Abstract
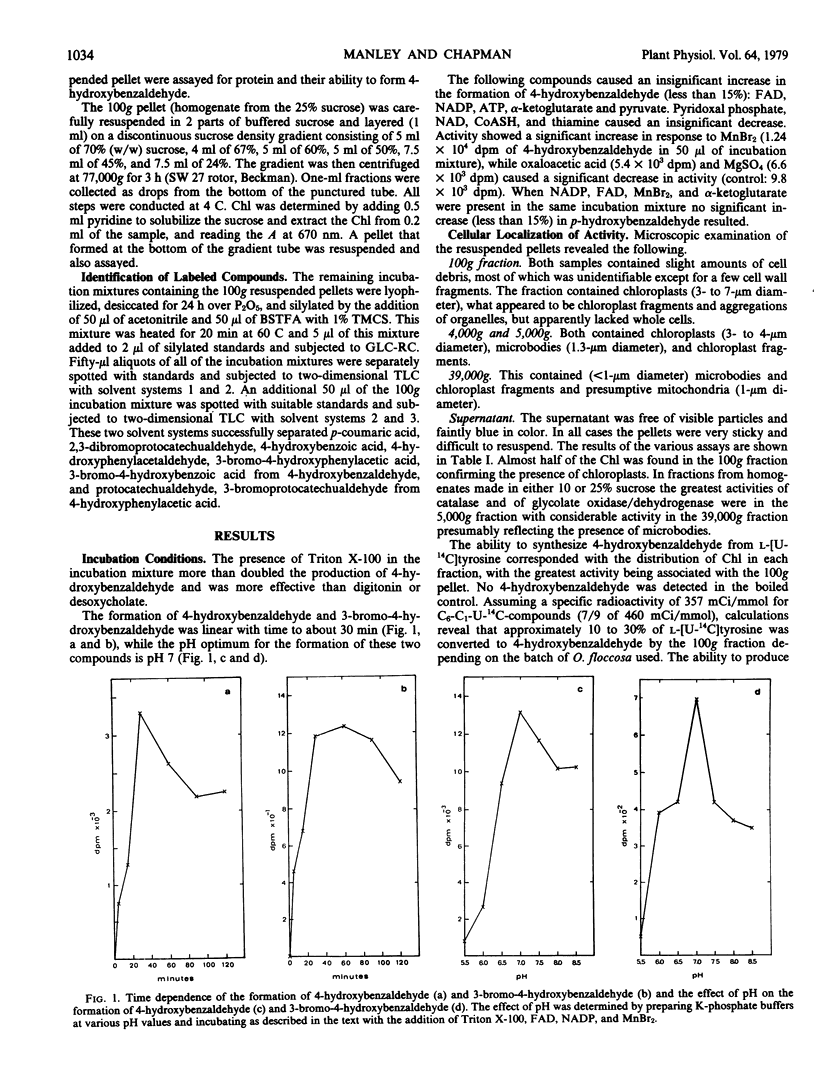
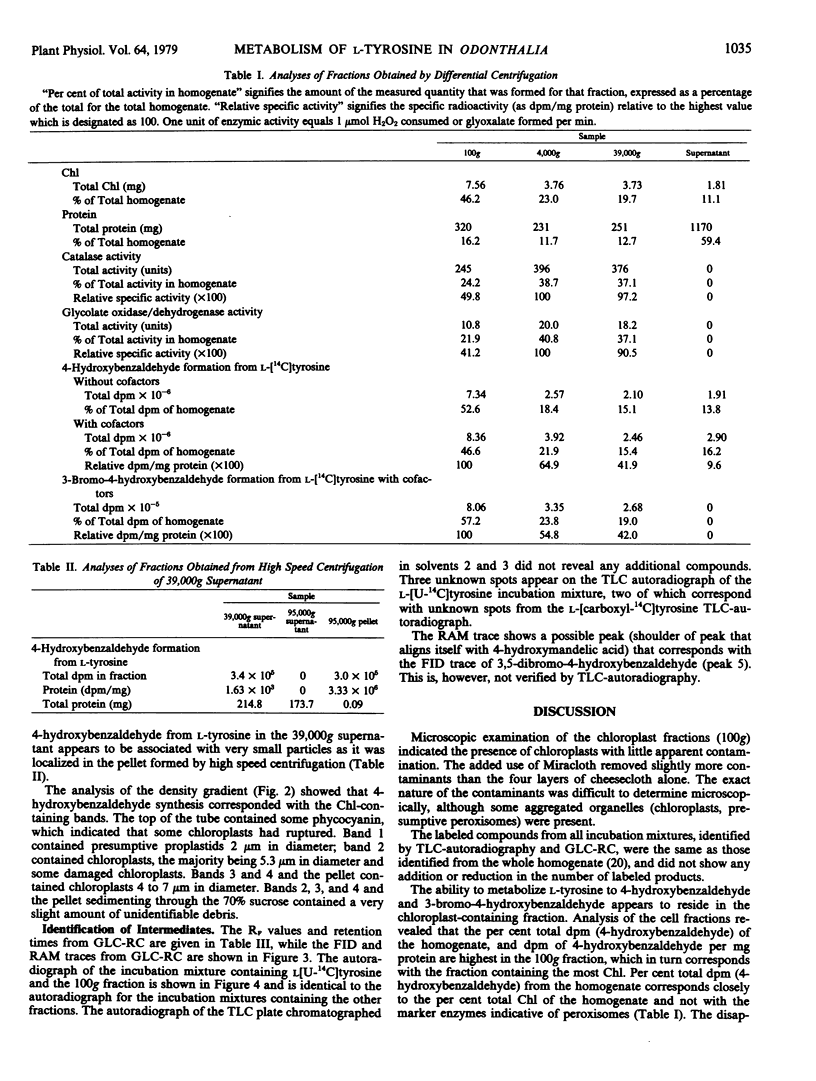
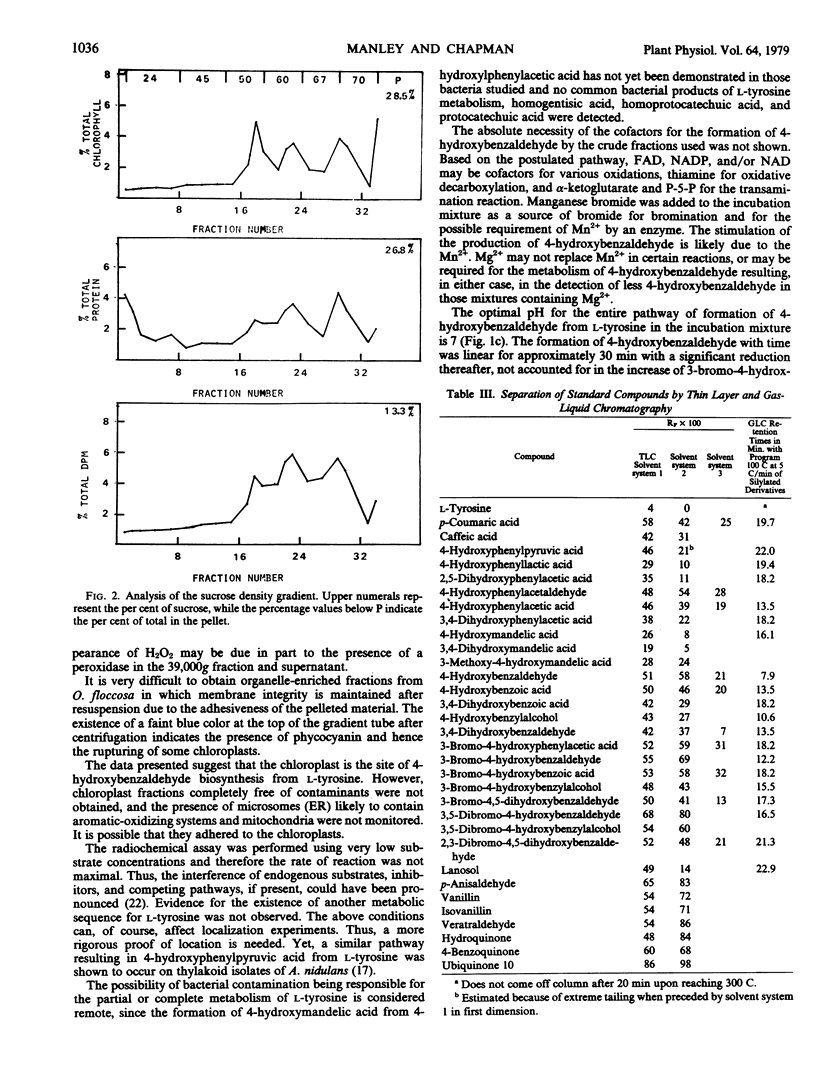
The biosynthesis of 4-hydroxybenzaldehyde and 3-bromo-4-hydroxybenzaldehyde from l-[U-14C]tyrosine has been demonstrated in chloroplast-containing fractions obtained by differential and isopycnic centrifugation from the marine red alga Odonthalia floccosa. Surfactant and high speed centrifugation studies indicate that the biosynthetic pathway involves a particulate enzyme system, possibly located on the thylakoid membranes. The following scheme, based upon identification of labeled 14C-intermediates, is proposed for the formation of aldehydes: l-tyrosine → 4-hydroxyphenylpyruvic acid → 4-hydroxyphenylacetic acid → 4-hydroxymandelic acid → 4-hydroxybenzaldehyde → 3-bromo-4-hydroxybenzaldehyde.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat S. G., Vaidyanathan C. S. Involvement of 4-hydroxymandelic acid in the degradation of mandelic acid by Pseudomonas convexa. J Bacteriol. 1976 Sep;127(3):1108–1118. doi: 10.1128/jb.127.3.1108-1118.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakley E. R. The catabolism of L-tyrosine by an Arthrobacter sp. Can J Microbiol. 1977 Sep;23(9):1128–1139. doi: 10.1139/m77-169. [DOI] [PubMed] [Google Scholar]
- Crowden R. K. Biosynthesis of the polyphenolic acid metabolites of Polyporus tumulosus Cooke. Can J Microbiol. 1967 Feb;13(2):181–197. doi: 10.1139/m67-025. [DOI] [PubMed] [Google Scholar]
- Hager L. P., Morris D. R., Brown F. S., Eberwein H. Chloroperoxidase. II. Utilization of halogen anions. J Biol Chem. 1966 Apr 25;241(8):1769–1777. [PubMed] [Google Scholar]
- Jamaluddin M., Rao P. V., Vaidyanathan C. S. Involvement of the protocatechuate pathway in the metabolism of mandelic acid by Aspergillus niger. J Bacteriol. 1970 Mar;101(3):786–793. doi: 10.1128/jb.101.3.786-793.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindl H. Biosynthesis and metabolism of hydroxyphenylacetic acids in higher plants. Eur J Biochem. 1969 Jan;7(3):340–347. doi: 10.1111/j.1432-1033.1969.tb19614.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Löffelhardt W. Formation of benzoic acid and p-hydroxybenzoic acid in the blue green alga Anacystis nidulans: a thylakoid-bound enzyme complex analogous to the chloroplast system. Z Naturforsch C. 1976 Nov-Dec;31(11-12):693–699. doi: 10.1515/znc-1976-11-1212. [DOI] [PubMed] [Google Scholar]
- Oldham K. G. Radiochemical enzyme assays: factors affecting their sensitivity and the selection of optimum conditions for assays. Int J Appl Radiat Isot. 1970 Jul;21(7):421–429. doi: 10.1016/0020-708x(70)90136-5. [DOI] [PubMed] [Google Scholar]
- Shephard D. C., Levin W. B. Biosynthesis in isolated Acetabularia chloroplasts. I. Protein amino acids. J Cell Biol. 1972 Aug;54(2):279–294. doi: 10.1083/jcb.54.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparnins V. L., Chapman P. J. Catabolism of L-tyrosine by the homoprotocatechuate pathway in gram-positive bacteria. J Bacteriol. 1976 Jul;127(1):362–366. doi: 10.1128/jb.127.1.362-366.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sumere C. F., Wolf G., Teuchy H., Kint J. A new thin-layer method for phenolic substances and coumarins. J Chromatogr. 1965 Oct;20(1):48–60. doi: 10.1016/s0021-9673(01)97365-0. [DOI] [PubMed] [Google Scholar]