Abstract
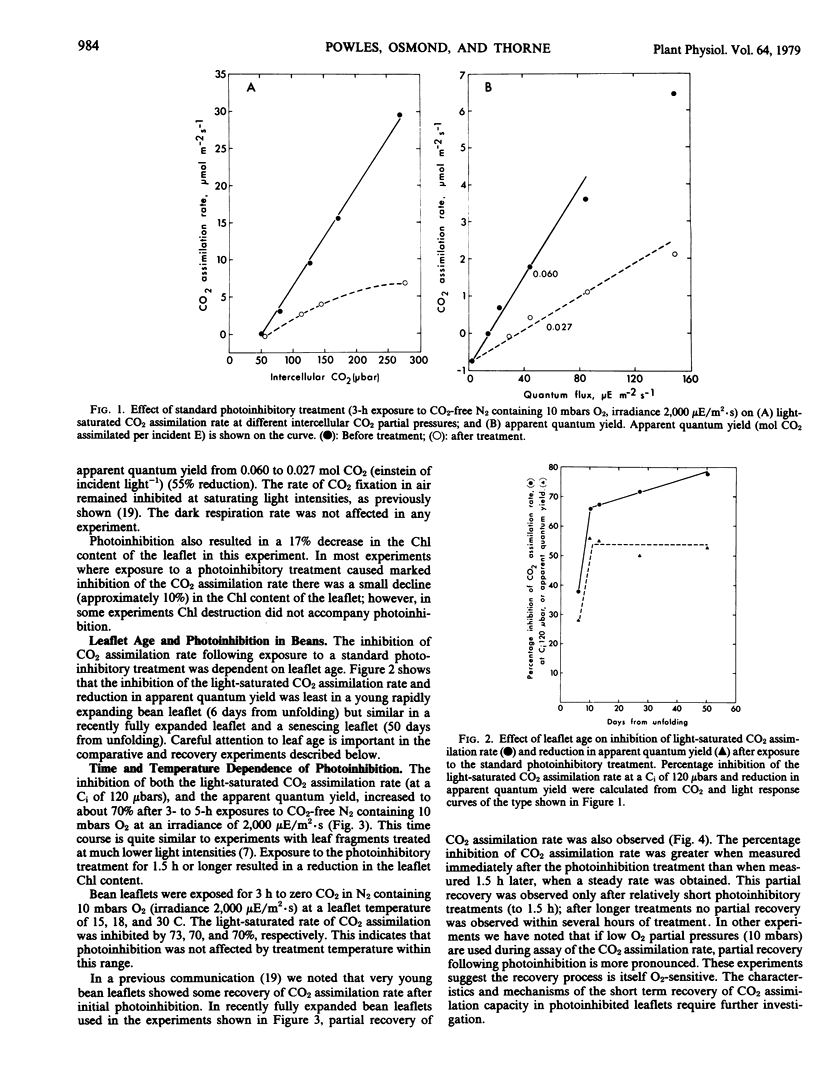
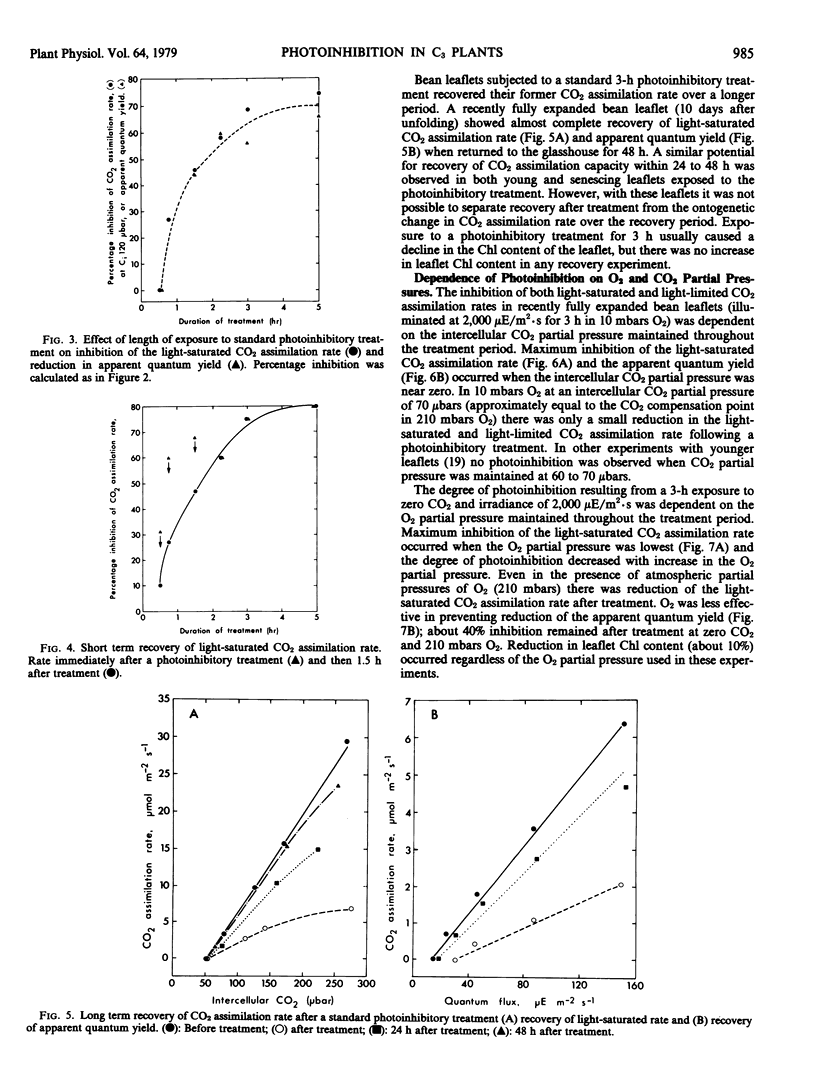
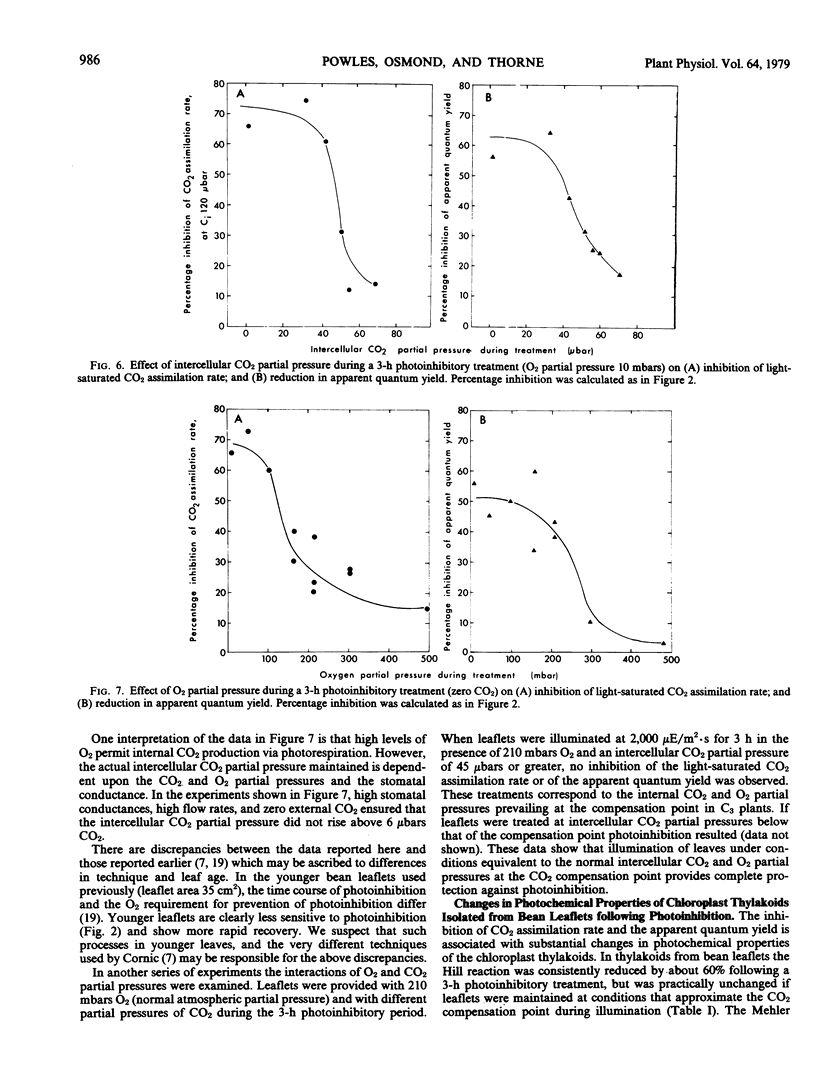
When leaflets of bean and leaves of other species of C3 plants are illuminated in the absence of CO2 and at low O2 partial pressure, the capacity for CO2 assimilation at saturating light and its efficiency at low light intensities are inhibited. This photoinhibition is dependent on leaflet age and period of illumination. In young leaflets and following short exposure to these photoinhibitory conditions, some recovery of CO2 assimilation capacity is observed immediately after treatment. Following substantial (70 to 80%) photoinhibition of CO2 assimilation, recovery in fully expanded leaflets is observed only after 48 hours in normal air. The photoinhibition is largely prevented by providing CO2 at partial pressures equivalent to the CO2 compensation point, or by >210 millibars O2 which permits internal CO2 production by photorespiration. If leaflets are illuminated in 60 microbars CO2 and 210 millibars O2 (the CO2 compensation point in air), no photoinhibition is observed. Electron transport processes and fluorescence emission associated with photosystem II are inhibited in chloroplast thylakoids isolated from leaflets after illumination in zero CO2 and 10 millibars O2. These studies support the hypothesis that CO2 recycling through photorespiration is one means of effectively dissipating excess photochemical energy when CO2 supply to illuminated leaves is limited.
Full text
PDF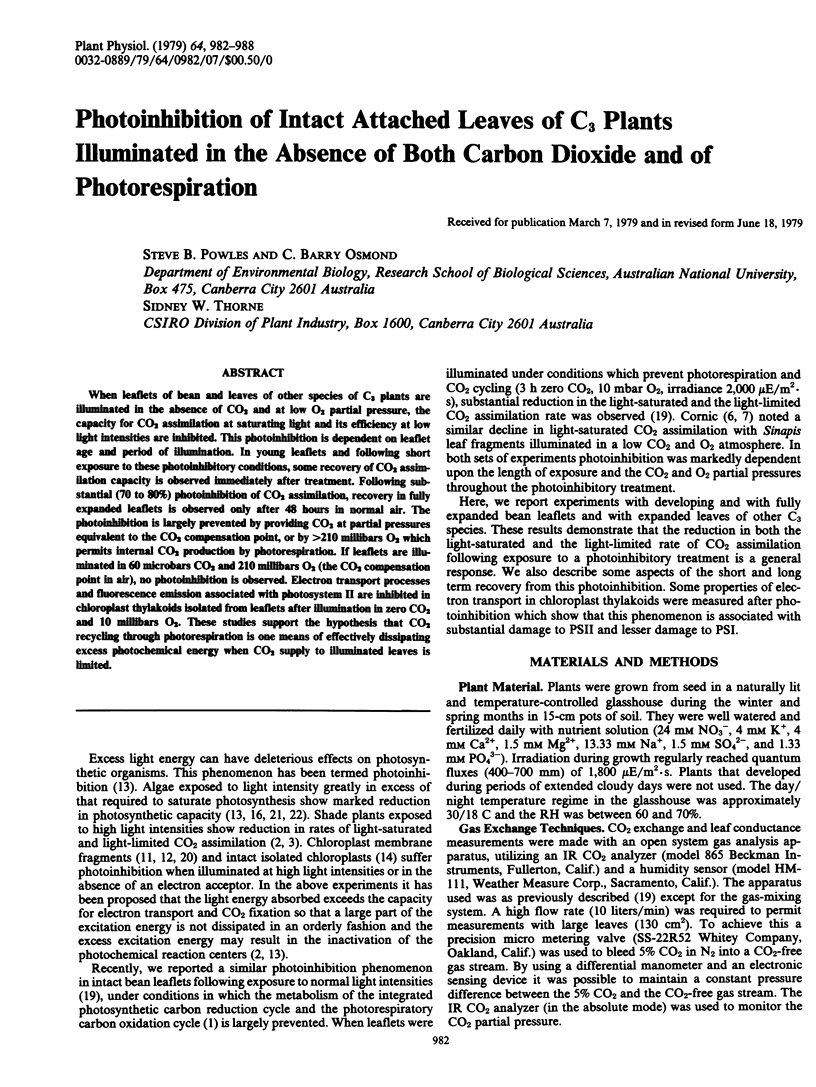


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry J. A., Osmond C. B., Lorimer G. H. Fixation of O(2) during Photorespiration: Kinetic and Steady-State Studies of the Photorespiratory Carbon Oxidation Cycle with Intact Leaves and Isolated Chloroplasts of C(3) Plants. Plant Physiol. 1978 Dec;62(6):954–967. doi: 10.1104/pp.62.6.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman N. K., Thorne S. W., Anderson J. M. Fluorescence properties of particles obtained by digitonin fragmentation of spinach chloroplasts. Proc Natl Acad Sci U S A. 1966 Aug;56(2):586–593. doi: 10.1073/pnas.56.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman N. K., Thorne S. W. Studies on a barley mutant lacking chlorophyll b. II. Fluorescence properties of isolated chloroplasts. Biochim Biophys Acta. 1968 Feb 12;153(2):448–458. doi: 10.1016/0005-2728(68)90086-8. [DOI] [PubMed] [Google Scholar]
- Jones L. W., Kok B. Photoinhibition of Chloroplast Reactions. II. Multiple Effects. Plant Physiol. 1966 Jun;41(6):1044–1049. doi: 10.1104/pp.41.6.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. W., Kok B. Photoinhibition of chloroplast reactions. I. Kinetics and action spectra. Plant Physiol. 1966 Jun;41(6):1037–1043. doi: 10.1104/pp.41.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]


