Abstract
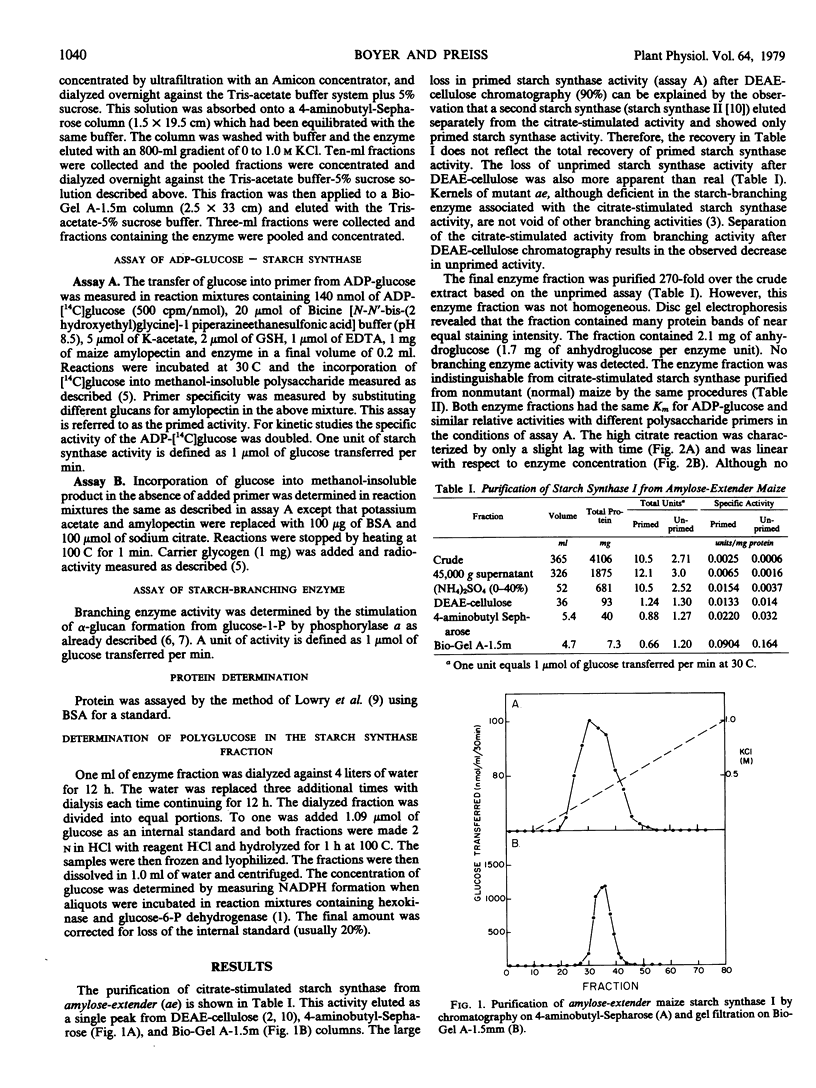
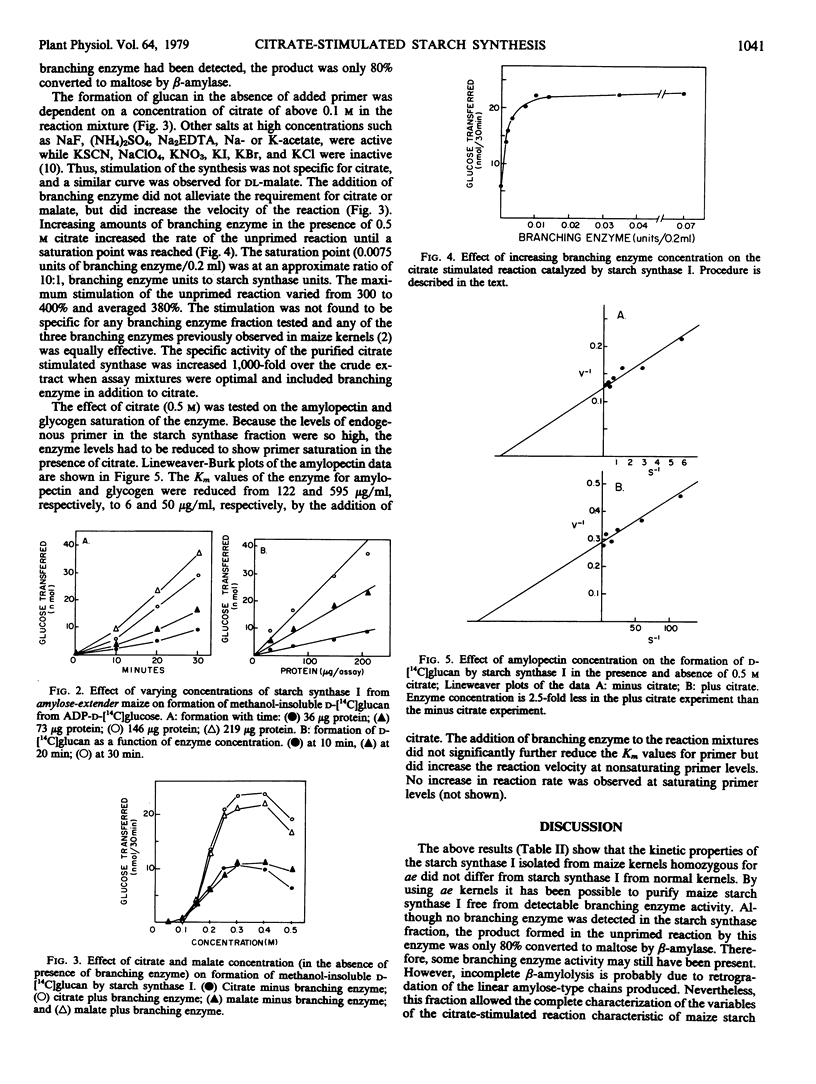
Chromatography of extracts of maize on diethylaminoethyl-cellulose resolves starch synthase activity into two fractions (Ozbun, Hawker, Preiss 1971 Plant Physiol 48: 785-769). Only starch synthase I is capable of synthesis in the absence of added primer and the presence of 0.5 molar citrate. This enzyme fraction has been purified about 1,000-fold from maize kernels homozygous for the endosperm mutant amylose-extender (ae). Because ae endosperm lacks the starch-branching enzyme which normally purifies with starch synthase I, the final enzyme fraction was free of detectable branching enzyme activity. This allowed a detailed characterization of the citrate-stimulated reaction. The citrate-stimulated reaction was dependent upon citrate concentrations of greater than 0.1 molar. However, the reaction is not specific for citrate and malate also stimulated the reaction. Branching enzyme increased the velocity of the reaction about 4-fold but did not replace the requirement for citrate. Citrate reduced the Km for the primers amylopectin and glycogen from 122 and 595 micrograms per milliliter, respectively, to 6 and 50 micrograms per milliliter, respectively. The enzyme was found to contain 1.7 milligrams of anhydroglucose units per enzyme unit. Thus reaction mixtures contained 1 to 5 micrograms (5 to 25 micrograms per milliliter) of endogenous primer. The citrate-stimulated reaction could be explained by an increased affinity for this endogenous primer. The starch synthase reaction in the absence of primer is dependent upon several factors including endogenous primer concentration, citrate concentration as well as branching enzyme concentration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer C. D., Preiss J. Multiple forms of starch branching enzyme of maize: evidence for independent genetic control. Biochem Biophys Res Commun. 1978 Jan 13;80(1):169–175. doi: 10.1016/0006-291x(78)91119-1. [DOI] [PubMed] [Google Scholar]
- Fox J., Kawaguchi K., Greenberg E., Preiss J. Biosynthesis of bacterial glycogen. Purification and properties of the Escherichia coli B ADPglucose:1,4-alpha-D-glucan 4-alpha-glucosyltransferase. Biochemistry. 1976 Feb 24;15(4):849–857. doi: 10.1021/bi00649a019. [DOI] [PubMed] [Google Scholar]
- Ghosh H. P., Preiss J. Biosynthesis of starch in spinach chloroplasts. Biochemistry. 1965 Jul;4(7):1354–1361. doi: 10.1021/bi00883a020. [DOI] [PubMed] [Google Scholar]
- Hawker J. S., Ozbun J. L., Ozaki H., Greenberg E., Preiss J. Interaction of spinach leaf adenosine diphosphate glucose alpha-1,4-glucan alpha-4-glucosyl transferase and alpha-1,4-glucan, alpha-1,4-glucan-6-glycosyl transferase in synthesis of branched alpha-glucan. Arch Biochem Biophys. 1974 Feb;160(2):530–551. doi: 10.1016/0003-9861(74)90430-5. [DOI] [PubMed] [Google Scholar]
- ILLINGWORTH B., BROWN D. H., CORI C. F. The de novo synthesis of polysaccharide by phosphorylase. Proc Natl Acad Sci U S A. 1961 Apr 15;47:469–478. doi: 10.1073/pnas.47.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi K., Fox J., Holmes E., Boyer C., Preiss J. De novo synthesis of Escherichia coli glycogen is due to primer associated with glycogen synthase and activation by branching enzyme. Arch Biochem Biophys. 1978 Oct;190(2):385–397. doi: 10.1016/0003-9861(78)90291-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ozbun J. L., Hawker J. S., Preiss J. Adenosine diphosphoglucose-starch glucosyltransferases from developing kernels of waxy maize. Plant Physiol. 1971 Dec;48(6):765–769. doi: 10.1104/pp.48.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbun J. L., Hawker J. S., Preiss J. Adenosine diphosphoglucose-starch glucosyltransferases from developing kernels of waxy maize. Plant Physiol. 1971 Dec;48(6):765–769. doi: 10.1104/pp.48.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltiel S., Er-El Z. Hydrophobic chromatography: use for purification of glycogen synthetase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):778–781. doi: 10.1073/pnas.70.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]


