 1 mL of BHI broth. The colony forming units (CFU) were counted.
Dentin samples were taken from dentinal walls using #3, 4 and 5 sterile Gates-Glidden drills (Dentsply-Maillefer, Ballaigues, Switzerland). Each drill removed a dentin layer from inner surface of the canal in thicknesses of 200 µm, 400 µm and 600 µm, respectively. The samples were immediately collected into separate test tubes containing BHI and CFU were counted. All experiments were repeated three times. The purity of the infection was checked as above.
Data analysis
After log10 transformation of CFU+1, data were analyzed using the Kruskal-Wallis test, followed by Mann Whitney U test. The level of significance was set at 0.05.
Results
The overall reduction of E. faecalis in CFU inside the root canal after biomechanical procedures is presented in Table 1.
Table 1.
Overall percentage reduction of E. faecalis in CFU
| Irrigant | Reduction of bacteria (%) |
|---|---|
| NaOCl 2.5% | 99.98 |
| NaOCl 5.25% | 99.99 |
| CHX 0.2% | 99.85 |
| CHX 2% | 99.96 |
| M.communis L. | 99.33 |
| E.galbie | 99.33 |
| Saline | 91.42 |
All the irrigants significantly reduced bacteria in comparison with positive control group (saline group) (P<0.01). The efficacy of NaOCl (5.25% and 2.5%) and CHX (2% and 0.2%) in reducing intracanal bacteria did not have significant differences (P>0.05). The average reduction of bacteria in the presence of E. galbie did not have any significant difference with 2.5% NaOCl, CHX 2%, CHX 0.2% and M. communis L. (P>0.05). The average reduction of bacteria in the presence of M. communis L. was significantly less than other groups except E. galbie and 0.2% CHX.
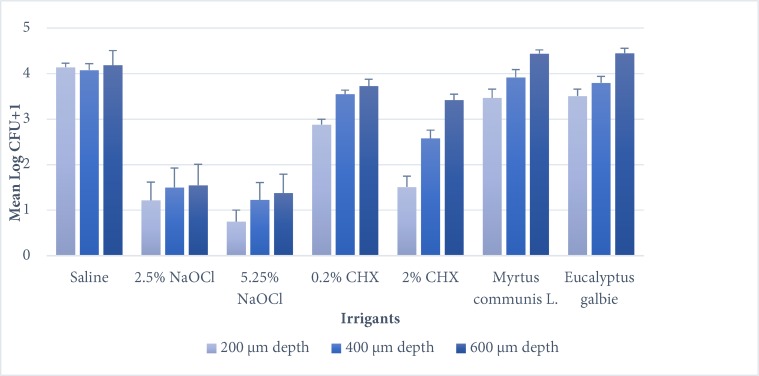
According to Mann-Whitney U analysis, the mean log CFU+1 for all experimental groups in all depths, was significantly less than the positive control group (saline group) (P<0.05). For all depths, 5.25% NaOCl was shown to be the most effective irrigant solution, followed by 2.5% NaOCl, without any significant difference between them (P>0.05). On the other hand, 2% CHX had significantly less effect than 5.25% NaOCl (all depth) and 2.5% NaOCl (600 µm depth) (P<0.05). The antibacterial effect of 0.2% CHX was significantly less than NaOCl (5.25% and 2.5%) in all depth and less than 2% CHX in 200 µm and 400 µm depths (P<0.05). E. galbie group had significantly less antibacterial effect in comparison with NaOCl (5.25% and 2.5%) and 2% CHX groups in all depths (P<0.05) and 0.2% CHX group only in 600 µm depth (P<0.01). M. communis L. had significantly less antibacterial effect than NaOCl (5.25% and 2.5%) and 2% CHX groups in all depths (P<0.01) and with 0.2% CHX group only in 600 µm depth (P<0.01). Moreover analysis indicated significant increase in log CFU+1 by increasing dentin depth (P<0.05) (Figure 1).
Figure 1.
Antibacterial action of the irrigants against E. faecalis in relation to the depth of dentin
Discussion
The purpose of endodontic therapy is disinfection of the root canal space [23]. Ability of bacteria to invade into dentinal tubules, limited penetration of medicaments into dentin and inactivation of agents by dentin, complicate cleaning of the root canal system [3]. As E. faecalis is the mostly responsible bacteria for failure of endodontically treated teeth; thus, ATCC 29212 as the standard strain of E. faecalis was selected in our study. It is well established that mechanical instrumentation reduced approximately 50% of bacteria from root canal space so irrigation is required to aid in eradication of bacteria especially in unreachable areas [2]. An ideal irrigating solution should have maximal antimicrobial and tissue solving properties with minimal toxic effects [3]. Sodium hypochlorite has long been known as the most common antibacterial agent in root canal treatment. Although, the antibacterial effect of NaOCl is superior to normal saline and other endodontic irrigating solutions, it is unable to completely eliminate bacteria, such as E. faecalis [24]. The major disadvantages of NaOCl are allergic potential [25] and cytotoxic effects on vital tissues [10].
Chlorhexidine gluconate (a cationic bisguanide) is another common antibacterial agent which adsorbs onto the cell wall of microorganisms, resulting in the leakage of intracellular components. At low concentrations of CHX, small elements (such as potassium and phosphorous) will leak out and bacteriostatic effect is developed. At higher concentrations, CHX has a bactericidal effect due to coagulation of cytoplasm [11]. CHX has some disadvantages, such as inability to dissolve tissue, discoloration of teeth and tongue [26] and rare adverse reactions, including desquamative gingivitis [26] and contact dermatitis [27].
Due to the growing existence of drug-resistant bacteria and possible side effects of chemical antibacterial agents, it is worthy to use an intracanal irrigant made of natural extracts [28]. The major advantages of herbal substitutes are their more shelf life, lower toxicity, lack of microbial resistance, availability and cost-effectiveness [29]. The two plant extracts used in our study, M. communis L. and E. galbie, have active components with valuable therapeutic and antimicrobial properties [16, 18]. Thus, they may have potential to replace conventional root canal irrigants such as NaOCl and CHX. Studies have indicated the susceptibility of both gram-positive and gram-negative bacteria (Streptococcus mutans, Lactobacillus and E. faecalis) and Candida albicans towards various Eucalyptus species extracts [30, 31]. According to the previous studies, components of Eucalyptus species can inhibit bacteria via interfering with the enzyme involved in fatty acid synthesis route [32]. Antimicrobial effect of Myrtus species has been demonstrated in many studies. Researches showed antibacterial effect of this plant against several oral bacteria [33]. Nabavizadeh et al. [34] evaluated the antibacterial effect of M. communis L. on Staphylococcus aureus, E. faecalis and Candida albicans using agar diffusion method and reported the inhibitory effect of this plant against these persistent endodontic pathogens. Although many studies have proven antibacterial effect of Eucalyptus and Myrtus, there is a lack of sufficient evidence regarding the antibacterial activity of these plants in endodontics.
As in the previous study, it was reported that 12.5 mg/mL E. galbie and 6.25 mg/mL M. communis L. had considerable antibacterial actions [20], we chose these concentrations in this study. Investigators have used various methods assessing the effects of endodontic irrigants on infected dentin. In the present study and some others, the microorganisms were entombed within dentinal tubules [2, 21, 35] so the agents do not essentially have direct connection with microorganisms. Similar to previous studies, dentin block model was used in our study [2, 21, 35, 36]. One of the advantages of this method is samples with standard length and diameter. By introduction of endodontic rotary instruments and techniques, time required for root canal preparation is reduced so the irrigating solution should express its action against pathogens of root canal and dentinal tubules, such as E. faecalis as quickly as possible [2].
We evaluated bacterial reduction in the root canal and dentinal tubules immediately after biomechanical preparation (10 min), which is similar to Berber et al. [2]. In the present study, microbial samples inside root canals, collected with paper points just after chemo-mechanical preparation. Our results demonstrated that instrumentation techniques along with saline irrigation for a period of 10 min, eliminated more than 90% of the bacterial cells from the root canal via flushing action, which is in accordance with Siqueira et al. [37], Berber et al. [2] and Dametto et al. [35]. In our study as the study by Berber et al. [2], all test groups reduced more than 99% of bacteria in the root canal. In addition, dentinal samples were obtained using Gates Glidden drills to evaluate the presence of bacterial cells inside the dentinal tubules immediately following biomechanical procedures. It is well known that E. faecalis has the ability to travel deeply into dentin even up to 800-1000 µm after 3 weeks of incubation [38].
In this study, a total depth of 200, 400 and 600 µm from pulp-dentin junction into the dentin was examined. As reported earlier, proportion of bacterial cells in the most superficial level (200 µm) of dentin were significantly lower than that for 400 µm and 600 µm depths, representing effective antimicrobial action of irrigants adjacent to the pulp-dentin junction [2]. Intra tubular efficacy of all irrigants was significantly more than saline solution (positive control). Efficiency of 5.25% NaOCl and 2.5% NaOCl was similar in all depths. Also 2% CHX was significantly more effective than 0.2% CHX only in 200 and 400 µm depths. Efficacy of 2% CHX was significantly less than 5.25% NaOCl (in all depths) and 2.5% NaOCl (only in 600 µm depth). Inhibitory effect of 0.2% CHX was less than NaOCl in all depths. Berber et al. [2] reported the significant difference between the concentrations of NaOCl (2.5% and 5.25%) and positive control group (saline group). In the deepest dentin, 0.5% NaOCl did not present significant difference in comparison with positive control group. In our study, intra tubular effect of E. galbie and M. communis L. had significant difference with positive control group. Their antibacterial effect was significantly lower than NaOCl (5.25% and 2.5%) and 2% CHX and similar to 0.2% CHX only in 200 and 400 µm depths. This means that the inhibitory effect of dentin on NaOCl and CHX was less than E. galbie and M. communis L. especially in deep dentin. Nevertheless, as shown earlier, even by increasing the concentration of irrigants to increase the penetration depth of the antibacterial effect, complete removal of bacteria from the dentinal tubules can scarcely be reached by needle-and-syringe irrigation so dentinal tubules may not be considered absolutely free of microorganisms [2]. Various factors should be considered for these results. A 10-min period may be inadequate for the plant extracts to exert their inhibitory effect on E. faecalis. However, no evidence exists about the specific time required for irrigants to have the antimicrobial action. Dentin buffering action might diminish the antibacterial effect of E. galbie and M. communis L. extracts. Also, inadequate penetration depth of irrigants, inactivation of the agents by dentin and microbial biofilms may be the reasons for the incomplete killing of bacteria.
Conclusion
Within limitations of the current study, Eucalyptus galbie and Myrtus communis L. revealed acceptable efficacy to eradicate E. faecalis. Although, their antibacterial activity was lower than NaOCl, these extracts seem to be promising in endodontics. Moreover, further in vitro and clinical studies should be done to evaluate their antibacterial effect with longer exposure periods and against other bacteria and their biocompatibility and toxicological aspects.
Acknowledgment
This study was funded by the Zanjan University of Medical Sciences (Grant no: A-12-804-1). The authors also thank Dr. Fakhri Haghi for cooperation in our study.
Conflict of interest
‘None declared’.
References
- 1.Sahebi S, Khosravifar N, SedighShamsi M, Motamedifar M. Comparison of the antibacterial effect of sodium hypochlorite and aloe vera solutions as root canal irrigants in human extracted teeth contaminated with enterococcus faecalis. J Dent. 2014;15(1):39–43. [PMC free article] [PubMed] [Google Scholar]
- 2.Berber V, Gomes B, Sena N, Vianna M, Ferraz C, Zaia A, Souza‐Filho F. Efficacy of various concentrations of NaOCl and instrumentation techniques in reducing Enterococcus faecalis within root canals and dentinal tubules. Int Endod J. 2006;39(1):10–7. doi: 10.1111/j.1365-2591.2005.01038.x. [DOI] [PubMed] [Google Scholar]
- 3.Kayaoglu G, Ørstavik D. Virulence factors of Enterococcus faecalis: relationship to endodontic disease. Crit Rev Oral Biol Med. 2004;15(5):308–20. doi: 10.1177/154411130401500506. [DOI] [PubMed] [Google Scholar]
- 4.Afkhami F, Akbari S, Chiniforush N. Entrococcus faecalis Elimination in Root Canals Using Silver Nanoparticles, Photodynamic Therapy, Diode Laser, or Laser-activated Nanoparticles: An In Vitro Study. J Endod. 2017;43(2):279–82. doi: 10.1016/j.joen.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 5.Zand V, Lotfi M, Soroush MH, Abdollahi AA, Sadeghi M, Mojadadi A. Antibacterial Efficacy of Different Concentrations of Sodium Hypochlorite Gel and Solution on Enterococcus faecalis Biofilm. Iran Endod J. 2016;11(4):315–9. doi: 10.22037/iej.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zehnder M. Root canal irrigants. J Endod. 2006;32(5):389–98. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Siqueira J, Machado A, Silveira R, Lopes H, Uzeda Md. Evaluation of the effectiveness of sodium hypochlorite used with three irrigation methods in the elimination of Enterococcus faecalis from the root canal, in vitro. Int Endod J. 1997;30(4):279–82. doi: 10.1046/j.1365-2591.1997.00096.x. [DOI] [PubMed] [Google Scholar]
- 8.Mohammadi Z, Shalavi S, Giardino L, Palazzi F, Asgary S. Impact of Ultrasonic Activation on the Effectiveness of Sodium Hypochlorite: A Review. Iran Endod J. 2015;10(4):216–20. doi: 10.7508/iej.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sim T, Knowles J, Ng YL, Shelton J, Gulabivala K. Effect of sodium hypochlorite on mechanical properties of dentin and tooth surface strain. Intel Endod J. 2001;34(2):120–32. doi: 10.1046/j.1365-2591.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- 10.Kleier DJ, Averbach RE, Mehdipour O. The sodium hypochlorite accident: experience of diplomates of the American Board of Endodontics. J Endod. 2008;34(11):1346–50. doi: 10.1016/j.joen.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Gomes B, Ferraz C, ME V, Berber V, Teixeira F, Souza‐Filho F. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J. 2001;34(6):424–8. doi: 10.1046/j.1365-2591.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- 12.Estrela C, Ribeiro RG, Estrela CR, Pécora JD, Sousa-Neto MD. Antimicrobial effect of 2% sodium hypochlorite and 2% chlorhexidine tested by different methods. Brazil Dent J. 2003;14(1):58–62. doi: 10.1590/s0103-64402003000100011. [DOI] [PubMed] [Google Scholar]
- 13.Green K, Livingston V, Bowman K, Hull DS. Chlorhexidine effects on corneal epithelium and endothelium. Arch Ophthalmol. 1980;98(7):1273–8. doi: 10.1001/archopht.1980.01020040125020. [DOI] [PubMed] [Google Scholar]
- 14.Henschen A, Olson L. Chlorhexidine-induced degeneration of adrenergic nerves. Acta Neuropathologica. 1984;63(1):18–23. doi: 10.1007/BF00688466. [DOI] [PubMed] [Google Scholar]
- 15.Palombo EA. Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evidence-Based Complementary and Alternative Medicine. 2011;2011 doi: 10.1093/ecam/nep067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva J, Abebe W, Sousa S, Duarte V, Machado M, Matos F. Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. J Ethnopharmacol. 2003;89(2):277–83. doi: 10.1016/j.jep.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Berka-Zougali B, Ferhat M-A, Hassani A, Chemat F, Allaf KS. Comparative study of essential oils extracted from Algerian Myrtus communis L leaves using microwaves and hydrodistillation. Int J Mol Sci. 2012;13(4):4673–95. doi: 10.3390/ijms13044673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mimica-Dukić N, Bugarin D, Grbović S, Mitić-Ćulafić D, Vuković-Gačić B, Orčić D, Jovin E, Couladis M. Essential oil of Myrtus communis L as a potential antioxidant and antimutagenic agents. Molecules. 2010;15(4):2759–70. doi: 10.3390/molecules15042759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raoof M, Eslaminejad Z, Sharififar F, Heidarizadeh M. Ex-vivo Evaluation of Antibacterial Effect of Methanolic Extract of Ten Medicinal Plants as Intracanal Medicament against Usual and Resistent Microorganisms in Root Canal Therapy. Kerman Dental School, Kerman University of Medical Sciences; 2008. [Google Scholar]
- 20.Raoof M, Eslaminejad Z, Sharififar F, Pouradeli S, Mohammadi N. Ex-Vivo Evaluation of Antibacterial Effect of Methanolic Extract of Eucalyptus galbie and Myrtus Communis L as Intracanal Medicament against Enterococcus Faecalis. Kerman Dental School, Kerman University of Medical Sciences; 2011. [Google Scholar]
- 21.Gomes B, Souza S, Ferraz C, Teixeira F, Zaia A, Valdrighi L, Souza-Filho F. Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentin in vitro. Int Endod J. 2003;36(4):267–75. doi: 10.1046/j.1365-2591.2003.00634.x. [DOI] [PubMed] [Google Scholar]
- 22.Ferraz CCR, de Almeida Gomes BPF, Zaia AA, Teixeira FB, de Souza-Filho FJ. In vitro assessment of the antimicrobial action and the mechanical ability of chlorhexidine gel as an endodontic irrigant. J Endod. 2001;27(7):452–5. doi: 10.1097/00004770-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Asgary S, Nourzadeh M, Eghbal MJ. Miniature Pulpotomy of Symptomatic Mature Permanent Teeth: A Report of Two Cases. Iran Endod J. 2016;11(1):75–8. doi: 10.7508/iej.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Önçağ Ö, Hoşgör M, Hilmioğlu S, Zekioğlu O, Eronat C, Burhanoğlu D. Comparison of antibacterial and toxic effects of various root canal irrigants. Int Endod J. 2003;36(6):423–32. doi: 10.1046/j.1365-2591.2003.00673.x. [DOI] [PubMed] [Google Scholar]
- 25.Neelakantan P, Jagannathan N, Nazar N. Ethnopharmacological approach in endodontic treatment: A focused review. J Dr NTR Univ Health Sci. 2011;3(4):68–77. [Google Scholar]
- 26.Prasad SD, Goda PC, Reddy KS, Kumar CS, Hemadri M, Reddy DSR. Evaluation of antimicrobial efficacy of neem and Aloe vera leaf extracts in comparison with 3% sodium hypochlorite and 2% chlorhexidine against E faecalis and C albicans. J Dr NTR Univ Health Sci. 2016;5(2):104–10. [Google Scholar]
- 27.Krautheim A, Jermann T, Bircher A. Chlorhexidine anaphylaxis: case report and review of the literature. Contact Dermatitis. 2004;50(3):113–6. doi: 10.1111/j.0105-1873.2004.00308.x. [DOI] [PubMed] [Google Scholar]
- 28.Prabhakar J, Senthilkumar M, Priya M, Mahalakshmi K, Sehgal P, Sukumaran V. Evaluation of antimicrobial efficacy of herbal alternatives (Triphala and green tea polyphenols), MTAD, and 5% sodium hypochlorite against Enterococcus faecalis biofilm formed on tooth substrate: an in vitro study. J Endod. 2010;36(1):83–6. doi: 10.1016/j.joen.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 29.Kamath U, Sheth H, Ramesh S, Singla K. Comparison of the antibacterial efficacy of tea tree oil with 3% sodium hypochlorite and 2% Chlorhexidine against E faecalis: An in vitro study. Jo Contem Dent. 2013;3(3):117–20. [Google Scholar]
- 30.Firas H Q, Al-Mizraqchi AS. The antimicrobial effect of aqueous & alcoholic extracts of eucalyptus leaves on oral Mutans streptococci, Lactobacilli & Candida albicans (an in vitro study) J Bagh College Dentistry. 2009;21(4):109–12. [Google Scholar]
- 31.Cock IE. Antimicrobial activity of Eucalyptus major and Eucalyptus baileyana methanolic extracts. Internet J Microbiol. 2015;6(1):1–14. [Google Scholar]
- 32.Takahashi T, Kokubo R, Sakaino M. Antimicrobial activities of eucalyptus leaf extracts and flavonoids from Eucalyptus maculata. Lett Appl Microbiol. 2004;39(1):60–4. doi: 10.1111/j.1472-765X.2004.01538.x. [DOI] [PubMed] [Google Scholar]
- 33.Houshmand B, Mortazavi H, Alikhani Y, Abdolsamadi H, AhmadiMotemayel F, ZareMahmoudabadi R. In Vitro Evaluation of Antibacterial Effect of Myrtus Extract with Different Concentrations on Some Oral Bacteria. J Mashad Dent Sch. 2011;35(2):123–30. [Google Scholar]
- 34.Nabavizadeh M, Abbaszadegan A, Gholami A, Sheikhiani R, Shokouhi M, Shams MS, Ghasemi Y. Chemical constituent and antimicrobial effect of essential oil from Myrtus communis leaves on microorganisms involved in persistent endodontic infection compared to two common endodontic irrigants: An in vitro study. J Conserv Dent. 2014;17(5):449–53. doi: 10.4103/0972-0707.139836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dametto FR, Ferraz CCR, de Almeida Gomes BPF, Zaia AA, Teixeira FB, de Souza-Filho FJ. In vitro assessment of the immediate and prolonged antimicrobial action of chlorhexidine gel as an endodontic irrigant against Enterococcus faecalis. Oral Sur Oral Med Oral Pathol, Oral Radiolo Endod. 2005;99(6):768–72. doi: 10.1016/j.tripleo.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 36.Bazvand L, Aminozarbian MG, Farhad A, Noormohammadi H, Hasheminia SM, Mobasherizadeh S. Antibacterial effect of triantibiotic mixture, chlorhexidine gel, and two natural materials Propolis and Aloe vera against Enterococcus faecalis: An ex vivo study. Dent Res J. 2014;11(4):469–74. [PMC free article] [PubMed] [Google Scholar]
- 37.Siqueira JF, Lima KC, Magalhães FA, Lopes HP, de Uzeda M. Mechanical reduction of the bacterial population in the root canal by three instrumentation techniques. J Endod. 1999;25(5):332–5. doi: 10.1016/S0099-2399(06)81166-0. [DOI] [PubMed] [Google Scholar]
- 38.Haapasalo M, Ørstavik D. In vitro infection and disinfection of dentinal tubules. J Dent Res. 1987;66(8):1375–9. doi: 10.1177/00220345870660081801. [DOI] [PubMed] [Google Scholar]