Abstract
The populations of many pathogen species consist of a collection of common and rare strains but the factors underlying this strain-specific variation in frequency are often unknown. Understanding frequency variation among strains is particularly challenging for vector-borne pathogens where the strain-specific fitness depends on the performance in both the vertebrate host and the arthropod vector. Two sympatric multiple-strain tick-borne pathogens, Borrelia afzelii and B. garinii, that use the same tick vector, Ixodes ricinus, but different vertebrate hosts were studied. 454-sequencing of the polymorphic ospC gene was used to characterize the community of Borrelia strains in a local population of I. ricinus ticks over a period of 11 years. Estimates of the reproduction number (R0), a measure of fitness, were obtained for six strains of B. afzelii from a previous laboratory study. There was substantial variation in prevalence among strains and some strains were consistently common whereas other strains were consistently rare. In B. afzelii, the strain-specific estimates of R0 in laboratory mice explained over 70% of the variation in the prevalences of the strains in our local population of ticks. Our study shows that laboratory estimates of fitness can predict the community structure of multiple-strain pathogens in the field.
Introduction
Many pathogen populations consist of multiple strains or mixed infections in their host1–3. From a public health perspective, the study of multiple-strain pathogens and parasites is important for a number of reasons. Strains belonging to the same pathogen species can show enormous variation in their ability to establish infection and cause disease4, 5. Mixed infections can induce more pathology and disease than single-strain infections6–8. Multiple-strain pathogens complicate the development of vaccines and anti-parasite drugs2, 9. The use of vaccines and anti-parasite drugs can induce strong selection on multiple-strain pathogen populations and result in the competitive release of strains that are not targeted by human medicine10–13. Multiple-strain infections are of further interest to evolutionary biologists because competition between strains influences the evolution of virulence3.
Studies on multiple-strain pathogens have shown that some strains are consistently more common than others over time and/or space14–17. A fundamental question is therefore to understand the genetic and phenotypic factors that underlie this variation in frequency among strains. For example, in Streptococcus pneumonia, the thickness and structure of the capsule were good predictors of whether a given serotype was common or not16. In the case of vector-borne pathogens, the search for predictive phenotypes is complicated by the fact that the frequency of a particular strain depends on its performance in both the vertebrate host and the arthropod vector. Recent developments in so-called next generation population matrix models now allow scientists to combine the relevant transmission components of vector-borne pathogens into the reproduction number (R0), an inclusive measure of fitness18, 19. In conjunction, the development of next generation sequencing methods has greatly enhanced our ability to detect multiple infections in individual hosts20, 21. In the present study, we combined these two next generation methods to better understand the strain-specific prevalence distribution in a tick-borne pathogen.
Borrelia afzelii and B. garinii are two species of tick-borne spirochete bacteria that cause Lyme borreliosis in humans22. In Europe, the main vector for both pathogen species is the hard tick Ixodes ricinus. The two immature tick stages, larva and nymph, take a single blood meal to develop into the next stage23. Larval ticks acquire spirochetes after feeding on an infected reservoir host and develop into infected nymphs that transmit the pathogen the following year to the next generation of reservoir hosts. B. afzelii and B. garinii are specialized on different classes of vertebrate hosts: rodents and birds, respectively23–26, and therefore rarely occur together in the same tick27–30. Local populations of both Borrelia species consist of multiple strains21, 31–34, which are often defined by the highly polymorphic, single-copy ospC gene35–38. In the present study, we used the ospC-typing system to study mixed-strain infections within each of the two Borrelia species, as others have done previously21, 31, 33, 34, 39–42.
The first purpose of the present study was to test whether laboratory estimates of strain-specific fitness could predict the strain structure of Borrelia pathogens in the field. We had recently used a laboratory Lyme borreliosis system that included Mus musculus mice and I. ricinus ticks to show that there was significant variation in fitness among six different ospC strains of B. afzelii 43. For each of the six strains, we measured the three most important fitness components of any vector-borne pathogen: vector-to-host transmission, host-to-vector transmission, and co-feeding transmission43. We then used next generation matrices to estimate the reproduction number (R0) for each of the six strains43. These strains originated from a field site near Neuchâtel where the local tick population had been sampled over a period of 11 years to create a collection of isolates of B. afzelii and B. garinii. We had recently estimated the diversity of ospC strains in these tick-derived isolates using 454-sequencing21. These two studies therefore provided a unique opportunity to test whether laboratory estimates of strain-specific fitness can predict the strain structure of Borrelia pathogens in the field.
The second purpose of the present study was to test whether the prevalences of the different ospC strains were stable or fluctuating over time. A review on the evolutionary ecology of LB pathogens emphasized the importance of studying temporal variation in the frequencies of Borrelia ospC strains22. A number of Lyme disease researchers have predicted that the frequencies of the ospC strains should cycle over time22, 44, 45. Thus long-term studies are important because they may improve our understanding of the ecological factors that shape the dynamics of multiple-strain Borrelia pathogens. In addition, for the laboratory estimates of fitness to have predictive value, the ospC strain structure should be stable over time so that some strains are consistently more common than others. In contrast, if the ospC strains cycle between being rare and being common, the laboratory estimates of R0 are unlikely to predict the long-term average strain-specific prevalences. The present study therefore provided a unique opportunity to test whether the prevalences of the ospC strains are stable or fluctuating over time in two sympatric Borrelia species.
Results
Background
The University of Neuchâtel has a large collection of B. afzelii and B. garinii isolates that were obtained from a local population of I. ricinus nymphs over a period of 11 years. We had previously characterized the community of ospC strains in a stratified random sample of these isolates using 454-sequencing21. The stratified random sample contained a maximum of 20 isolates for each of the 22 combinations of Borrelia species and year, resulting in a total of 193 isolates of B. afzelii and 190 isolates of B. garinii 21. We had previously assigned the ospC gene sequences to a limited set of distinct clusters, the so-called ospC major groups (oMGs), of which 10 belonged to B. afzelii and 11 to B. garinii 21. For each oMG strain, the proportion of isolates carrying at least one spirochete of that particular strain was calculated for each year and for the duration of the study. These proportions give the prevalence of each oMG strain in the subset of infected ticks. We use the term ‘relative prevalence’ to indicate that the proportion was calculated over the subset of infected ticks that yielded an isolate of that particular Borrelia species and not the whole sample of ticks (i.e., uninfected ticks were excluded in the calculation of this proportion). The prevalence can range from 0.00 to 1.00 for any given oMG strain in any given year.
Relative prevalences differ among the oMG strains in B. afzelii and B. garinii
Proportion tests were used to determine whether the long-term average prevalences differed among oMG strains within each Borrelia species. The mean relative prevalences were significantly different between the 10 oMG strains in B. afzelii (proportion test: χ2 = 280.512, df = 9, p < 0.001; Fig. 1) and between the 11 oMG strains in B. garinii (proportion test: χ2 = 179.018, df = 10, p < 0.001; Fig. 1). In B. afzelii, the most common oMG (A10) was 11.7 times more common than the least common oMG (A3). In B. garinii, the most common oMG (G8) was 26.7 times more common than the least common oMG (G10).
Figure 1.
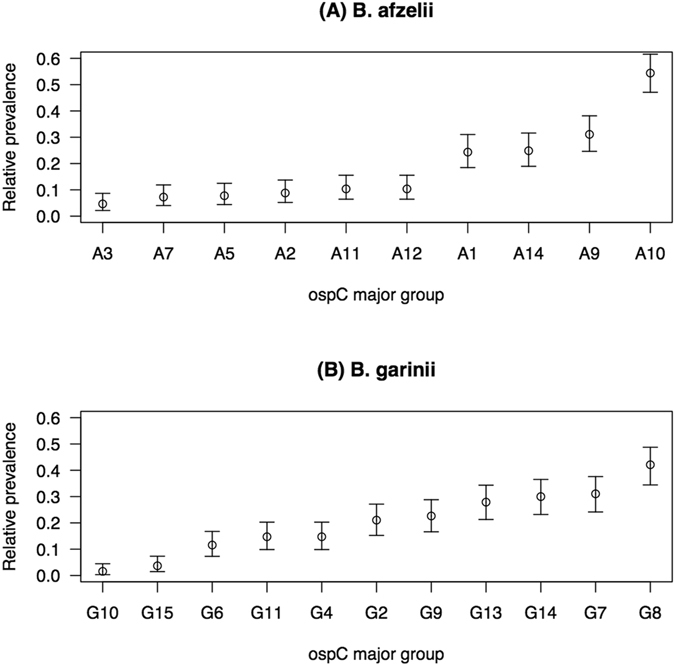
The relative prevalences differ significantly among the oMGs in (A) B. afzelii and in (B) B. garinii. For each species, the oMGs are ranked from least common to most common. The relative prevalence of an oMG strain is defined as the proportion of B. afzelii-infected nymphs that are infected with that particular oMG strain.
Directional change in the relative prevalences of the oMG strains over time
The relative prevalence distribution of the oMG strains for the first six years of the study was significantly correlated with the relative prevalence distribution for the last five years of the study in both B. afzelii (Pearson correlation test: r = 0.869, p = 0.001; Fig. 2) and B. garinii (Pearson correlation test: r = 0.945, p < 0.001; Fig. 2). This result suggests that the community of oMG strains was the same between the two halves of the study, and that previously common strains did not become rare or vice versa. There was significant directional change in the relative prevalence between the start (2000 to 2005) and the end (2006 to 2010) of the study for 2 of the 20 oMGs (Table S2). B. afzelii oMGs A11 and A14 increased their relative prevalence by a factor of 4.0 (p = 0.009) and 1.8 (p = 0.027), respectively, between the start and the end of the study. However, after Bonferroni correction, none of the directional changes in relative prevalence were statistically significant. In summary, there was no directional change in the relative prevalences between the start and the end of the study.
Figure 2.
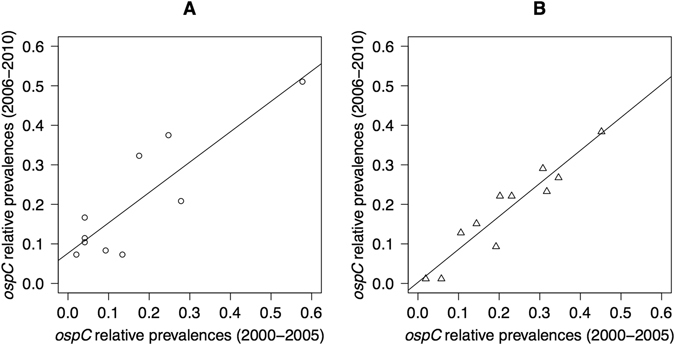
The community of oMG strains is stable over time. The relationship between the relative prevalences of the oMG strains of the first 6 years versus the last 5 years of the survey is shown for both (A) B. afzelii and (B) B. garinii. The Pearson correlation of the relative prevalence distribution of the oMG strains between the two time periods was highly significant for both Borrelia species. The relative prevalence of an oMG strain is defined as the proportion of B. afzelii-infected nymphs that are infected with that particular oMG strain.
Stability of the relative prevalences of the oMG strains over time
The relative prevalences of the oMG strains were stable over time in both B. afzelii and B. garinii (Figures S1, S2, S3 and S4). There were 110 proportion tests for B. afzelii (10 oMGs * 11 years) and 99 proportion tests for B. garinii (9 oMGs * 11 years). Assuming a type I error rate of 0.05, we expect that there should be 0.05 * 209 = 10.45 proportion tests that are statistically significant for the two Borrelia species. Of the 209 proportion tests, only 11 annual prevalences were significantly different (p < α = 0.05) from the average prevalence over the duration of the study (these years are marked with an asterisk in Figures S1, S2, S3 and S4). These 11 significant prevalences occurred in 6 different years and were distributed over 9 different oMG strains. The observed number of significant prevalences (11) was equal to the expected number of type I errors (10.45). This result supports the idea that the oMG strains did not fluctuate over time (Figures S3 and S4).
Relationship between the R0-values and the relative prevalences of the oMG strains in B. afzelii
For the six B. afzelii oMG strains for which we had data43, there was a positive relationship between the strain-specific reproduction number (R0) in laboratory mice and the strain-specific relative prevalence in the questing I. ricinus nymphs (GLM with binomial errors; χ2 = 135.25, dof = 1, p < 0.001; Fig. 3). After correcting for overdispersion, the relationship remained statistically significant (GLM with quasibinomial errors; F1, 4 = 10.019, p = 0.034). The strain-specific R0 value explained 70.24% of the variation in the strain-specific relative prevalences (Fig. 3). Thus estimates of R0 using laboratory mice were a good predictor of the relative prevalences of the B. afzelii oMG strains in a wild population of I. ricinus nymphs.
Figure 3.
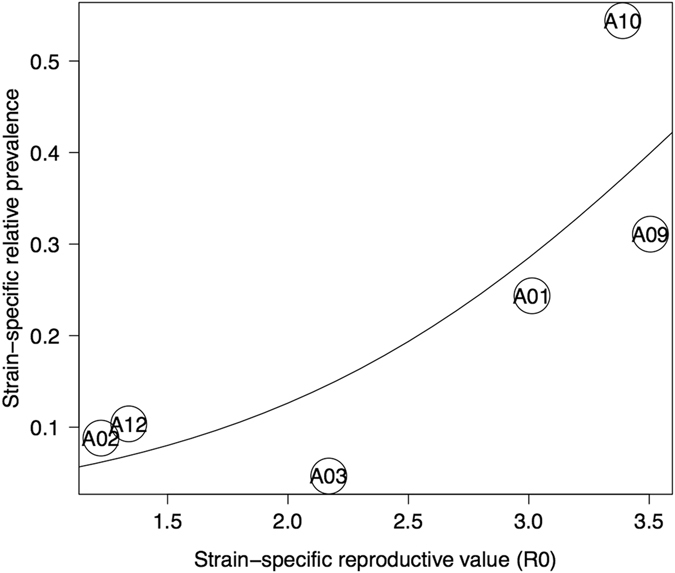
The reproductive numbers (R0) of six B. afzelii oMG strains determine the strain-specific relative prevalences in a wild population of I. ricinus nymphs. Each data point is labeled with the name of the oMG of the B. afzelii strain. The strain-specific R0 values were estimated from an experimental infection study using laboratory mice43. The strain-specific relative prevalences were estimated over the 11-year duration of the present study. The relative prevalence of an oMG strain is defined as the proportion of B. afzelii-infected nymphs that are infected with that particular oMG strain.
Discussion
The most interesting result from this study was that our estimates of R0 for six oMG strains of B. afzelii explained ~70% of the differences in prevalences among these strains in the field (Fig. 3). The strain-specific estimates of R0 were calculated using the three most important fitness components for any vector-borne pathogen: vector-to-host transmission, systemic (host-to-vector) transmission, and co-feeding transmission. These pathogen fitness components, in turn, were estimated using a laboratory Lyme borreliosis system that used Mus musculus mice as the reservoir host and Ixodes ricinus ticks as the vector. Thus laboratory estimates of transmission combined into a single measure of fitness (R0) were able to predict the community structure of this multiple-strain tick-borne pathogen in nature. Previous studies have used next generation matrix methods to estimate and compare R0 values between different tick-borne pathogens (e.g. Borrelia burgdorferi versus tick-borne encephalitis virus) to enhance our understanding of their ecology and epidemiology18, 19, 46, 47. Our study is an important demonstration that laboratory estimates of R0 can predict the strain-specific prevalences of a vector-borne pathogen in the field.
We had previously tested the relationship between our laboratory estimates of R0 and the relative prevalences of the oMG strains in the field, but the p-values were not significant43. In that study43, the prevalences of the oMG strains were obtained from the study by Pérez et al.33, who had collected nymph-derived isolates from two field sites in Switzerland, which are different from the Bois de l’Hôpital site in the present study. The major improvements in the present study are as follows. First, the prevalences of the B. afzelii oMG strains are based on a larger sample size at a single site, whereas the study by Pérez et al.33 had smaller sample sizes at two different sites. Second, we used 454-sequencing and the prevalences of the B. afzelii oMG strains were based on 114,432 ospC sequences, whereas in the study by Pérez et al.33, the prevalences of the oMG strains were based on cold single-strand conformational polymorphism analysis. We have previously pointed out that the sensitivity of single-strand conformational polymorphism analysis is much lower than that of 454-sequencing21, 42. For example, for the subset of B. afzelii-infected questing nymphs, Pérez et al.33 found that 1.5% carried multiple oMG strains, whereas we found that 78.8% carried multiple oMG strains21. Third, an implicit assumption underlying Fig. 3 is that the genetic backgrounds of the oMG strains used in the infection experiment of Tonetti et al.43 are similar to the oMG strains in our local tick population. This assumption is more likely to be met in the present study, because 3 of the 6 B. afzelii oMG strains (A2, A9, and A10) used to estimate the strain-specific R0 values in the infection experiment of Tonetti et al.43 came from the same Bois de l’Hôpital field site used to estimate the oMG strain prevalences in Fig. 3.
Studies on human pathogens consisting of multiple-strains such as Neisseria meningitidis and Streptococcus pneumoniae have shown that the frequencies of these strains can remain constant over time14–16. Similarly, we found that the community of oMG strains was stable over time and that some strains were consistently more common than others (Figs 2 and 3, S1 and S2). This result was particularly striking in B. afzelii where A10 was the most common oMG strain in 10 of the 11 years of the study (Fig. 2). We point out that the present study finds no evidence for the prediction found in the Lyme borreliosis literature that common oMG strains should decrease in frequency over time because they are preferentially targeted by the vertebrate immune system22, 44, 45. Previous studies on B. burgdorferi s. s. in I. scapularis ticks in the northeastern United States have documented rapid shifts in the prevalence distribution of the oMGs17, 48, 49. However, these studies were either done in tick populations where B. burgdorferi s. s. was emerging48 or over shorter time periods (3 years) using less reliable methods (single-strand conformational polymorphism analysis) for detecting multiple oMG strains in ticks17, 49. The present study of two sympatric Borrelia species is exceptional because of its long duration and because of its focus on a local population at a single small field site where Lyme borreliosis is endemic.
There are two hypotheses that explain how the ospC gene polymorphism of B. burgdorferi s. l. pathogens is maintained in nature: balancing or frequency-dependent selection17, 35, 49, 50 and multiple niche polymorphism45, 51, 52. In the frequency-dependent selection hypothesis, the vertebrate immune system is the mechanism that preferentially targets the most common strains and thereby prevents them from becoming too common22, 35, 45, 50. The OspC protein is an immunodominant antigen that induces a strong antibody response that protects the vertebrate host from secondary infection53–57. Protection is highly specific, and immunization with a given oMG antigen will only protect against infection with strains carrying the same oMG allele58–60. In summary, an immune-based explanation for the ospC polymorphism is intuitive because the OspC protein resembles other highly variable pathogen surface antigens that are under immune-based selection61–64.
Multiple niche polymorphism is the other hypothesis for the ospC polymorphism in Borrelia species45, 51. In this hypothesis, the different oMG strains of the Borrelia pathogen are adapted to different vertebrate host species, which represent different ecological niches45, 51, 65. The multiple niche polymorphism hypothesis was developed for B. burgdorferi s. s. in North America, which has a large host range22, 23, but the evidence for this hypothesis is conflicted51, 52, 66. B. afzelii has a much narrower host range than B. burgdorferi s. s. and is mostly associated with rodents31, 33, 34. Host blood meal analysis of I. ricinus ticks at our field site in Switzerland found that the most important rodent species were Apodemus mice, the bank vole (Myodes glareolus), and the red squirrel (Sciurus vulgaris)67, 68. With the exception of a system in France that contains an introduced species of chipmunk40, 41, other studies on B. afzelii in Europe have found little support for the multiple niche polymorphism hypothesis33, 34, 39. In the present study, the strain-specific estimates of R0 were based on a single rodent species (Mus musculus) but were still highly predictive of the strain structure in the field. This result shows that it was not necessary to consider multiple host species and provides an indirect argument against the multiple niche polymorphism hypothesis.
A number of reviews on the ecology of Lyme borreliosis have predicted that the frequencies of the oMG strains should cycle under frequency-dependent selection22, 44, 45. The present study found no evidence of cycles, but this result should not be interpreted as evidence against the frequency-dependent selection hypothesis. We point out here that immune-based models of selection on multiple-strain pathogens can produce all kinds of dynamics. Gupta and colleagues developed a number of theoretical models that explore how cross-reactive immune responses directed against immunodominant pathogen antigens (such as the OspC protein) influence the dynamics and community structure of multiple-strain pathogens69–71. A key finding of these models is that strong selection on immunodominant antigens by the host immune system will cause the pathogen population to organize into a set of unique serotypes that minimizes cross-reactive acquired immunity71. In such systems, the community of strains can be stable over long periods of time and the prevalence of each strain depends on its reproduction number (R0) as observed in the present study69, 70. With respect to another vector-borne parasite, the human malaria parasite Plasmodium falciparum, Gupta and colleagues suggested that, “many features of its epidemiology can be explained by assuming that it is a construct of ‘independently transmitted strains69, 72’”. Similarly, Qiu et al.73 suggested that the oMGs “could be viewed as evolutionarily stable “clonal complexes” within B. burgdorferi populations”. In summary, we suggest that immune-mediated selection on the OspC antigen is the best explanation for the maintenance of the ospC polymorphism in nature.
Due to its critical role in host invasion74, 75, the ospC gene has received much interest from a public health perspective. Genetic analysis of human isolates of B. burgdorferi s. l. revealed that only a subset of oMG strains is capable of infecting and causing disease in humans4, 76, 77. In the United States, oMG strains A, B, K, I, and N are most commonly associated with disseminated infections in humans but these strains are also the most common in questing I. scapularis ticks76. In the present study, strains carrying oMG A10 dominated the B. afzelii population in Neuchatel over the last decade (Fig. 1). Recent studies have shown that oMG strain A10 is common in other parts of Switzerland33 and Sweden31. In contrast, genetic screening of human isolates has never recovered oMG A10 from a human patient36–38. However, these genetic screens were based on a limited number of tissue biopsies (245 ospC sequences) that may have been sampled from areas in Europe where oMG A10 was not locally common36–38. Future studies should screen human isolates of B. afzelii to test whether strains carrying this oMG are infectious to humans.
In conclusion, our study on two common Lyme borreliosis pathogens in a local population of I. ricinus ticks showed that the community of Borrelia oMG strains was stable over a period of 11 years. In both B. afzelii and B. garinii, some oMG strains were consistently common whereas other oMG strains were consistently rare. In B. afzelii, the strain-specific estimates of R0 in laboratory rodents explained over 70% of the variation in the strain-specific prevalences in the field. Our results are consistent with theoretical models of how cross-reactive acquired immunity in the vertebrate host can determine the strain structure of pathogen populations69–71. The present study shows the importance of studying local pathogen populations over long periods of time to better understand their epidemiology.
Methods
Field sampling and molecular methods
The sampling of the I. ricinus ticks in the field, the testing for Borrelia infection, and the 454-sequencing of the single-copy ospC gene was described in a previous study21. Briefly, I. ricinus nymphs were sampled in a deciduous forest at the Bois de l’Hôpital site near the city of Neuchâtel (47°00′55.6″N, 6°94′16.7″E; surface of ~1 ha) over a period of 11 years (2000 to 2010). Nymphs were screened for spirochete infection using immunofluorescence microscopy and Borrelia-infected nymphs were incubated in BSK II medium at 34 °C. DNA was extracted from all spirochete-positive BSK cultures and the Borrelia species was identified using a PCR-reverse line blot assay that targets the 23S-5S spacer gene78. The experimental design was described in a previous study21. Only those isolates that were singly infected with B. afzelii or B. garinii were selected for 454-sequencing of the ospC gene. For each Borrelia species, a maximum of 20 isolates was randomly selected for each of the 11 years of the survey for a total of 193 B. afzelii isolates and 190 B. garinii isolates. For each of these 383 isolates, the ospC gene was amplified using the PCR protocol of Bunikis et al.36. 454-sequencing of the amplicons in the forward direction produced 240,410 useable ospC gene sequences (reads) and each sequence was 521 bp long. For each nymphal-tick derived isolate, the mean coverage was 632 ospC gene sequences (reads).
Identification of the ospC major groups
The ospC gene sequences can be classified into what are called ospC major groups (oMGs). The oMGs have a highly discrete pattern of genetic variation where each oMG is ≥8% different in DNA sequence from all other oMGs35–38, 42, 51. For the three Lyme borreliosis pathogens that have been most studied to date, B. burgdorferi s. s., B. afzelii, and B. garinii, each Borrelia species contains ~20 oMGs worldwide, with local populations often having 50% or more of this diversity17, 21, 35, 36, 38, 40–42, 51. We had previously shown that all 240,410 ospC gene sequences clustered into 23 distinct oMGs that were 8% divergent from each other in DNA sequence21. Within each oMG, the DNA sequence variation was <2%21. We did not find any ospC gene sequences that were intermediately divergent (2–8%)21. This finding is important because it shows that the oMG alleles are real biological categories that are relatively robust to errors in sequencing or to changes in the clustering protocol. For example, changing the similarity threshold of our clustering protocol from 93–98% did not affect the number of unique oMGs in our dataset21.
Nomenclature of the oMGs of B. afzelii and B. garinii
In the literature, there are two different nomenclatures for the oMGs of B. afzelii and B. garinii 36, 38. In the present study, we used the nomenclature system that was developed by Bunikis et al.36, and which has been used by others and we21, 31, 34, 42. According to this nomenclature system, the 23 oMGs were as follows: 10 for B. afzelii (A1, A2, A3, A5, A7, A9, A10, A11, A12, and A14), 11 for B. garinii (G2, G4, G6, G7, G8, G9, G10, G11, G13, G14, and G15), 1 for B. burgdorferi s. s. (Q), and 1 for B. valaisiana (V1)21. The present study is restricted to the oMGs belonging to B. afzelii and B. garinii. In what follows, we will refer to a tick-derived isolate carrying a particular oMG allele as an oMG strain.
Calculation of the relative prevalences of the oMG strains over time
The community of oMG strains was determined for each of the 383 nymph-derived spirochete isolates as described previously21. An oMG was considered as present as long as the isolate contained a single sequence belonging to that group21. For each oMG strain, the annual relative prevalence was calculated as the proportion of nymph-derived spirochete isolates that carried that particular oMG that year21. In our previous study, we investigated patterns of oMG strain diversity in ticks and patterns of co-occurrence of oMG strains within ticks21. Those analyses combined all the data over the 11 years of the study and ignored temporal variation in the prevalences of the oMG strains, which is the focus of the present study.
Important assumptions of this study
This study makes three important assumptions. The first assumption is that the ospC gene is a reliable genetic marker for a given strain. The second assumption is that the step of culturing the Borrelia isolates in BSK media did not change the composition of the oMG strains. The third assumption is that the PCR protocol used to amplify the ospC gene was equally effective at amplifying all of the different oMG alleles. We address each of these three assumptions in the supplementary information.
Statistical Methods
Directional change in the relative prevalences of the oMG strains over time
To visualize whether the community of oMG strains changed between the first and second halves of the study, we compared the relative prevalences of the oMGs for the first six years (2000 to 2005) and for the last five years of the study (2006 to 2010). We used a Pearson’s correlation test to determine whether there was a correlation in the relative prevalences of the oMG strains between these two periods of time. This test was done separately for B. afzelii and B. garinii. We used a proportion test to determine whether the relative prevalence of any of the oMG strains had changed between the first six years (2000 to 2005) and the last five years of the study (2006 to 2010). This approach allows us to detect large directional changes in relative prevalence over the duration of the study but not scenarios where the prevalences of the strains cycle within the 11-year period of the study.
Stability of the relative prevalences of the oMG strains over time
Previous reviews suggested that the oMG strains should cycle over time22, 44, 45. If true, we would expect the years where a given strain is common or rare to deviate significantly from the long-term average of that strain over the course of the study. For each of the 10 B. afzelii oMGs (A1, A2, A3, A5, A7, A9, A10, A11, A12, and A14), we used a proportion test to determine whether its relative prevalence in a given year was significantly different from the average long-term relative prevalence of that strain over the duration of the study. We did the same for each of the 9 most common B. garinii oMGs (G2, G4, G6, G7, G8, G9, G11, G13, and G14; oMGs G10 and G15 were too rare to be analysed). To test for the stability of the relative prevalences of the oMG strains, we summed the number of significant deviations from the long-term average for all the oMG strains belonging to the same Borrelia species. The observed number of significant deviations was then compared to the null hypothesis that deviations were caused by random binomial sampling error.
Relationship between R0 and the relative prevalences of the oMG strains
The R0 of an infection can be thought of as the number of cases one case generates, on average, over the course of its infectious period, in an otherwise uninfected population. In the absence of immunity-mediated competition between strains, theory predicts that the R0 value of each strain will determine its prevalence in nature70. We recently conducted a study where laboratory mice were experimentally infected via tick bite with one of six B. afzelii oMG strains: A1, A2, A3, A9, A10, and A1243. To avoid confusion, we point out that Tonetti et al.43 used the nomenclature developed by Lagal et al.38 and Pérez et al.33 and the six B. afzelii oMG strains in that study are therefore referred to as A2, ME, A3, A1, YU, and A4, respectively. The purity of these six strains was recently confirmed by 454-sequencing of the ospC gene (Table S1). Importantly, three of these isolates, A2, A9, and A10, were obtained from the Neuchâtel area, whereas isolates A1, A3, and A12 were obtained from Thune (Switzerland), Austria, and Germany respectively. For each of the six strains, the following three fitness components were measured: tick-to-host transmission, systemic (host-to-tick) transmission, and co-feeding transmission43.
We used next generation matrix methods18, 46, 47 to combine these transmission components into the reproduction number (R0) for each of the six B. afzelii ospC strains43. The study of Tonetti et al.43 assumed that the efficiency of vertical transmission (r A) was 0.10 and that the proportion of competent hosts (h c) was 0.50. Recent work suggests that transovarial transmission of B. burgdorferi s. l. does not occur in Ixodes ticks79, 80 and the value of r A was therefore set to 0.00 in the present study. Host blood meal analysis in our local Lyme borreliosis system suggests that only 28.0% of questing immature I. ricinus ticks obtained their blood meal from B. afzelii-competent rodent reservoir hosts68, and the value of h c was therefore set to 0.28 in the present study. Thus the estimates of R0 for the six strains of B. afzelii are similar but not identical between Tonetti et al.43 and the present study. We used a generalized liner model (GLM) with binomial errors to test whether there was a positive relationship between the R0 values of the six B. afzelii ospC strains and the strain-specific relative prevalences in the local I. ricinus population (averaged over the entire course of the study). We calculated the associated r2 using the McFadden’s pseudo r2 method.
Electronic supplementary material
Acknowledgements
This work was supported by a grant from the Swiss National Science Foundation (SNSF) to Maarten Voordouw (FN 31003A_141153). The University of Neuchâtel supported this work by an SNSF overhead grant to Maarten Voordouw (U.01851.01 project 4.5). The members of the working group ‘Tiques et Maladies à Tiques’ (GDR REID) provided insightful discussions. Thanks to Marco Pagni from the Swiss Institute of Bioinformatics for helping with the clustering analysis. Thanks to Dustin Brisson, Jacob Koella, Steve Perlman, and Lars Raberg for comments on this manuscript. This study is part of the PhD thesis of Jonas Durand.
Author Contributions
J.D. and M.J.V. designed the study, analysed the data, and wrote the manuscript. L.G. designed the tick sampling survey, screened the isolates for Borrelia infection, and edited the manuscript. O.R. sampled the ticks in the field and determined Borrelia infection via the reverse line blot assay. J.D. performed the library preparation and the clustering analysis of the ospC gene sequences into the oMGs. M.J. participated in the library preparation of the samples. All authors read and approved the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01821-1
Accession Codes: The ospC gene sequence data have been deposited in the Sequence Read Archive under BioProject PRJNA293785 with the accession number SRP063760.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Balmer O, Tanner M. Prevalence and implications of multiple-strain infections. Lancet Infectious Diseases. 2011;11:868–878. doi: 10.1016/S1473-3099(11)70241-9. [DOI] [PubMed] [Google Scholar]
- 2.Schmid-Hempel, P. Evolutionary Parasitology: The Integrated Study of Infections, Immunology, Ecology, and Genetics. (Oxford University Press, 2011).
- 3.Alizon S, de Roode JC, Michalakis Y. Multiple infections and the evolution of virulence. Ecol. Lett. 2013;16:556–567. doi: 10.1111/ele.12076. [DOI] [PubMed] [Google Scholar]
- 4.Seinost G, et al. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect. Immun. 1999;67:3518–3524. doi: 10.1128/iai.67.7.3518-3524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yazdankhah SP, et al. Distribution of serogroups and genotypes among disease-associated and carried isolates of Neisseria meningitidis from the Czech Republic, Greece, and Norway. J. Clin. Microbiol. 2004;42:5146–5153. doi: 10.1128/JCM.42.11.5146-5153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottlieb GS, et al. Dual HIV-1 infection associated with rapid disease progression. Lancet. 2004;363:619–622. doi: 10.1016/S0140-6736(04)15596-7. [DOI] [PubMed] [Google Scholar]
- 7.Henning L, et al. A prospective study of Plasmodium falciparum multiplicity of infection and morbidity in Tanzanian children. Trans. R. Soc. Trop. Med. Hyg. 2004;98:687–694. doi: 10.1016/j.trstmh.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Mueller I, et al. Force of infection is key to understanding the epidemiology of Plasmodium falciparum malaria in Papua New Guinean children. Proc. Natl. Acad. Sci. USA. 2012;109:10030–10035. doi: 10.1073/pnas.1200841109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipsitch M, O’Hagan JJ. Patterns of antigenic diversity and the mechanisms that maintain them. J. Royal Soc. Interface. 2007;4:787–802. doi: 10.1098/rsif.2007.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wargo AR, Huijben S, de Roode JC, Shepherd J, Read AF. Competitive release and facilitation of drug-resistant parasites after therapeutic chemotherapy in a rodent malaria model. Proc. Natl. Acad. Sci. USA. 2007;104:19914–19919. doi: 10.1073/pnas.0707766104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Read AF, Day T, Huijben S. The evolution of drug resistance and the curious orthodoxy of aggressive chemotherapy. Proc. Natl. Acad. Sci. USA. 2011;108:10871–10877. doi: 10.1073/pnas.1100299108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollitt, L. C. et al. Rapid response to selection, competitive release and increased transmission potential of artesunate-selected Plasmodium chabaudi malaria parasites. PLOS Pathog. 10 (2014). [DOI] [PMC free article] [PubMed]
- 13.Read, A. F. & Mackinnon, M. In Evolution in Health and Disease (eds Stearns, S. C. & Koella, J. C.) (Oxford University Press, 2007).
- 14.Buckee CO, Gupta S, Kriz P, Maiden MCJ, Jolley KA. Long-term evolution of antigen repertoires among carried meningococci. P. Roy. Soc. B-Biol. Sci. 2010;277:1635–1641. doi: 10.1098/rspb.2009.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bambini, S. et al. An analysis of the sequence variability of meningococcal fHbp, NadA and NHBA over a 50-year period in the Netherlands. PLOS ONE8 (2013). [DOI] [PMC free article] [PubMed]
- 16.Weinberger, D. M. et al. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLOS Pathog. 5 (2009). [DOI] [PMC free article] [PubMed]
- 17.Qiu WG, Dykhuizen DE, Acosta MS, Luft BJ. Geographic uniformity of the Lyme disease spirochete (Borrelia burgdorferi) and its shared history with tick vector (Ixodes scapularis) in the northeastern United States. Genetics. 2002;160:833–849. doi: 10.1093/genetics/160.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartemink NA, Randolph SE, Davis SA, Heesterbeek JAP. The basic reproduction number for complex disease systems: Defining R-0 for tick-borne infections. Am. Nat. 2008;171:743–754. doi: 10.1086/587530. [DOI] [PubMed] [Google Scholar]
- 19.Matser A, Hartemink N, Heesterbeek H, Galvani A, Davis S. Elasticity analysis in epidemiology: an application to tick-borne infections. Ecol. Lett. 2009;12:1298–1305. doi: 10.1111/j.1461-0248.2009.01378.x. [DOI] [PubMed] [Google Scholar]
- 20.Juliano JJ, et al. Exposing malaria in-host diversity and estimating population diversity by capture-recapture using massively parallel pyrosequencing. Proc. Natl. Acad. Sci. USA. 2010;107:20138–20143. doi: 10.1073/pnas.1007068107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durand J, et al. Cross-immunity and community structure of a multiple-strain pathogen in the tick vector. Appl. Environ. Microbiol. 2015;81:7740–7752. doi: 10.1128/AEM.02296-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtenbach, K. et al. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat. Rev. Microbiol. 4 (2006). [DOI] [PubMed]
- 23.Piesman J, Gern L. Lyme borreliosis in Europe and North America. Parasitology. 2004;129:S191–S220. doi: 10.1017/S0031182003004694. [DOI] [PubMed] [Google Scholar]
- 24.Gern, L. & Humair, P.-F. In Lyme borreliosis: biology, epidemiology, and control 149-174 (CABI Publishing, 2002).
- 25.Kurtenbach K, et al. Host association of Borrelia burgdorferi sensu lato - the key role of host complement. Trends Microbiol. 2002;10:74–79. doi: 10.1016/S0966-842X(01)02298-3. [DOI] [PubMed] [Google Scholar]
- 26.Heylen DJA, et al. Inefficient co-feeding transmission of Borrelia afzelii in two common European songbirds. Scientific Reports. 2017;7:39596. doi: 10.1038/srep39596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrmann C, Gern L, Voordouw M. Species co-occurrence patterns among Lyme borreliosis pathogens in the tick vector Ixodes ricinus. Appl. Environ. Microbiol. 2013;79:7273–7280. doi: 10.1128/AEM.02158-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurtenbach K, et al. Distinct combinations of Borrelia burgdorferi sensu lato genospecies found in individual questing ticks from Europe. Appl. Environ. Microbiol. 2001;67:4926–4929. doi: 10.1128/AEM.67.10.4926-4929.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gern L, Douet V, Lopez Z, Rais O, Moran Cadenas F. Diversity of Borrelia genospecies in Ixodes ricinus ticks in a Lyme borreliosis endemic area in Switzerland identified by using new probes for reverse line blotting. Ticks Tick Borne Dis. 2010;1:23–29. doi: 10.1016/j.ttbdis.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Rauter C, Hartung T. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: a metaanalysis. Appl. Environ. Microbiol. 2005;71:7203–7216. doi: 10.1128/AEM.71.11.7203-7216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersson M, Scherman K, Raberg L. Multiple-strain infections of Borrelia afzelii: a role for within-host interactions in the maintenance of antigenic diversity? Am. Nat. 2013;181:545–554. doi: 10.1086/669905. [DOI] [PubMed] [Google Scholar]
- 32.Heylen D, Matthysen E, Fonville M, Sprong H. Songbirds as general transmitters but selective amplifiers of Borrelia burgdorferi sensu lato genotypes in Ixodes rinicus ticks. Environ. Microbiol. 2014;16:2859–2868. doi: 10.1111/1462-2920.12304. [DOI] [PubMed] [Google Scholar]
- 33.Pérez D, Kneubühler Y, Rais O, Jouda F, Gern L. Borrelia afzelii ospC genotype diversity in Ixodes ricinus questing ticks and ticks from rodents in two Lyme borreliosis endemic areas: Contribution of co-feeding ticks. Ticks Tick Borne Dis. 2011;2:137–142. doi: 10.1016/j.ttbdis.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Strandh, M. & Raberg, L. Within-host competition between Borrelia afzelii ospC strains in wild hosts as revealed by massively parallel amplicon sequencing. Philos. T. Roy. Soc. B370 (2015). [DOI] [PMC free article] [PubMed]
- 35.Wang IN, et al. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics. 1999;151:15–30. doi: 10.1093/genetics/151.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bunikis J, et al. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology-Sgm. 2004;150:1741–1755. doi: 10.1099/mic.0.26944-0. [DOI] [PubMed] [Google Scholar]
- 37.Baranton G, Seinost G, Theodore G, Postic D, Dykhuizen D. Distinct levels of genetic diversity of Borrelia burgdorferi are associated with different aspects of pathogenicity. Res. Microbiol. 2001;152:149–156. doi: 10.1016/S0923-2508(01)01186-X. [DOI] [PubMed] [Google Scholar]
- 38.Lagal V, Postic D, Ruzic-Sabljic E, Baranton G. Genetic diversity among Borrelia strains determined by single-strand conformation polymorphism analysis of the ospC gene and its association with invasiveness. J. Clin. Microbiol. 2003;41:5059–5065. doi: 10.1128/JCM.41.11.5059-5065.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hellgren O, Andersson M, Raberg L. The genetic structure of Borrelia afzelii varies with geographic but not ecological sampling scale. J. Evol. Biol. 2011;24:159–167. doi: 10.1111/j.1420-9101.2010.02148.x. [DOI] [PubMed] [Google Scholar]
- 40.Jacquot M, et al. High-throughput sequence typing reveals genetic differentiation and host specialization among populations of the Borrelia burgdorferi species complex that infect rodents. PLOS ONE. 2014;9:e88581. doi: 10.1371/journal.pone.0088581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacquot, M. et al. Multiple independent transmission cycles of a tick-borne pathogen within a local host community. Scientific Reports6 (2016). [DOI] [PMC free article] [PubMed]
- 42.Durand, J. et al. Multi-strain infections of the Lyme borreliosis pathogen in the tick vector. Appl. Environ. Microbiol. 83 (2016). [DOI] [PMC free article] [PubMed]
- 43.Tonetti N, Voordouw MJ, Durand J, Monnier S, Gern L. Genetic variation in transmission success of the Lyme borreliosis pathogen Borrelia afzelii. Ticks Tick Borne Dis. 2015;6:334–343. doi: 10.1016/j.ttbdis.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Tsao, J. Reviewing molecular adaptations of Lyme borreliosis spirochetes in the context of reproductive fitness in natural transmission cycles. Vet. Res. 40 (2009). [DOI] [PMC free article] [PubMed]
- 45.Brisson D, Drecktrah D, Eggers C, Samuels DS. Genetics of Borrelia burgdorferi. Annu. Rev. Genet. 2012;46:515–536. doi: 10.1146/annurev-genet-011112-112140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrison A, Montgomery WI, Bown KJ. Investigating the persistence of tick-borne pathogens via the R-0 model. Parasitology. 2011;138:896–905. doi: 10.1017/S0031182011000400. [DOI] [PubMed] [Google Scholar]
- 47.Harrison A, Bennett N. The importance of the aggregation of ticks on small mammal hosts for the establishment and persistence of tick-borne pathogens: an investigation using the R-0 model. Parasitology. 2012;139:1605–1613. doi: 10.1017/S0031182012000893. [DOI] [PubMed] [Google Scholar]
- 48.MacQueen D, et al. Genotypic diversity of an emergent population of Borrelia burgdorferi at a coastal Maine island recently colonized by Ixodes scapularis. Vector Borne and Zoonotic Diseases. 2012;12:456–461. doi: 10.1089/vbz.2011.0811. [DOI] [PubMed] [Google Scholar]
- 49.Qiu WG, et al. A population genetic study of Borrelia burgdorferi sensu stricto from eastern Long Island, New York, suggested frequency-dependent selection, gene flow and host adaptation. Hereditas. 1997;127:203–216. doi: 10.1111/j.1601-5223.1997.00203.x. [DOI] [PubMed] [Google Scholar]
- 50.Dykhuizen DE, Baranton G. The implications of a low rate of horizontal transfer in Borrelia. Trends Microbiol. 2001;9:344–350. doi: 10.1016/S0966-842X(01)02066-2. [DOI] [PubMed] [Google Scholar]
- 51.Brisson D, Dykhuizen DE. ospC diversity in Borrelia burgdorferi: different hosts are different niches. Genetics. 2004;168:713–722. doi: 10.1534/genetics.104.028738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vuong HB, et al. Occurrence and transmission efficiencies of Borrelia burgdorferi ospC types in avian and mammalian wildlife. Infect. Genet. Evol. 2014;27:594–600. doi: 10.1016/j.meegid.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dressler F, Whalen JA, Reinhardt BN, Steere AC. Western blotting in the serodiagnosis of Lyme disease. J. Infect. Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 54.Engstrom SM, Shoop E, Johnson RC. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J. Clin. Microbiol. 1995;33:419–427. doi: 10.1128/jcm.33.2.419-427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fung BP, McHugh GL, Leong JM, Steere AC. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect. Immun. 1994;62:3213–3221. doi: 10.1128/iai.62.8.3213-3221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mbow ML, Gilmore RD, Titus RG. An OspC-specific monoclonal antibody passively protects mice from tick-transmitted infection by Borrelia burgdorferi B31. Infect. Immun. 1999;67:5470–5472. doi: 10.1128/iai.67.10.5470-5472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barthold SW. Specificity of infection-induced immunity among Borrelia burgdorferi sensu lato species. Infect. Immun. 1999;67:36–42. doi: 10.1128/iai.67.1.36-42.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacquet M, Durand J, Rais O, Voordouw MJ. Cross-reactive acquired immunity influences transmission success of the Lyme disease pathogen, Borrelia afzelii. Infect. Genet. Evol. 2015;36:131–140. doi: 10.1016/j.meegid.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 59.Probert WS, Crawford M, Cadiz RB, LeFebvre RB. Immunization with outer surface protein (Osp) A, but not OspC, provides cross-protection of mice challenged with North American isolates of Borrelia burgdorferi. J. Infect. Dis. 1997;175:400–405. doi: 10.1093/infdis/175.2.400. [DOI] [PubMed] [Google Scholar]
- 60.Earnhart CG, Buckles EL, Dumler JS, Marconi RT. Demonstration of OspC type diversity in invasive human Lyme disease isolates and identification of previously uncharacterized epitopes that define the specificity of the OspC murine antibody response. Infect. Immun. 2005;73:7869–7877. doi: 10.1128/IAI.73.12.7869-7877.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conway DJ, Polley SD. Measuring immune selection. Parasitology. 2002;125:S3–S16. doi: 10.1017/S0031182002002214. [DOI] [PubMed] [Google Scholar]
- 62.Abal-Fabeiro JL, Maside X, Bello X, Llovo J, Bartolome C. Multilocus patterns of genetic variation across Cryptosporidium species suggest balancing selection at the gp60 locus. Mol. Ecol. 2013;22:4723–4732. doi: 10.1111/mec.12425. [DOI] [PubMed] [Google Scholar]
- 63.Polley SD, Conway DJ. Strong diversifying selection on domains of the Plasmodium falciparum apical membrane antigen 1 gene. Genetics. 2001;158:1505–1512. doi: 10.1093/genetics/158.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Urwin R, et al. Distribution of surface protein variants among hyperinvasive meningococci: Implications for vaccine design. Infect. Immun. 2004;72:5955–5962. doi: 10.1128/IAI.72.10.5955-5962.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanincova K, Kurtenbach K, Diuk-Wasser M, Brei B, Fish D. Epidemic spread of Lyme borreliosis, Northeastern United States. Emerg. Infect. Dis. 2006;12:604–611. doi: 10.3201/eid1204.051016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mechai, S. et al. Evidence for host-genotype associations of Borrelia burgdorferi sensu stricto. PLOS ONE11 (2016). [DOI] [PMC free article] [PubMed]
- 67.Humair P-F, et al. Molecular identification of bloodmeal source in Ixodes ricinus ticks using 12S rDNA as a genetic marker. J. Med. Entomol. 2007;44:869–880. doi: 10.1093/jmedent/44.5.869. [DOI] [PubMed] [Google Scholar]
- 68.Morán Cadenas FM, et al. Identification of host bloodmeal source and Borrelia burgdorferi sensu lato in field-collected Ixodes ricinus ticks in Chaumont (Switzerland) J. Med. Entomol. 2007;44:1109–1117. doi: 10.1093/jmedent/44.6.1109. [DOI] [PubMed] [Google Scholar]
- 69.Gupta S, Anderson RM. Population structure of pathogens: The role of immune selection. Parasitol. Today. 1999;15:497–501. doi: 10.1016/S0169-4758(99)01559-8. [DOI] [PubMed] [Google Scholar]
- 70.Gupta S, Ferguson N, Anderson R. Chaos, persistence, and evolution of strain structure in antigenically diverse infectious agents. Science. 1998;280:912–915. doi: 10.1126/science.280.5365.912. [DOI] [PubMed] [Google Scholar]
- 71.Gupta S, et al. The maintenance of strain structure in populations of recombining infectious agents. Nature Medicine. 1996;2:437–442. doi: 10.1038/nm0496-437. [DOI] [PubMed] [Google Scholar]
- 72.Gupta S, Trenholme K, Anderson RM, Day KP. Antigenic diversity and the transmission dynamics of Plasmodium falciparum. Science. 1994;263:961–963. doi: 10.1126/science.8310293. [DOI] [PubMed] [Google Scholar]
- 73.Qiu WG, et al. Genetic exchange and plasmid transfers in Borrelia burgdorferi sensu stricto revealed by three-way genome comparisons and multilocus sequence typing. Proc. Natl. Acad. Sci. USA. 2004;101:14150–14155. doi: 10.1073/pnas.0402745101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grimm D, et al. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. USA. 2004;101:3142–3147. doi: 10.1073/pnas.0306845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tilly K, et al. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 2006;74:3554–3564. doi: 10.1128/IAI.01950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dykhuizen DE, et al. Short report: The propensity of different Borrelia burgdorferi sensu stricto genotypes to cause disseminated infections in humans. Am. J. Trop. Med. Hyg. 2008;78:806–810. [PMC free article] [PubMed] [Google Scholar]
- 77.Wormser GP, et al. Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J. Infect. Dis. 2008;198:1358–1364. doi: 10.1086/592279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burri C, Cadenas FM, Douet V, Moret J, Gern L. Ixodes ricinus density and infection prevalence of Borrelia burgdorferi sensu lato along a north-facing altitudinal gradient in the Rhone Valley (Switzerland) Vector-Borne Zoonot. 2007;7:50–58. doi: 10.1089/vbz.2006.0569. [DOI] [PubMed] [Google Scholar]
- 79.Rollend L, Fish D, Childs JE. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: A summary of the literature and recent observations. Ticks Tick Borne Dis. 2013;4:46–51. doi: 10.1016/j.ttbdis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 80.Richter D, Debski A, Hubalek Z, Matuschka FR. Absence of Lyme disease spirochetes in larval Ixodes ricinus ticks. Vector-Borne Zoonot. 2012;12:21–27. doi: 10.1089/vbz.2011.0668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


