Abstract
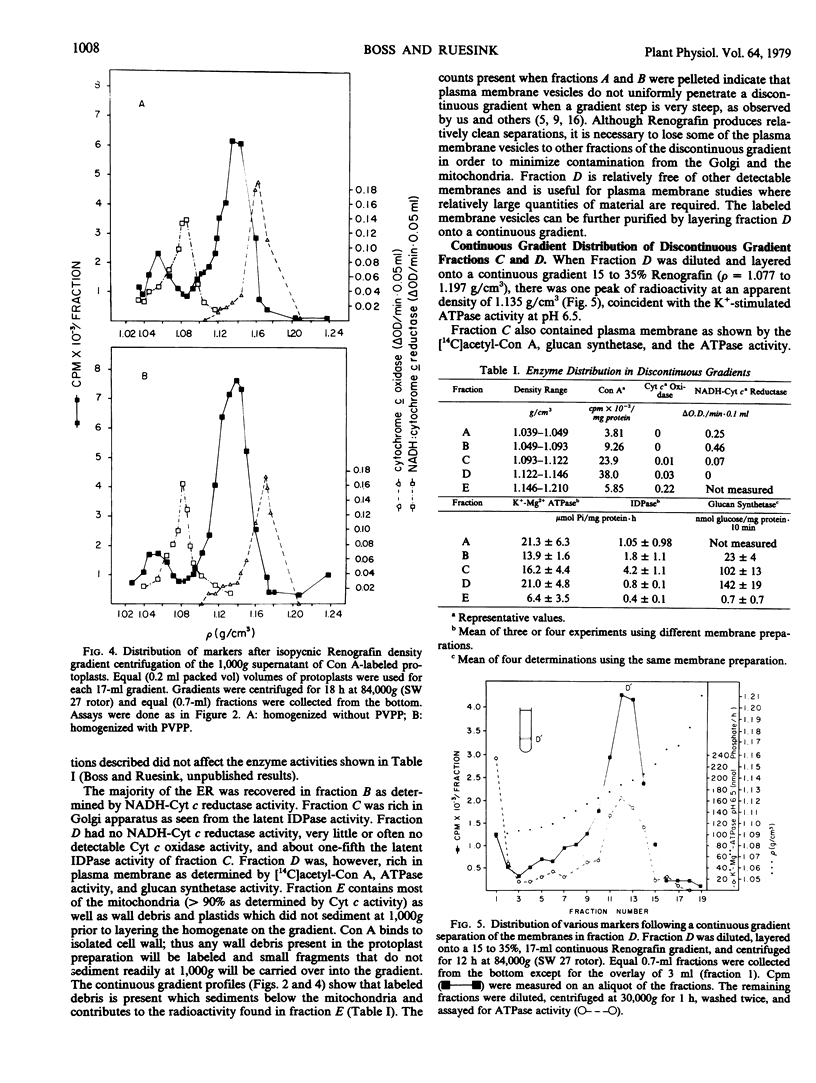
The plasma membranes of protoplasts released from carrot suspension culture cells were labeled with [14C]acetyl-concanavalin A. After homogenization a single labeled membrane fraction was isolated in a continuous isopycnic Renografin gradient. The labeled membranes peaked at an apparent density of 1.14 grams per cubic centimeter between the Golgi fraction at a density of 1.11 grams per cubic centimeter as determined by latent IDPase activity and the mitochondria at a density of 1.16 grams per cubic centimeter as determined by the cytochrome c oxidase activity. This method provided a very discrete peak of putative plasma membrane. On discontinuous Renografin gradients a relatively pure fraction of labeled plasma membranes could be readily isolated at the 1.122 to 1.146 grams per cubic centimeter interface. The labeled fraction was enriched in both an ATPase (pH 6.5) and a glucan synthetase with a pH optimum of 6.5 whose activity was promoted by magnesium and cellobiose. Enzyme activities were not altered by the membrane label.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. L., Ray P. M. Labeling of the Plasma Membrane of Pea Cells by a Surface-localized Glucan Synthetase. Plant Physiol. 1978 May;61(5):723–730. doi: 10.1104/pp.61.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D. J., Kauss H. Characterization, enzymatic and lectin properties of isolated membranes from Phaseolus aureus. Biochim Biophys Acta. 1976 Sep 7;443(3):360–374. doi: 10.1016/0005-2736(76)90456-9. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Galbraith D. W., Northcote D. H. The isolation of plasma membrane from protoplasts of soybean suspension cultures. J Cell Sci. 1977 Apr;24:295–310. doi: 10.1242/jcs.24.1.295. [DOI] [PubMed] [Google Scholar]
- Helgerson S. L., Cramer W. A., Morré D. J. Evidence for an increase in microviscosity of plasma membranes from soybean hypocotyls induced by the plant hormone, indole-3-acetic Acid. Plant Physiol. 1976 Oct;58(4):548–551. doi: 10.1104/pp.58.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leonard R. T., Vanderwoude W. J. Isolation of plasma membranes from corn roots by sucrose density gradient centrifugation: an anomalous effect of ficoll. Plant Physiol. 1976 Jan;57(1):105–114. doi: 10.1104/pp.57.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Wagner G. J., Siegelman H. W., Hind G. Membrane-bound ATPase of intact vacuoles and tonoplasts isolated from mature plant tissue. Biochim Biophys Acta. 1977 Feb 14;465(1):110–117. doi: 10.1016/0005-2736(77)90359-5. [DOI] [PubMed] [Google Scholar]
- Noonan K. D., Burger M. M. An assay for labeled lectin binding to cell surfaces. Methods Enzymol. 1974;32:621–625. doi: 10.1016/0076-6879(74)32063-0. [DOI] [PubMed] [Google Scholar]
- Quail P. H., Browning A. Failure of lactoperoxidase to iodinate specifically the plasma membrane of cucurbita tissue segments. Plant Physiol. 1977 Apr;59(4):759–766. doi: 10.1104/pp.59.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Auxin-binding Sites of Maize Coleoptiles Are Localized on Membranes of the Endoplasmic Reticulum. Plant Physiol. 1977 Apr;59(4):594–599. doi: 10.1104/pp.59.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Control of plant cell enlargement by hydrogen ions. Curr Top Dev Biol. 1977;11:187–214. doi: 10.1016/s0070-2153(08)60746-2. [DOI] [PubMed] [Google Scholar]
- Scarborough G. A. Isolation and characterization of Neurospora crassa plasma membranes. J Biol Chem. 1975 Feb 10;250(3):1106–1111. [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]


