Abstract
Xerostomia in head and neck (H&N) cancer patients significantly affects their quality of life (QoL). The aim of the present study was to investigate the associations among QoL, xerostomia and quantity of saliva in a sample of H&N cancer patients who had received conventional radiotherapy (RT). A total of 60 H&N adult patients were enrolled in this prospective study. The patients completed the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (QLQ-C30), the Quality of Life Questionnaire Head and Neck Module (QLQ-H&N35) and the Greek version of the XQ questionnaire at 4 timepoints: At the beginning of RT, at the end of RT, 6 months after RT completion and 1 year after RT completion. Patients with distant metastases or serious comorbidities were excluded from the study. Salivary pH, and stimulated and unstimulated salivary flow rate were assessed. All functional scales and symptom scales, apart from cognitive functioning in QLQ-C30 and feeding tube in H&N35 exhibited an abrupt deterioration at timepoint 3 and were then gradually restored over time. The difference was statistically significant (P<0.001). XQ scores at different timepoints exhibited a statistically significant negative correlation with salivary flow rates. Salivary flow rate and XQ scores almost parallelled one another. Flow rates recovered at a mean level of 20% below baseline values at the end of the follow-up period. The subjective symptom of xerostomia parallelled salivary flow and QoL. Despite receiving conventional RT, the participants exhibited a considerable preservation of salivary gland function after 12 months, allowing some optimism regarding the course of xerostomia in selected patients.
Keywords: head and neck cancer, quality of life, salivary flow, xerostomia
Introduction
Xerostomia in head and neck (H&N) cancer patients following radiation treatment (RT) is caused by locoregional and permanent damage to the salivary glands in the RT field (1). Xerostomia is a common complaint, significantly affecting the quality of life (QoL) of the patients. The prevalence of xerostomia has been reported to range between 73.5 and 93% (2,3). Unfortunately, precise evaluation of radiation-induced xerostomia requires morphological and functional assessment methods, such as histological evaluation, computed tomography, magnetic resonance (MR) imaging and ultrasonography, MR sialography and scintigraphy. As these methods are technically complex and practically non-applicable in the clinical setting, and xerostomia is primarily a subjective symptom, its assessment mainly relies on patient self-reports. Longitudinal studies in H&N cancer patients support the hypothesis that the feeling of xerostomia increases and QoL decreases during and directly after treatment and is restored to pretreatment levels at least after 12 months, despite any remaining functional disability (4). Despite extensive research in this field, only a limited number of studies have simultaneously investigated the association between QoL, the general subjective feeling of xerostomia, and the amount of saliva during and after RT, whereas it remains uncertain whether and to what extent objective signs are in accordance with subjective symptoms (5,6). Modern RT techniques, such as intensity-modulated RT (IMRT), which are able to spare a large volume of the salivary glands, have been developed (2). However, a significant number of patients cannot benefit from these techniques, mainly due to financial restraints, with the most representative example being the current state of the public hospitals in Greece. The financial crisis Greece has been experiencing over the last decade has adversely affected the availability of medical resources and services. New RT techniques are currently not available in Greece due to the lack of expertise and the fact that there are no available funds for the replacement of the existing linear accelerators with new ones and for training the medical staff in the new technology. However, QoL and xerostomia must be assessed with widely accepted and adequately validated tools for valid and reliable data to be obtained. In that context, the aim of the present study was to investigate the associations among these three variables (QoL, xerostomia and quantity of saliva) in a sample of Greek H&N cancer patients who were treated with conventional RT.
Materials and methods
Patients
The present study was conducted at the outpatient clinics of the Department of Radiation Therapy of the University Hospital of Larissa (Larissa, Greece), the ‘Theagenio’ Cancer Hospital (Thessaloniki, Greece) and the ‘AHEPA’ University Hospital of Thessaloniki (Thessaloniki, Greece) between 2014 and 2015. Patients with H&N cancer comprised the study population. The exclusion criteria were as follows according to the literature: i) Salivary gland cancer, ii) psychiatric morbidity, iii) distant metastases, iv) past RT for H&N irrelevant to the present disease, v) Sjögren syndrome and other autoimmune diseases, such as systemic lupus erythematosus and rheumatoid arthritis, vi) diabetes mellitus, vii) use of antihistamines and anticholinergic drugs or antipsychotics and viii) use of pilocarpine. Due to the limited resources, 60 patients were randomly selected from the eligible subjects to participate in the study.
The patients completed the questionnaires in a private clinic room in the presence of the primary researcher. All the patients were adults (aged >18 years), capable of fluent communication in Greek, with histologically confirmed H&N cancer and an Eastern Cooperative Oncology Group performance status of 0–2.
The Radiation Therapy Oncology Group (RTOG)/ European Organisation for Research and Treatment of Cancer (EORTC) late xerostomia grading was determined during follow-up visits at the clinic by 3 physicians at each visit. Each of the observers assigned the score independently and was blinded to the scores assigned by the others. The scores ranged from 0 to 3 (0, none; 1, slight dryness of the mouth, good response on stimulation; 2, moderate dryness, poor response on stimulation; and 3, complete dryness, no response on stimulation). A mean RTOG/EORTC score was calculated from the scores provided by the 3 observers and was used for correlations with the patient reported scores.
Questionnaires
The patients answered three questionnaires at the same time: The Greek version of the XQ Instrument (7,8) the EORTC Quality of Life Questionnaire-Core 30 (QLQ-C30) and the Quality of Life Questionnaire Head and Neck Module (QLQ-H&N35) (9) from the EORTC group. All the questionnaires have been validated in the Greek population. The QLQ-C30 encompasses 30 questions grouped into five functional scales, nine scales associated with symptoms and a global scale. The QLQ-H&N35 includes 35 questions, 30 of which are grouped in 13 scales and 5 are simple-answer. This questionnaire addresses symptoms associated with specific tumor location, side effects associated with the treatment provided and additional QoL aspects affected by the disease or its treatment. The answers were converted into a linear scoring scale, with values between 0 and 100, as per advocated by EORTC (9,10). The results were expressed as mean values with confidence intervals. A high score in the questions associated with the symptoms reflects more prominent symptoms, while a high score in the questions associated with function reflects a better condition of the patient. The XQ provides a measure of the severity of radiation-induced xerostomia that affects the patients' QoL. This questionnaire consists of 8 questions, 4 of which are associated with patient-reported dryness while eating or chewing and the remaining 4 are associated with dryness in the absence of eating or chewing. The XQ is a self-administered tool and patients are asked to rate each symptom on an 11-point ordinal Likert scale of 0–10, with higher scores indicating more severe dryness or discomfort due to dryness. Each item score is added, and the sum is linearly transformed to produce the final summary score ranging from 0 to 100, with higher scores representing higher levels of xerostomia. The results of this questionnaire are correlated with more global QoL parameters (7,8). The XQ has recently been validated in the Greek population by the research team conducting the present study, in an attempt to yield a reliable xerostomia tool for Greek patients. The Greek version of XQ exhibited excellent validity and reliability, as well as high correlation with the observers' estimation of the salivary output (11).
Assessment of unstimulated salivary flow rate
The unstimulated salivary flow rate was assessed using the spitting method. Patients were instructed to collect their saliva for 5 min in a graded tube. The volume of the saliva was then determined and divided by 5 to obtain a flow reading per minute. Stimulated salivary flow was determined in the same manner. Saliva secretion was stimulated by chewing a piece of parafilm, to avoid chewing gum containing mannitol or other substances that may interfere with saliva production.
All measurements but QoL ones were conducted at 6 timepoints: i) Initiation of RT, ii) during RT, iii) at the end of RT, iv) 3 months after RT completion, v) 6 months after RT completion and vi) 1 year after RT completion.
pH measurement
For pH measurement, commercial kit strips (Simplex Health, Wollaston, UK) were used. The manufacturers' instructions for sample preparation were strictly followed: The measurement was conducted at least 2 h after eating, drinking or brushing teeth. The patients were instructed not to rinse their mouth and strictly avoid any beverages/coffee to avoid altering the pH. The patients spat saliva on a spoon. The pH strip was dipped into the fluid for ~3 sec until both pads on the test strip were sufficiently covered with liquid. After 15 sec, the color change was compared with the color chart. The test was repeated three times to obtain an average reading.
Ethics
All the procedures were conducted in accordance with the ethical standards of the University Hospital and National Committees on human experimentation, and with the principles of the Helsinki Declaration of 1975, as revised in 2000.
Statistical analysis
The distribution and normality of the collected data were tested using the Shapiro-Wilk test and the Kolmogorov-Smirnov test. Item analysis was performed using the mean and standard deviation data of the items. Pearson's and Spearman's correlations were used as appropriate. The Friedman's test was performed for repeated measures analysis. Statistical significance was set at P<0.05. SPSS 22.0 software (IBM Corp., Armonk, NY, USA) was used to analyze data. SigmaPlot 12.5 and Microsoft Excel 2007 (version 12.00) were used for graph construction.
Therefore, clinicians should be confident with well-validated instruments in the assessment of xerostomia and adjust therapeutic regimens as appropriate.
Results
Patient characteristics
The socio-demographic and clinical characteristics of the participants are summarized in Table I. The mean age of the patients was 61.08 years [standard deviation (SD), 10.07 years] and 80% (48/60) were men. Half of the patients had laryngeal/hypopharyngeal cancer and 78.3% had undergone chemotherapy (Table I).
Table I.
Socio-demographic and clinical characteristics of the study subjects (n=100).
| Variables | Values |
|---|---|
| Gender, n | |
| Male | 48 |
| Female | 12 |
| Age, years | |
| Mean | 61.08 |
| Standard deviation | 10.07 |
| Tumor location, n | |
| Oropharynx | 9 |
| Nasopharynx | 9 |
| Larynx | 28 |
| Hypopharynx | 2 |
| Tongue | 12 |
| Chemotherapy | |
| Yes | 47 |
| No | 13 |
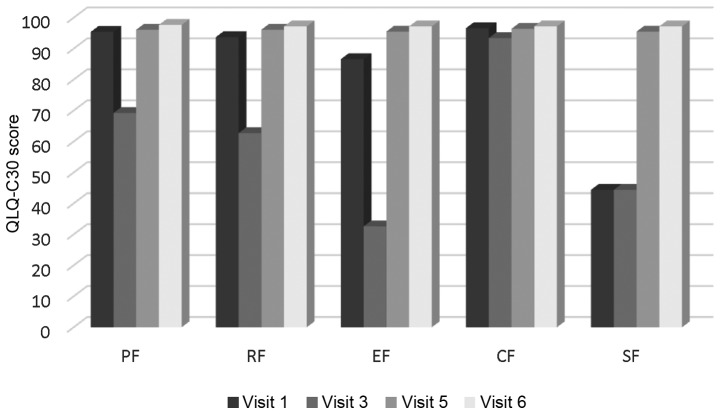
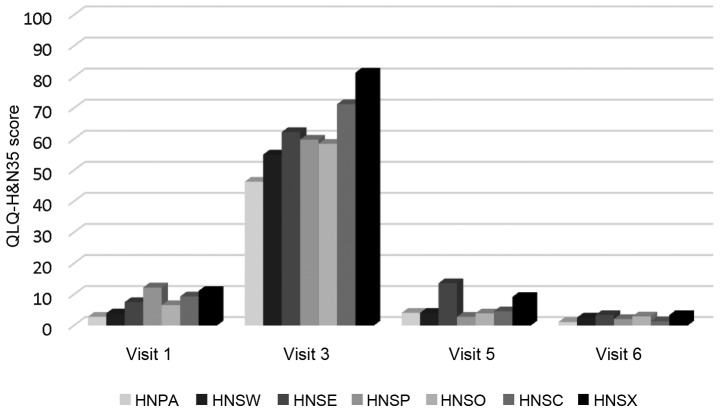
Changes in functional and symptom scales over time. All functional scales and symptom scales, apart from cognitive functioning in QLQ-C30 and feeding tube in H&N35, exhibited an abrupt deterioration at timepoint 3 and were gradually restored over time. The difference was statistically significant (P<0.001). The mean XQ values at timepoint 2 were 47.27 (SD, 18.26) indicating a low-to-medium grade of xerostomia (Figs. 1 and 2). XQ scores at different timepoints exhibited a statistically significant negative correlation with salivary flow rates, while the difference in XQ score between the start and endpoint of the study was significantly correlated only with the corresponding difference in stimulated salivary flow (r=0.333, P=0.009).
Figure 1.
QLQ-C30 functional scales through time. QLQ-C30, Quality of Life Questionnaire-Core 30. PF, physical functioning (revised); RF, role functioning (revised); EF, emotional functioning; CF, cognitive functioning; SF, social functioning.
Figure 2.
QLQ-H&N symptom scales through time. QLQ-H&N35, Quality of Life Questionnaire Head and Neck Module; HNPA, pain; HNSW, swallowing, HNSE, senses problems; HNSP, speech problems; HNSO, trouble with social eating; HNSC; trouble with social contact; HNSX, low sexuality.
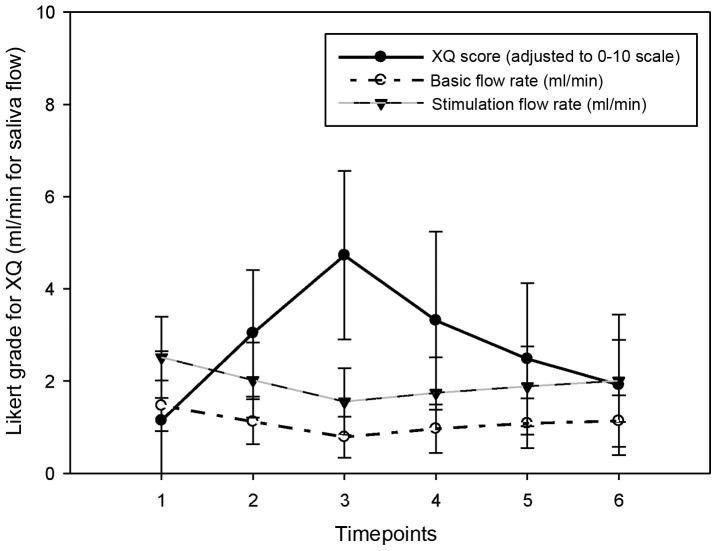
Changes in biochemical indices over time. Salivary flow rate and XQ scores almost parallelled one another (Fig. 3). The peak in XQ and dip in the flow rate both occured at timepoint 3. The unstimulated mean flow rate was at its minimum at timepoint 2 [immediately after RT: 1,12 (SD, 0.49)], while the minimum stimulated flow rate was 2.02 (SD, 0.55) at the same time. The values gradually recovered to a level of 20% below baseline values by the end of the follow-up period (Table II).
Figure 3.
Changes in XQ score and salivary flow rates over time.
Table II.
Changes in biochemical indices over time.
| Timepointsa | |||||
|---|---|---|---|---|---|
| Indices | 1 | 3 | 5 | 6 | P-value |
| Unstimulated saliva volume, ml | 7.33 | 3.93 | 5.42 | 5.68 | <0.001 |
| Stimulated saliva volume, ml | 12.57 | 7.77 | 9.42 | 10.03 | <0.001 |
| pH | 6.35 | 6.52 | 6.39 | 6.45 | <0.001 |
Only timepoints exhibiting statistically significant differences are presented.
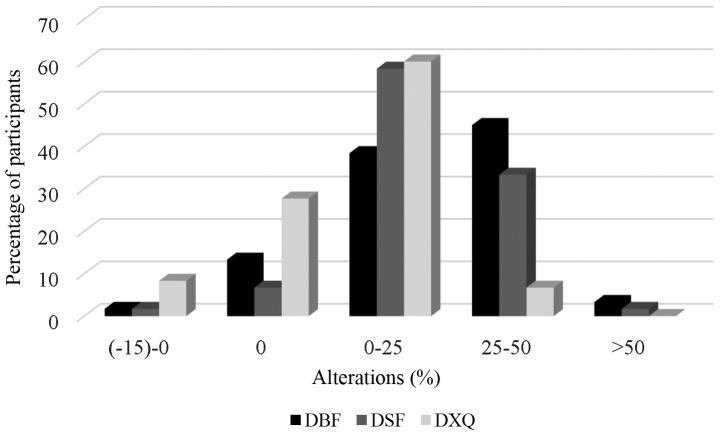
A total of 8.4 and 15% of the patients exhibited a completely restored or even increased stimulated and unstimulated salivary flow rate, respectively, while 3.3 and 1.7% of the participants exhibited >50% loss of unstimulated and stimulated saliva production, respectively (Fig. 4).
Figure 4.
Changes in salivary flow and xerostomia over time (%). DBF, difference (points 1–6) for baseline (unstimulated) flow rates; DSF: difference (points 1–6) for stimulated flow rates; DXQ: difference (points 1–6) for xerostomia questionnaire.
Discussion
The findings of the present study strongly support the hypothesis that the subjective feeling of xerostomia, as measured by certain instruments (XQ in this study), may be well-parallelled with physiological indices, such as salivary flow. Furthermore, QoL may be particularly compromised following RT, gradually improving over time and to a significant extent within the first year after treatment initiation. Data allow some optimism regarding patients' recovery, as salivary flow after stimulation shows a significant improvement over time and the functional scales suggest an almost full recovery of the patients' daily activities. To the best of our knowledge, this is the first study in Greece to investigate the associations of QoL with the general subjective feeling of xerostomia, and the amount of saliva production prior to and following RT.
There is no consensus on the extent of the association between the subjective feeling of xerostomia and objective measures and, despite the validated tools used to evaluate the salivary gland function, xerostomia is only a subjective symptom (12). Patients complaining of xerostomia do not always suffer from hyposalivation (13), while qualitative changes in saliva may well affect the feeling of xerostomia, despite adequate saliva production (14). Moreover, methodological issues, such as sample selection bias, may affect the conclusions. The inclusion of patients with advanced disease or comorbidities may negatively affect the results, while salivary flow stimulation with not purely mechanical methods may overestimate saliva production. In the present study, a purely mechanical method was used for salivary flow stimulation, in order to avoid misinterpretations. In that context, our results are likely more representative of the patients' daily symptoms, best reflecting their basic saliva production. Examining mean cohort levels of xerostomia may also be misleading, as patients who have a flow ratio below the critical cut-off (<25%) appeared to complain the most of a dry mouth (14,15). In the present study, hyposalivation was not prominent (minimum baseline salivary flow rate >0.1 ml/min following RT) and, due to the exclusion criteria, patients with severe comorbidities or metastatic disease were excluded, whereas no deaths were reported during the follow-up period. Therefore, terminally ill patients were not included in the present study.
It is estimated that at least 1 year of follow-up is required to determine the effect of RT on QoL (12,16). Thus, the follow-up period in the present study may be considered as sufficient to provide adequate information in terms of QoL following RT. Of note, when estimating xerostomia and QoL, psychological issues should also be taken into consideration. The psychological shock early in the treatment course may contribute to the subjective feeling of xerostomia, even in the absence of a considerable decrease of the amount of saliva, although in the majority of the patients salivary production decreases significantly after RT. However, long-term follow-up (>6 months) indicates that QoL improves over time, although xerostomia may not be directly associated with QoL. Furthermore, all QoL aspects may not improve, at least not to the same extent. In the study of Filho et al, despite some symptom scale deterioration, functional scale scores remained high throughout the 3-month follow-up, without significant changes over time, and the overall QoL did not exhibit any significant changes (17); however, the authors commented on significant symptom variability. Melo Filho et al (18) reported loss of physical, social and emotional function and role performance, while Braam et al (16) observed that, compared to baseline, patients reported better emotional functioning and worse social functioning at 12 months, while a better overall health status was reported at 24 months. The authors of that study emphasized the contribution of the patients' own beliefs regarding their illness in terms of QoL prediction after 2 years. When the patients believed their own behaviour did not affect their outcome, it predicted better functioning and better overall health and vice versa.
In the present study, salivary flow rate, QoL and subjective feeling of xerostomia, as measured by XQ, were almost parallel. XQ has been previously validated in a Greek H&N cancer population by the same research team and yielded excellent psychometric properties (11); its association with salivary flow rate allows clinicians to reliably estimate xerostomia and patients' perspective during the follow-up period, without resorting to laboratory methods.
In the present study, all the functional and symptom scales declined following RT and significantly improved after the third timepoint, while salivary flow rates approached or even surpassed baseline levels in a considerable number of patients. The sample homogeneity (mostly laryngeal and nasopharyngeal cancer patients) and the exclusion of terminally ill patients may partly account for these results. However, financial restraints did not allow for a larger study sample and the patients, although randomly selected, may not be representative of all H&N patients attending public hospitals. Despite receiving conventional radiation therapy (not IMRT), the participants exhibited a considerable preservation of salivary gland function after 12 months, allowing for some optimism regarding the course of xerostomia in selected patients.
References
- 1.Guobis Z, Baseviciene N, Paipaliene P, Sabalys G, Kubilius R. Xerostomia: Clinic, etiology, diagnosis and treatment. Medicina (Kaunas) 2006;42:171–179. (In Lithuanian) [PubMed] [Google Scholar]
- 2.Pow EH, Kwong DL, McMillan AS, Wong MC, Sham JS, Leung LH, Leung WK. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: Initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66:981–991. doi: 10.1016/j.ijrobp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Liu XK, Zeng ZY, Hong MH, Zhang AL, Cui NJ, Chen FJ. [Clinical analysis of xerostomia in patients with nasopharyngeal carcinoma after radiation therapy] Ai Zheng. 2004;23:593–596. [PubMed] [Google Scholar]
- 4.Ledeboer QC, Velden LA, Boer MF, Feenstra L, Pruyn JF. Physical and psychosocial correlates of head and neck cancer: An update of the literature and challenges for the future (1996–2003) Clin Otolaryngol. 2005;30:303–319. doi: 10.1111/j.1365-2273.2005.01035.x. [DOI] [PubMed] [Google Scholar]
- 5.Heutte N, Plisson L, Lange M, Prevost V, Babin E. Quality of life tools in head and neck oncology. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131:33–47. doi: 10.1016/j.anorl.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Meirovitz A, Murdoch-Kinch CA, Schipper M, Pan C, Eisbruch A. Grading xerostomia by physicians or by patients after intensity-modulated radiotherapy of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;66:445–453. doi: 10.1016/j.ijrobp.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Pacholke HD, Amdur RJ, Morris CG, Li JG, Dempsey JF, Hinerman RW, Mendenhall WM. Late xerostomia after intensity-modulated radiation therapy versus conventional radiotherapy. Am J Clin Oncol. 2005;28:351–358. doi: 10.1097/01.coc.0000158826.88179.75. [DOI] [PubMed] [Google Scholar]
- 8.Jabbari S, Kim HM, Feng M, Lin A, Tsien C, Elshaikh M, Terrel JE, Murdoch-Kinch C, Eisbruch A. Matched case-control study of quality of life and xerostomia after intensity-modulated radiotherapy or standard radiotherapy for head-and-neck cancer: Initial report. Int J Radiat Oncol Biol Phys. 2005;63:725–731. doi: 10.1016/j.ijrobp.2005.02.045. [DOI] [PubMed] [Google Scholar]
- 9.Bjordal K, Hammerlid E, Ahlner-Elmqvist M, de Graeff A, Boysen M, Evensen JF, Biörklund A, de Leeuw JR, Fayers PM, Jannert M, et al. Quality of life in head and neck cancer patients: Validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H&N35. J Clin Oncol. 1999;17:1008–1019. doi: 10.1200/JCO.1999.17.3.1008. [DOI] [PubMed] [Google Scholar]
- 10.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 11.Memtsa PT, Tolia M, Tzitzikas I, et al. Validity and reliability of the Greek version of the xerostomia questionnaire in head and neck cancer patients. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2016 doi: 10.1007/s00520-016-3471-0. (In press) [DOI] [PubMed] [Google Scholar]
- 12.Kakoei S, Haghdoost AA, Rad M, Mohammadalizadeh S, Pourdamghan N, Nakhaei M, Bahador M. Xerostomia after radiotherapy and its effect on quality of life in head and neck cancer patients. Arch Iran Med. 2012;15:214–218. [PubMed] [Google Scholar]
- 13.Shahdad SA, Taylor C, Barclay SC, Steen IN, Preshaw PM. A double-blind, crossover study of Biotène Oralbalance and BioXtra systems as salivary substitutes in patients with post-radiotherapy xerostomia. Eur J Cancer Care (Engl) 2005;14:319–326. doi: 10.1111/j.1365-2354.2005.00587.x. [DOI] [PubMed] [Google Scholar]
- 14.Pinna R, Campus G, Cumbo E, Mura I, Milia E. Xerostomia induced by radiotherapy: An overview of the physiopathology, clinical evidence, and management of the oral damage. Ther Clin Risk Manag. 2015;11:171–188. doi: 10.2147/TCRM.S70652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roesink JM, Schipper M, Busschers W, Raaijmakers CP, Terhaard CH. A comparison of mean parotid gland dose with measures of parotid gland function after radiotherapy for head-and-neck cancer: Implications for future trials. Int J Radiat Oncol Biol Phys. 2005;63:1006–1009. doi: 10.1016/j.ijrobp.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Braam PM, Roesink JM, Moerland MA, Raaijmakers CP, Schipper M, Terhaard CH. Long-term parotid gland function after radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:659–664. doi: 10.1016/j.ijrobp.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Scharloo M, de Baatenburg Jong RJ, Langeveld TP, van Velzen-Verkaik E, den Doorn-Op Akker MM, Kaptein AA. Illness cognitions in head and neck squamous cell carcinoma: Predicting quality of life outcome. Support Care Cancer. 2010;18:1137–1145. doi: 10.1007/s00520-009-0728-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filho Melo MR, Rocha BA, Pires MB, Fonseca ES, Freitas EM, Junior Martelli H, Santos FB. Quality of life of patients with head and neck cancer. Braz J Otorhinolaryngol. 2013;79:82–88. doi: 10.5935/1808-8694.20130014. [DOI] [PMC free article] [PubMed] [Google Scholar]