Abstract
Methodology has been developed for the synthesis of 3-propanaldehydes through a five-step process in 11–67% yield from aldehydes. Aldehydes were reacted with Meldrum’s acid through a Knoevenagel condensation to give materials that upon reduction with sodium borohydride and subsequent hydrolysis decarboxylation generated the corresponding 3-propanoic acid derivatives. The -propanoic acid derivatives were reduced to give 3-propanol derivatives, which were readily oxidised to target 3-propanal derivatives.
Introduction
Aryl-3-propanaldehydes have demonstrated themselves as synthetically useful in the synthesis of natural products1, chiral tetrahydroquinolines2, 3 chemosensors4, 5 and in the perfume industry6. As such, facile synthesis of a range of these derivatives would be advantageous.
The chemoselective reduction of cinnamaldehydes to hydrocinnamaldehydes has been reported by Hashizume et al. and List et al. via either a palladium catalysed reduction7 or the organocatalysed Hantzsch’s ester reduction8, respectively. The synthesis of cinnamaldehydes has been reported utilising a range of conditions including the Wittig reaction7 from aryl aldehydes and the Heck cross-coupling of aryl halides7, 9–13. Alternatively, the products from the Knoevenagel condensation of aldehydes with Meldrum’s acid can be converted to hydrocinnamaldehydes. Frost et al. reported the hydrosilylation of Meldrum’s acid derivatives (3) either through a one-step14 or two-step15 process, using palladium or molybdenum catalysts and reagents.
A study by Andrews et al. (Glaxo-Smith-Kline (GSK)) reported a four-step synthesis of 3-(anthracen-9-yl)propan-1-ol (6d) on a 20-gram scale. Upon oxidation, this material would give the corresponding aldehyde (7d)16. However, this route was reported to have been carried out on a single substrate, starting with 9-anthraldehyde (1d) affording 3-(anthracen-9-yl)propan-1-ol (6d) in an overall yield of 84% over four steps.
Herein we provide alternative methodology to the established literature and build on previous studies16 for the synthesis of 3-propanal derivatives (Fig. 1) utilising a Knoevenagel condensation, olefin reduction, decarboxylation, carboxylic acid reduction and an alcohol oxidation. Substrate scope is expanded and a range of versatile hydrocinnamaldehyde derivatives are synthesised.
Figure 1.
General route for the synthesis of hydrocinnamaldehydes.
Results and Discussion
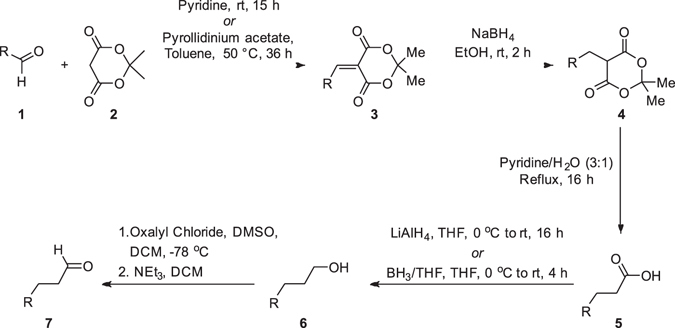
The synthesis of condensation products para-nitro (3a), para-dimethylamino (3b) and para-methoxy (3c) could be achieved via the literature reported Knoevenagel condensation of aldehydes 1a–c with Meldrum’s acid (2) in 74–87% yields14. Whilst this method successfully delivered 3a–c in our hands, the use of an aqueous solvent system prevented us from successfully applying the same conditions to substrates with low water solubility such as 9-anthryl (3d, Fig. 2, entry 7). The issue was overcome utilising the method reported by Andrews et al. (GSK) for the synthesis of 3d, where pyridine is used as the reaction solvent16. Pleasingly, in contrast to the aqueous solvent system the reaction proceeded smoothly with the 9-anthryl derivative 3d in 93% yield. We expanded the substrate scope of these conditions to include electron rich (3b,c,h,I, Fig. 2, entry 4,6,12,13), electron poor (3a, Fig. 2, entry 2), heterocyclic (3e,l, Fig. 2, entry 9,16), alkyl (3g, Fig. 2, entry 11) and hindered (3f,j,k,m, Fig. 2, entry 10,14,15,17) groups yielding the desired products in good to moderate yields (34–93%). On the other hand, extremely electron-deficient substrates such as para-trifluoromethyl (3n, Fig. 2, entry 18) were amenable to this procedure, e.g., decomposition of the starting material was observed.
Figure 2.
Substrate scope for the Knoevenangel condensation of aldehydes with Meldrum’s acid.
A literature reported method for the synthesis of 3n was used17, for which we carried out minor solvent modifications to avoid the use of benzene (Fig. 2, entry 19) giving the desired Knoevenagel condensation products. The same procedure also yielded the novel pentafluorophenyl derivative (3o, Fig. 2, entry 20), Both the para-trifluoromethyl (3n) and pentafluoro (3o) derivatives were not purified at this stage due to instability of the substrates during attempted purification protocol, which included recrystallisation and flash column chromatography. Instead, when full conversion was determined to have been reached by 1H NMR spectroscopic analysis of the crude reaction mixtures for these reactions, they were taken forward to the next step18.
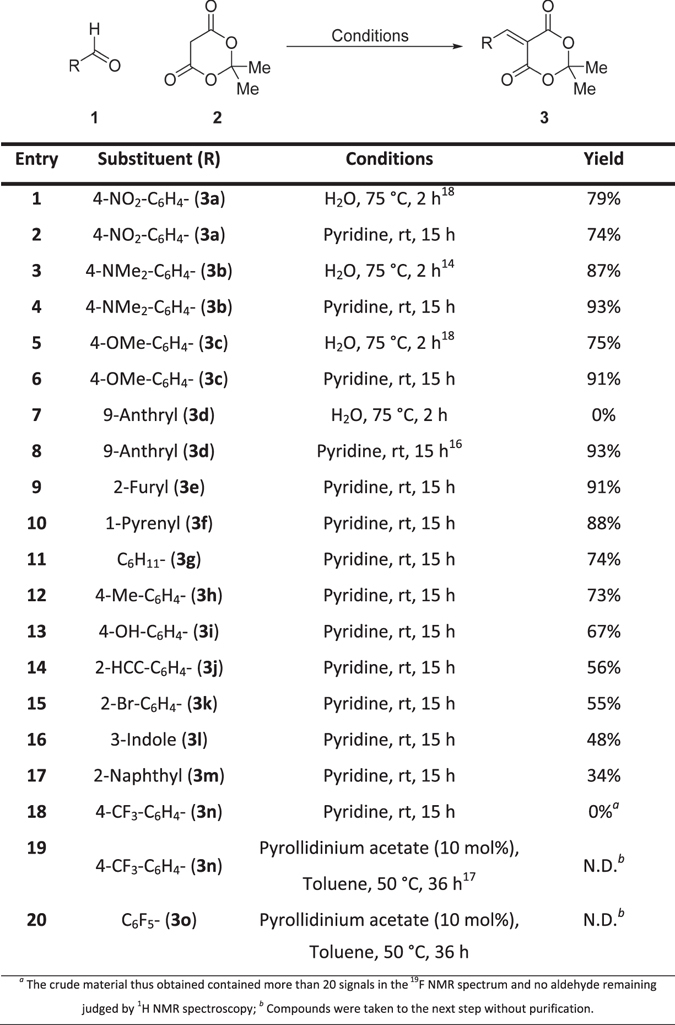
With alkene containing compounds 3a–o in hand, the next step was reduction of the conjugated double bonds introduced through the Knoevenagel condensation. This was successfully carried out according to the method reported for the synthesis of 4d by Andrews et al.16 giving high yields (87–99%) for 4a,c–h,j–l,n–o. The 4-dimethylamino derivative (4b) gave a lower than expected yield of 75%, minor decomposition was observed. In the case of compounds 4c (Fig. 3, entry 3) and 4h (Fig. 3, entry 8) methanol led to ring opening of the Meldrum’s moiety to the dimethyl malonate, whereas under otherwise identical conditions the use of ethanol furnished the desired compounds. Therefore, ethanol was selected as the preferable solvent for manipulation of 3 to 4 from this point.
Figure 3.
Reduction of Knoevenangel products to afford saturated Meldrum’s derivatives.
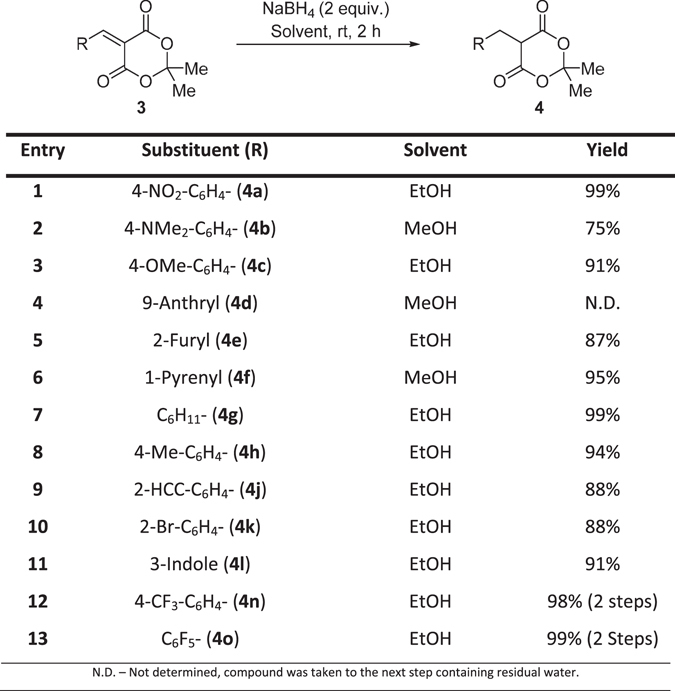
The hydrolysis and decarboxylation of derivatives 4 was required in order to synthesise 5, this was achieved with the method reported for the synthesis of 5d by Andrews et al.16 in acceptable to good yields (48–98%, Fig. 4) for 5a–h,j–l,n–o.
Figure 4.
Decarboxylation to synthesise hydrocinnamic acid derivatives.
For the synthesis of the para-methyl (5h) and para-methoxy (5c) derivatives from 4h and 4c, respectively, undesired side-products were detected. In order to minimise the formation of the side-products, the reaction was run initially at room temperature for one hour, followed by heating to reflux for a further 4 hours. The desired compounds were obtained after work-up without requiring further purification. Furthermore, under the standard reaction conditions the synthesis of 2-furyl derivative 5e from 4e led to the formation of the desired compound alongside a minor undesired side-product, the desired compound was poorly soluble in common laboratory solvents and therefore this impurity was taken through to the LiAlH4 reduction. The low yield for the synthesis of 3-indole derivative 5l was most likely due to product loss during reaction work-up because of the probable zwitterionic nature of 5l having some water solubility.
In order to synthesis 7, isolated 5a–h,j–l,n–o should first be converted to the corresponding primary alcohols 6a–h,j–l,n–o before oxidation to aldehydes 7a–h,j–l,n–o. The reduction of 5b–d, f–h,j–l to 6b–d, f–h,j–l was carried out with lithium aluminium hydride (LiAlH4) in THF to give the primary alcohols in 83% to 99% yields (Fig. 5). The reduction of 5e to 6e was attempted with lithium aluminium hydride (LiAlH4) in THF led to the formation of a number of unidentified decomposition products.
Figure 5.
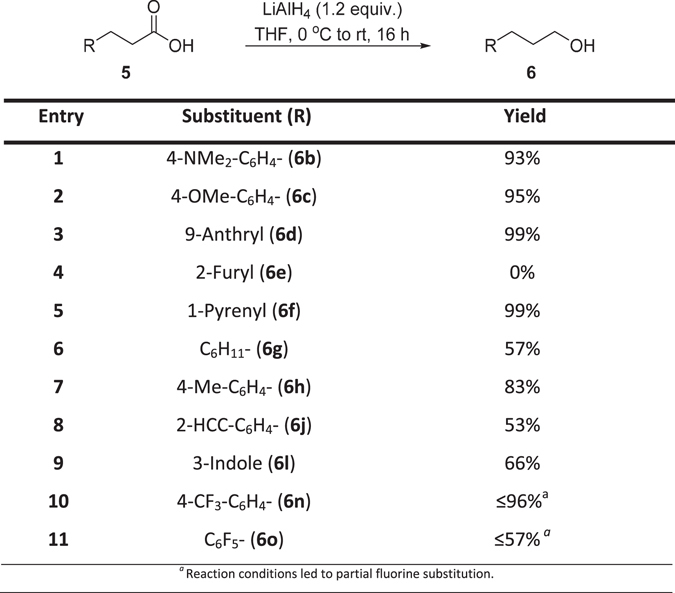
Reduction of carboxylic acids to afford hydrocinnamyl alcohol.
The reduction of 5n and 5o to 6n and 6o was attempted with lithium aluminium hydride (LiAlH4) however partial fluorine displacement was observed. Pentafluoro derivative 5o underwent a nucleophilic aromatic substitution (SNAr) displacing one of the fluorine substituents to give 8o in an approximate 4:1 ratio 6o:8o (Fig. 6), similar observations are reported in the literature with related substrates19. When para-trifluoromethyl derivative 6n was exposed to LiAlH4 it underwent a hydride-fluorine exchange to give the para-difluoromethyl compound 8n (Fig. 6) in an approximate 1:1 ratio 6n:8n by 1H NMR spectroscopic analysis. Fluorine substitution by hydride within trifluoromethyl groups has been previously reported with related substrates20.
Figure 6.
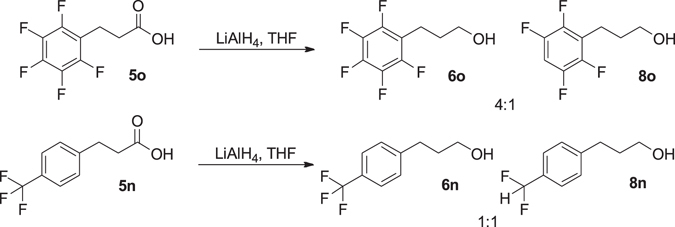
By-products formed during the lithium aluminium hydride reduction of fluorinated hydrocinnamic acids.
Reduction of 5a and 5k to 6a and 6k was carried out using borane to give the desired compounds in 86% and 74%, respectively (Fig. 7). This procedure provides an alternative, milder, method to reduce carboxylic acids when incompatible with LiAlH4. Thus, this procedure should also be applicable to fluorinated derivatives 5n and 5o and has previously been demonstrated in the literature21, 22.
Figure 7.
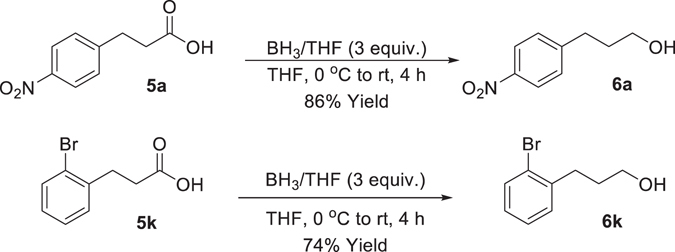
Borane reduction of 4-nitro 5b and 2-bromo 5k derivatives.
Hydrocinnamyl alcohol derivatives 6 a,c,d,f–h,j,k,o were converted to aldehydes 7a,c,d,f–h,j,k,o using a Swern oxidation in 29–89% yield (Fig. 8). The oxidation of 4-dimethylamino derivative 6b to 7b and 3-indole derivative 6l to 7l was unsuccessful, a complex mixture of unidentifiable by-products alongside the desired compound precluded satisfactory synthesis and isolation. Oxidation of a mixture of 6o and 8o led to the formation of the desired aldehyde 7o in acceptable yield (29%) and the by-product from the oxidation of 8o could be separated with column chromatography.
Figure 8.
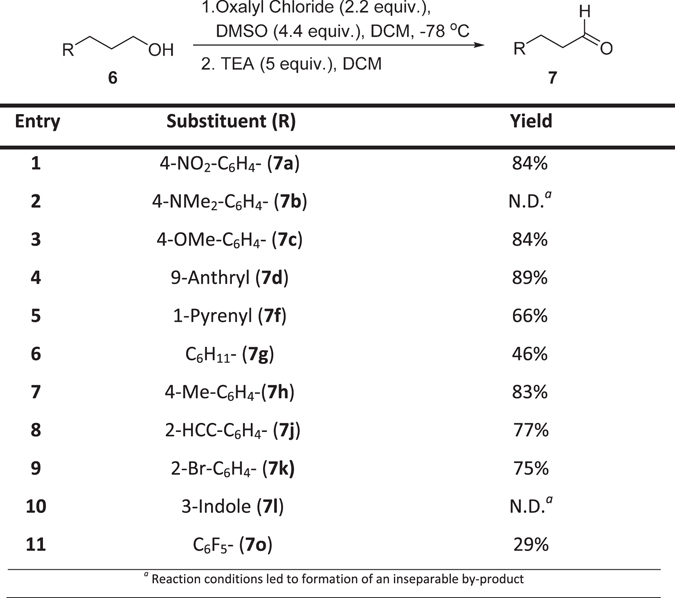
Swern oxidation of cinnamoyl alcohols to give corresponding hydrocinnamaldehyde derivatives.
The outlined five-step synthesis of aldehydes 7 was successful in providing a range of derivatives in acceptable yields (11–67%, Fig. 9). Our studies found that a single set of conditions were not applicable to all substrates but tailoring of reaction conditions can give a diverse range of derivatives. By-products were observed in the LiAlH4 reduction of 6n and 6o, the decarboxylation of 4d and 4h but modifications to the synthetic procedure can minimise their formation23. Experimental procedures are detailed in the Supplementary Information.
Figure 9.
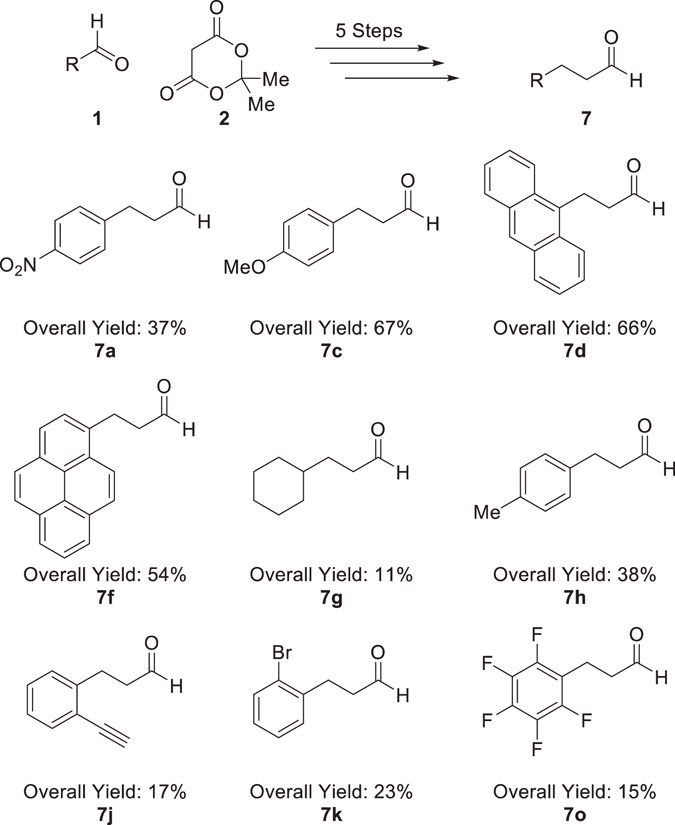
Summary of five step synthesis of hydrocinnamaldehyde derivatives with overall yields.
Electronic supplementary material
Acknowledgements
The authors would like to thank Louise Male, Chi Tsang, Allen Bowden, Peter Ashton and Cécile S. Le Duff for analytical support. J.S.F. would like to thank the Royal Society for an Industrial Fellowship and the EPSRC for funding (EP/J003220/1). J.S.F., Y.Z. and DTP thank the University of Birmingham for support. D.T.P. thanks the Royal Society of Chemistry and the Society for Chemical Industry for travel support. The Catalysis and Sensing for our Environment (CASE) group is thanked for providing essential networking opportunities.
Author Contributions
All authors contributed to designing experiments, preparing the manuscript, suggested modifications and analysed the data. J.S.F. led the project, D.T.P. and Y.Z. conducted the experiments.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01950-7
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Daniel T. Payne, Email: dxp854@bham.ac.uk
John S. Fossey, Email: j.s.fossey@bham.ac.uk
References
- 1.Yadav JS, et al. Stereoselective total synthesis of 4-((3S,5R)-3,5-dihydroxynonadecyl)phenol. Tetrahedron Letters. 2014;55(8):1395–1397. doi: 10.1016/j.tetlet.2013.12.056. [DOI] [Google Scholar]
- 2.Rawat V, Kumar BS, Sudalai A. Proline catalyzed sequential [small alpha]-aminooxylation or -amination/reductive cyclization of o-nitrohydrocinnamaldehydes: a high yield synthesis of chiral 3-substituted tetrahydroquinolines. Organic & Biomolecular Chemistry. 2013;11(22):3608–3611. doi: 10.1039/c3ob40320c. [DOI] [PubMed] [Google Scholar]
- 3.Kang YK, Kim DY. Enantioselective organocatalytic oxidative enamine catalysis-1,5-hydride transfer-cyclization sequences: asymmetric synthesis of tetrahydroquinolines. Chemical Communications. 2014;50(2):222–224. doi: 10.1039/C3CC46710D. [DOI] [PubMed] [Google Scholar]
- 4.Richter, I. et al. Intramolecular cation-pi interactions control the conformation of nonrestricted (phenylalkyl)pyridines. Chem. Commun. 9, 1082–1084, doi:10.1039/b716937j. [DOI] [PubMed]
- 5.Chen W, et al. A pyridinium cation-pi interaction sensor for the fluorescent detection of alkyl halides. Chem. Commun. 2010;47(1):253–255. doi: 10.1039/C0CC01420F. [DOI] [PubMed] [Google Scholar]
- 6.Pybus, D. H.; Sell, C. S. The Chemistry of Fragrances. Royal Society of Chemistry: (1999).
- 7.Hashizume H, et al. Synthesis and Biological Activity of New 3-Hydroxy-3-methylglutaryl Coenzyme A (HMG-CoA) Synthase Inhibitors: 2-Oxetanones with a Side Chain Mimicking the Folded Structure of 1233A. CHEMICAL & PHARMACEUTICAL BULLETIN. 1994;42(3):512–520. doi: 10.1248/cpb.42.512. [DOI] [PubMed] [Google Scholar]
- 8.Yang JW, Hechavarria Fonseca MT, List B. A Metal-Free Transfer Hydrogenation: Organocatalytic Conjugate Reduction of α,β-Unsaturated Aldehydes. Angewandte Chemie International Edition. 2004;43(48):6660–6662. doi: 10.1002/anie.200461816. [DOI] [PubMed] [Google Scholar]
- 9.Battistuzzi G, Cacchi S, Fabrizi G. An Efficient Palladium-Catalyzed Synthesis of Cinnamaldehydes from Acrolein Diethyl Acetal and Aryl Iodides and Bromides. Organic Letters. 2003;5(5):777–780. doi: 10.1021/ol034071p. [DOI] [PubMed] [Google Scholar]
- 10.Noël S, Djakovitch L, Pinel C. Influence of the catalytic conditions on the selectivity of the Pd-catalyzed Heck arylation of acrolein derivatives. Tetrahedron Letters. 2006;47(23):3839–3842. doi: 10.1016/j.tetlet.2006.03.186. [DOI] [Google Scholar]
- 11.Noël S, Luo C, Pinel C, Djakovitch L. Efficient Heterogeneously Palladium-Catalysed Heck Arylation of Acrolein Diethyl Acetal. Selective Synthesis of Cinnamaldehydes or 3-Arylpropionic Esters. Advanced Synthesis & Catalysis. 2007;349(7):1128–1140. doi: 10.1002/adsc.200600593. [DOI] [Google Scholar]
- 12.Alacid E, Nájera C. Acrolein Diethyl Acetal: A Three-Carbon Homologating Reagent for the Synthesis of β-Arylpropanoates and Cinnamaldehydes by Heck Reaction Catalyzed by a Kaiser Oxime Resin Derived Palladacycle. European Journal of Organic Chemistry. 2008;2008(18):3102–3106. doi: 10.1002/ejoc.200800047. [DOI] [Google Scholar]
- 13.Pan K, Noël S, Pinel C, Djakovitch L. Heck arylation of acrolein acetals using the 9-bromoanthracene: A case of study. Journal of Organometallic Chemistry. 2008;693(17):2863–2868. doi: 10.1016/j.jorganchem.2008.05.042. [DOI] [Google Scholar]
- 14.Frost CG, Hartley BC. Tandem Molybdenum Catalyzed Hydrosilylations: An Expedient Synthesis of β-Aryl Aldehydes. Organic Letters. 2007;9(21):4259–4261. doi: 10.1021/ol701812w. [DOI] [PubMed] [Google Scholar]
- 15.Frost CG, Hartley BC. Lewis Base-Promoted Hydrosilylation of Cyclic Malonates: Synthesis of β-Substituted Aldehydes and γ-Substituted Amines. The Journal of Organic Chemistry. 2009;74(9):3599–3602. doi: 10.1021/jo900390d. [DOI] [PubMed] [Google Scholar]
- 16.Andrews SP, Ladlow M. Convenient Preparation and Use of a New Analytical Construct for the Analysis and Development of Solid-Phase Chemistries. The Journal of Organic Chemistry. 2003;68(14):5525–5533. doi: 10.1021/jo034340p. [DOI] [PubMed] [Google Scholar]
- 17.Dumas AM, Seed A, Zorzitto AK, Fillion E. A general and practical preparation of alkylidene Meldrum’s acids. Tetrahedron Letters. 2007;48(40):7072–7074. doi: 10.1016/j.tetlet.2007.08.012. [DOI] [Google Scholar]
- 18.Bigi F, et al. Clean synthesis in water. Part 2: Uncatalysed condensation reaction of Meldrum’s acid and aldehydes. Tetrahedron Letters. 2001;42(31):5203–5205. doi: 10.1016/S0040-4039(01)00978-9. [DOI] [Google Scholar]
- 19.Zhang D, Chen Z, Cai H, Zou X. A new synthetic route to polyfluorobenzyl alcohol. Journal of Fluorine Chemistry. 2009;130(10):938–941. doi: 10.1016/j.jfluchem.2009.07.008. [DOI] [Google Scholar]
- 20.Fuchibe K, Ohshima Y, Mitomi K, Akiyama T. Low-Valent Niobium-Catalyzed Reduction of α,α,α-Trifluorotoluenes. Organic Letters. 2007;9(8):1497–1499. doi: 10.1021/ol070249m. [DOI] [PubMed] [Google Scholar]
- 21.Brooke, G. M.; Wallis, D. I., Partially fluorinated heterocyclic compounds. Part 14. Syntheses of 4,5,6,7-tetrafluoro-2,3-dihydro-2-methyl-1-benzothiophen and 5,6,7,8-tetrafluorothiochroman from pentafluorophenyl prop-2-enyl sulphide via the Claisen rearrangement intermediate and the related reaction of prop-2-enyl 2,3,5,6-tetrafluorophenyl sulphide. Reactions which appear to proceed via homolytic fission of an aliphatic carbon-fluorine bond. Journal of the Chemical Society, Perkin Transactions 11981 (0), 1659–1664.
- 22.Nguyen J, Duncan N, Lalic G. Direct β-Selective Cross-Coupling of Alkenyl Gold Complexes with Alkyl Electrophiles. European Journal of Organic Chemistry. 2016;2016(35):5803–5806. doi: 10.1002/ejoc.201601149. [DOI] [Google Scholar]
- 23.Payne DT, Fossey JS, Elmes RBP. Catalysis and Sensing for our Environment (CASE2015) and the Supramolecular Chemistry Ireland Meeting (SCI 2015): Dublin and Maynooth, Ireland. 8th–11th July. Supramolecular Chemistry. 2016;28(11-12):921–931. doi: 10.1080/10610278.2016.1150595. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


