Abstract
Tumor-associated macrophages (TAMs) promote cancer cell proliferation, invasion, and metastasis by producing various mediators. Although preclinical studies demonstrated that TAMs preferentially express CD163 and CD204, the TAM subsets in oral squamous cell carcinoma (OSCC) remain unknown. In this study, we examined the expression and role of TAM subsets in OSCC. Forty-six patients with OSCC were analyzed for expression of TAMs in biopsy samples by immunohistochemistry. We examined TAM subsets and their production of immune suppressive molecules (IL-10 and PD-L1) in peripheral blood mononuclear cells from three OSCC patients by flow cytometry. CD163 was detected around the tumor or connective tissue, while CD204 was detected in/around the tumors. Flow cytometric analysis revealed that CD163+CD204+ TAMs strongly produced IL-10 and PD-L1 in comparison with CD163+CD204− and CD163−CD204+ TAMs. Furthermore, the number of activated CD3+ T cells after co-culture with CD163+CD204+ TAMs was significantly lower than that after co-culture with other TAM subsets. In clinical findings, the number of CD163+CD204+ TAMs was negatively correlated with that of CD25+ cells and 5-year progression-free survival. These results suggest that CD163+CD204+ TAMs possibly play a key role in the invasion and metastasis of OSCC by T-cell regulation via IL-10 and PD-L1 production.
Introduction
Monocytes/macrophages are important contributors to cancer-associated inflammation. The heterogeneity of macrophages has been discussed with regard to different responses to various microenvironmental stimuli. Macrophages are classified into two distinct subtypes: the classically activated (M1) macrophage stimulated by microbial products and interferon-γ, and the alternatively activated (M2) macrophage stimulated by IL-4, IL-13, and IL-101–4. Several studies have shown that M2 macrophages infiltrating into the tumor microenvironment contribute to cancer progression and are associated with tumor progression, angiogenesis, metastasis and immunosuppression. This macrophage phenotype is referred to as the tumor-associated macrophage (TAM)5–7.
CD163 and CD204-positive macrophages are positively correlated with the histological gradient of malignancy in human ovarian tumors8 and thus CD163 and CD204 are useful markers for activation of TAMs in human samples. Furthermore, in malignant lymphoma, glioma, and kidney cancer, higher CD163 expression on TAMs is associated with worse clinical prognosis; however, no correlation exists between clinical prognosis and the number of CD204-expressing TAMs9, 10, CD204, also known as Class A scavenger receptor (SRA), has been shown to participate in the pathogenesis of atherosclerosis and the pattern recognition of pathogen infection11. CD163 is a hemoglobin scavenger receptor exclusively expressed in the monocyte-macrophage system. Furthermore, recent data indicate that soluble CD163 may be a valuable diagnostic parameter for monitoring macrophage activation in inflammatory conditions12.
Immune tolerance in the tumor microenvironment is closely involved in tumor progression caused by T-cell regulation via inhibitory signals of immune suppressive cytokine (IL-10), immune checkpoint molecules (programmed death-1 ligand 1 (PD-L1)), transforming growth factor-β, and prostaglandin E213. PD-L1 is widely expressed by leukocytes and tumor cells, and a recent study demonstrated that PD-L1 is expressed on TAMs in almost all malignant lymphomas including adult T cell leukemia/lymphoma, follicular lymphoma, and diffuse large B-cell lymphoma14, 15.
In the present study, we investigated the localization of CD163- and CD204-positive cells in oral squamous cell carcinoma (OSCC). We also examined the levels of immune suppressive molecules produced by each TAM subset (CD163+CD204+, CD163+CD204+, and CD163+CD204+ TAMs) and association with clinical outcome.
Materials and Methods
Ethics Statement
The study design and methods were approved by the Institutional Review Board of Center for Clinical and Translational Research of Kyushu University Hospital (IRB serial number: 27–362). The methods were carried out in accordance with the approved guidelines. All patients or their relatives gave their informed consent within written treatment contract on admission and therefore prior to their inclusion in the study.
Patients
We enrolled 46 patients with primary OSCC who were treated in the Department of Oral and Maxillofacial Surgery at Kyushu University Hospital from 2005 to 2015. The average age of the patients was 66.5 ± 10.3 years (range, 19–89). Twenty-seven patients were males and nineteen were females. Following the initial biopsy, all the specimens were fixed in 4% buffered formalin solution and embedded in paraffin blocks. The paraffin-embedded specimens were processed into 5 μm thick sections, stained with hematoxylin and eosin (HE) and examined by experienced oral pathologists to confirm the diagnosis and histologic grade. The tumor stage was classified according to the TNM classification of the International Union Against Cancer. Tumor histologic grade was defined according to the WHO classification. The mode of tumor invasion was determined from H&E stained specimens according to the Yamamoto-Kohama criteria as follows: grade 1 = well-defined borderline; grade 2 = cords, less-marked borderline; grade 3 = groups of cells, no distinct borderline; and grade 4 = diffuse invasion (4 C = cord-like type; 4D = widespread type). Patients and tumor characteristics are shown in Table 1.
Table 1.
Association of tumor-associated macrophages (TAMs) with clinicopathologic characteristics in OSCC.
| Case (%) | CD163+ cells (/HPF) | P-value | CD204+ cells (/HPF) | P-value | CD163+CD204+ cells (/HPF) | P-value | CD25+ cells (/HPF) | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| Age† | |||||||||
| ≤65 | 17 (37.0) | 24.6 ± 15.2 | N.S. | 43.8 ± 28.1 | N.S. | 40.0 ± 28.4 | N.S. | 48.8 ± 28.5 | N.S. |
| 65< | 29 (63.0) | 27.8 ± 22.5 | 42.0 ± 23.1 | 38.0 ± 29.7 | 62.8 ± 31.6 | ||||
| Gender† | |||||||||
| Male | 27 (58.7) | 30.7 ± 18.0 | N.S. | 49.1 ± 27.3 | N.S. | 43.6 ± 24.7 | N.S. | 58.2 ± 28.4 | N.S. |
| Female | 19 (41.3) | 32.1 ± 23.6 | 44.7 ± 25.8 | 38.5 ± 22.2 | 49.5 ± 34.5 | ||||
| Primary site† | |||||||||
| Tongue | 22 (47.8) | 28.4 ± 23.7 | N.S. | 41.8 ± 25.8 | N.S. | 34.7 ± 22.3 | N.S. | 56.2 ± 33.4 | N.S. |
| Gingiva | 16 (34.8) | 35.2 ± 21.1 | 48.6 ± 29.2 | 45.8 ± 24.9 | 54.4 ± 30.3 | ||||
| Buccal mucosa | 6 (13.0) | 32.6 ± 13.3 | 66.0 ± 22.8 | 57.5 ± 21.7 | 49.6 ± 25.1 | ||||
| Oral floor | 2 (4.3) | 26.8 ± 3.6 | 42.0 ± 15.1 | 36.5 ± 12.0 | 57.6 ± 46.3 | ||||
| Clinical stage* | |||||||||
| I | 14 (30.0) | 33.3 ± 28.1 | N.S. | 39.6 ± 25.7 | N.S. | 33.5 ± 23.6 | N.S. | 61.5 ± 27.4 | 0.024 |
| II | 16 (34.8) | 25.3 ± 23.7 | 44.5 ± 26.1 | 36.7 ± 23.0 | 68.7 ± 21.8 | r = −0.33 | |||
| III | 8 (17.4) | 31.9 ± 11.3 | 51.5 ± 29.5 | 50.4 ± 20.4 | 35.8 ± 28.3 | ||||
| IV | 8 (17.4) | 39.2 ± 23.3 | 61.5 ± 23.8 | 54.9 ± 21.5 | 37.5 ± 39.4 | ||||
| T classification* | |||||||||
| T1 | 15 (32.6) | 44.2 ± 22.7 | N.S. | 41.1 ± 30.6 | 0.017 | 33.5 ± 25.1 | 0.004 | 64.9 ± 27.5 | 0.018 |
| T2 | 15 (32.6) | 46.1 ± 23.4 | 43.0 ± 19.3 | r = 0.34 | 36.6 ± 20.5 | r = 0.40 | 63.1 ± 23.6 | r = −0.34 | |
| T3 | 8 (17.4) | 53.1 ± 14.3 | 48.7 ± 28.2 | 48.0 ± 17.9 | 37.3 ± 29.9 | ||||
| T4 | 8 (17.4) | 43.1 ± 19.5 | 66.5 ± 23.7 | 59.9 ± 21.6 | 37.8 ± 39.2 | ||||
| Cervical nodal metastasis† | |||||||||
| + | 9 (19.6) | 48.8 ± 22.1 | N.S. | 49.5 ± 26.5 | N.S. | 47.8 ± 19.9 | 0.029 | 19.6 ± 32.8 | N.S. |
| − | 37 (80.4) | 44.3 ± 20.1 | 46.9 ± 26.5 | 36.9 ± 25.1 | 26.4 ± 27.4 | ||||
| Local recurrence† | |||||||||
| + | 12 (26.1) | 31.6 ± 16.4 | N.S. | 55.3 ± 23.4 | N.S. | 48.6 ± 22.5 | N.S. | 51.0 ± 26.4 | N.S. |
| − | 34 (73.9) | 31.1 ± 22.5 | 44.6 ± 27.3 | 39.1 ± 23.5 | 56.1 ± 32.5 | ||||
| Distant metastasis† | |||||||||
| + | 5 (10.9) | 49.9 ± 21.2 | N.S. | 78.2 ± 32.8 | 0.028 | 72.3 ± 30.8 | 0.023 | 25.5 ± 22.5 | 0.031 |
| − | 41 (89.1) | 45.8 ± 21.1 | 43.6 ± 23.4 | 37.9 ± 19.7 | 58.4 ± 30.0 | ||||
| Histological grade† | |||||||||
| Grade 1 | 31 (67.4) | 21. 9 ± 18.7 | N.S. | 21.2 ± 26.6 | N.S. | 20.8 ± 24.1 | N.S. | 61.5 ± 27.7 | N.S. |
| Grade 2 | 15 (32.6) | 26.8 ± 20.5 | 28.2 ± 26.6 | 28.9 ± 20.2 | 40.9 ± 33.4 | ||||
| Grade 3 | 0 (0.0) | ||||||||
| Mode of invasion* (YK criteria) | |||||||||
| Grade 1 | 3 (6.5) | 33.0 ± 8.0 | N.S. | 15.2 ± 16.6 | 0.002 | 10.0 ± 14.7 | 0.001 | 62.8 ± 35.6 | N.S. |
| Grade 2 | 7 (15.2) | 45.8 ± 27.6 | 32.1 ± 15.5 | r = 0.44 | 24.7 ± 16.0 | r = 0.43 | 60.4 ± 29.3 | ||
| Grade 3 | 21 (45.7) | 45.1 ± 22.1 | 49.7 ± 26.1 | 45.7 ± 20.1 | 57.4 ± 36.6 | ||||
| Grade 4C | 15 (32.6) | 50.8 ± 12.5 | 53.8 ± 24.4 | 45.6 ± 21.6 | 46.8 ± 22.1 | ||||
*Spearman’s rank correlation coefficient, †Mann-Whitney U-test and Wilcoxon signed-rank test. Not significant: N.S.
Immunohistochemical analysis
After deparaffinization/hydration of sections, the sections were washed three times in TBST for 5 min each. The slides were boiled in 10 mM sodium citrate buffer, pH 6.0 and maintained at 121 °C for 10 min. The slides were cooled on the bench top for 30 min and washed in TBST three times for 5 min each. The sections were incubated in 3% H2O2 for 30 min and then washed in TBST three times for 5 min each. Sections were blocked with 100–400 µl blocking solution for 30 min at room temperature, followed by incubation with primary antibody overnight at 4 °C. We used mouse anti-CD163 (Clone 10D6; Novocastra, Newcastle, UK, 1:400 dilution), mouse anti-CD204 (Clone SRA-E5; Transgenic, Kumamoto, Japan, 1:200 dilution), rabbit anti-CD25 (Clone ab128955; Abcam, Cambridge, UK 1:200 dilution), rabbit anti-IL-10 (Clone ab34843; Abcam, 1:50 dilution), rabbit anti-PD-L1 (E1L3N; Cell Signaling Technology, USA, 1:200 dilution), and goat anti-CD69 (clone H-20; SANTA CRUZ, Heidelberg, Germany 1:50 dilution). Antibody was removed and 100–400 µl DAB (Peroxidase Stain DAB Kit®, Nacalai Tesque, Japan) was added to each section. We performed counterstaining with hematoxylin and washed the sections in dH2O two times for 5 min each. After dehydration, we mounted the sections with coverslips.
Double immunofluorescence analysis
We first incubated sections with Blocking Buffer for 60 min and then incubated the sections with primary antibodies (as listed above). Apply these antibodies and the respective groups were CD163 IL-10, CD163 PD-L1, CD204 IL-10, CD204 PD-L1 and incubated for 3 h at room temperature. We rinsed the samples three times in TBST for 5 min each and then incubated the samples in fluorochrome-conjugated secondary antibody (Alexa Fluor® 594; Thermo Fisher Scientific, Waltham, MA, USA) diluted in Antibody Dilution Buffer for 1–2 h at room temperature in the dark. We rinsed the samples in TBST Coverslip slides with DAPI (Vectashield with DAPI®; VECTOR LABORATORIES, USA). About double staining of CD163 and CD204 was same of host animal so that we labeled FITC (Fluorescein Labelling Kit-NH2®; Dojindo Laboratories, Kumamoto, Japan).
Evaluation of macrophages and activated T cells
The numbers of CD25, CD163 and CD204 positive cells in immunohistochemical staining were counted in 4 mm2 sections from five independent high-power microscopic fields (400×; 0.0625 μm2) of cancer nest in immunohistochemistry. The numbers of CD163+CD204+ cells in double immunofluorescence staining were counted in the same way.
Culture and purification of TAMs
PBMCs (5 × 105 cells/ml) from the OSCC patients were cultured in PBS and stimulated with PMA 40 ng/ml (phorbol 12-myristate 13-acetate; Wako, Tokyo, Japan) and ionomycin 4 μg/ml (Ionomycin Calcium; Wako) for 6 h. CD163- and CD204-positive macrophages were isolated from the cultured PBMCs by positive selection with magnetic beads (PE or FITC Microbeads; Miltenyi Biotec Inc., Auburn, CA) according to the manufacturer’s manual. CD3+ T cells were purified from PBMCs without culture by negative selection with magnetic beads (CD3+ Microbeads, Miltenyi Biotec Inc.).
Co-culture of TAMs and CD3+ T cells
CD3+ T cells (5 × 105 cells/ml) were pre-incubated for 5 days in complete COSMEDIUM 006X® medium supplemented with 5% autoserum, and then pre-incubated for 6 h in PBS for additional PMA and ionomycin. These activated CD3+ T cells (5 × 105 cells/ml) co-cultured with TAMs (5 × 105 cells/ml) for 3 days in Lymphocyte Preservation Assist® (Takara Bio, Otsu, Japan) with 5% autoserum. The cells were examined under the microscope or harvested for flow cytometric analysis for expression of surface markers. In some cases, cell cultures were carried out on glass slides for observation under confocal microscopy.
Flow cytometric analysis
Harvested cells were washed with PBS supplemented with 1% BSA. After washing, the cells were incubated at room temperature for 20 min with PE anti-human CD163 antibodies (Clone GHI/61, IgG1,κ; BioLegend, San Diego, CA, USA), FITC anti-human CD204 antibodies (Clone REA460, IgG1; Milteni Biotec, Bergisch Gladbach, Germany), APC anti-human PD-L1 (Clone 29E.2A3, IgG2b,κ; BioLegend), and PerCP/Cy5.5 anti-human IL-10 (Clone JES3–9D7, IgG1,κ; BioLegend). PE mouse IgG1,κ (BioLegend), FITC REA Control antibodies IgG (Milteni Biotec), APC mouse IgG2b,κ (BioLegend), PerCP/Cy5.5 Rat IgG1,κ (BioLegend) and APC mouse IgG1,κ (BioKegend) were used as negative control antibodies. CD3+ T cells and TAMs in co-culture were analyzed using gating for CD3+, CD163+ CD204+ or CD163−CD204+ cells, respectively. Apoptosis of CD3+ T cells following culture with TAMs was measured by staining with 7-aminoactinomycin D (7-ADD) (BioLegend). Activation of CD3+ T cells following culture with TAMs was measured by staining with APC anti-human CD69 antibodies (Clone FN50, IgG1; BioLegend).
Statistical analysis
All statistical analyses were performed by JMP software version 11 (SAS Institute, NC, USA). Mann–Whitney U test, Wilcoxon test and Spearman’s rank correlation coefficient was used to assess the significant differences between each group. Progression-free survival and overall survival were estimated by the Kaplan–Meier method and curve comparisons were calculated using the log-rank test. In all analyses, P values ≤ 0.05 were considered statistically significant.
Results
Expression of TAM markers in OSCC
We first performed immunohistochemical staining to evaluate the distribution of TAMs (CD163, CD204) and activated immune cell markers (CD25 and CD69) in OSCC tissues. Expression of CD163 was detected in tumor stroma and around tumors, while that of CD204 was strongly detected in/around tumors. Expression of CD25 was diffusely detected in/around tumors (Fig. 1A). The number of CD163- and CD204-positive cells did not correlate with that of CD25-positive cells (Fig. 1B). Moreover, double immunofluorescence analysis found that CD163+CD204+ cells were frequently detected around tumors (Fig. 2A) and showed a correlation with the number of CD25-positive cells (r = −0.445; P < 0.05) (Fig. 2B) and CD69-positive cells (r = −0.359; P < 0.05) (Supp. Fig. 1).
Figure 1.
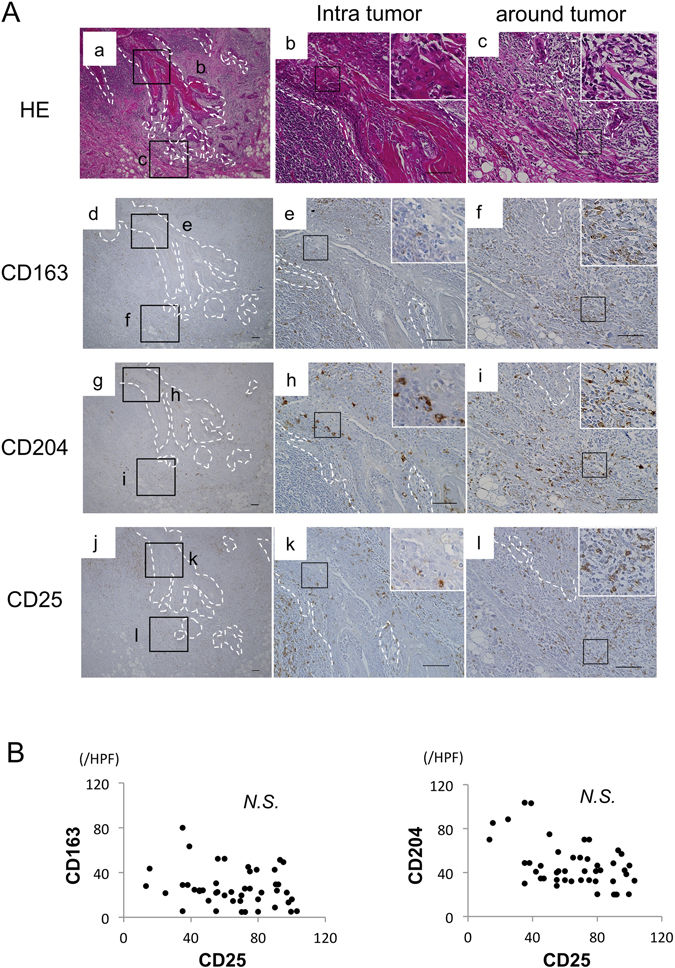
Correlation between tumor-associated macrophages (TAMs) and activated immune cells in OSCC patients. (A) Representative images of paraffin sections in/around tumor stained with H&E (a–c), CD25 (j–l), CD163 (d–f) and CD204 (g–i) antibodies (brown). Counterstaining with Mayer’s hematoxylin is shown in blue. Scale bars, 100 μm. (B) Correlation between the number of CD163+ or CD204+ and CD25+ cells in 46 OSCC patients. Statistically significant differences between groups were determined by Spearman’s rank correlation.
Figure 2.
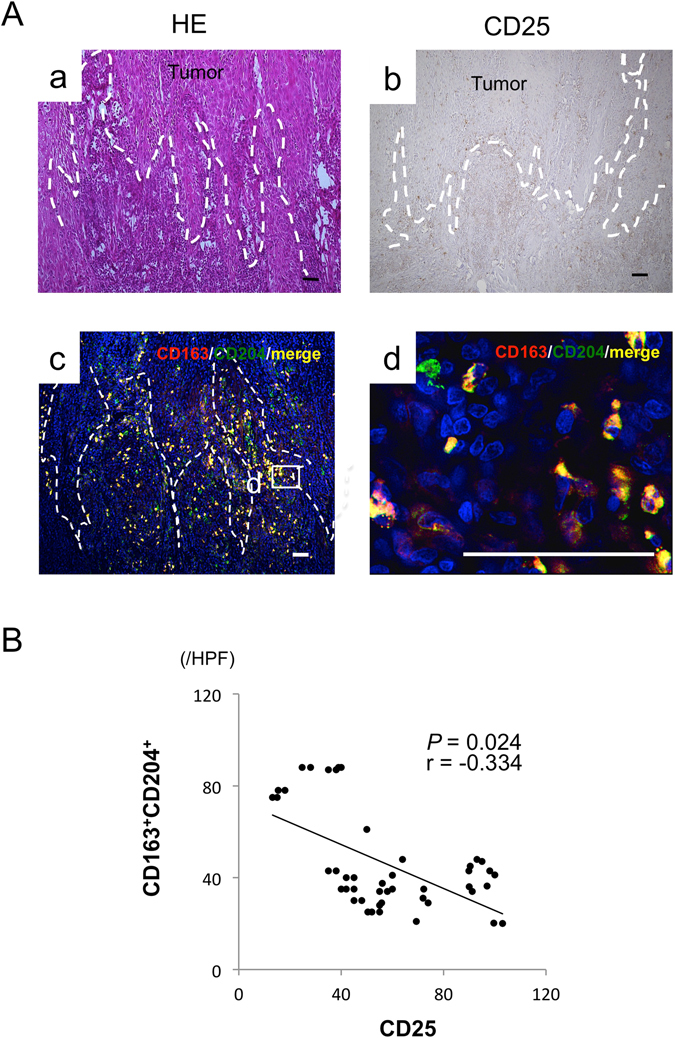
Co-localization of TAM markers in OSCC patients. (A) Representative images of paraffin sections in/around tumor stained with H&E (a) and CD25 (b) antibodies (brown). Counterstaining with Mayer’s hematoxylin is shown in blue. Double immunofluorescence staining performed with CD163 (red), CD204 (green), and DAPI for staining nuclei (blue) at low (c) and high magnification (d). (c,d) Merged CD163 and CD204 images (yellow). Scale bars, 50 μm. (B) Correlation between the number of CD163+CD204+ and CD25+ cells in 46 OSCC patients. Statistically significant differences between groups were determined by Spearman’s rank correlation.
Co-localization of TAM markers and immunosuppressive molecules in OSCC
To clarify whether TAMs express immunosuppressive molecules, double immunofluorescence staining with TAM markers and IL-10 or PD-L1 was performed. As shown in Fig. 3A, CD163- and CD204-positive cells (red) were co-localized with IL-10- and PD-L1-positive cells (green). Moreover, number of PD-L1-positive cells was positively correlated with that of IL-10-positive cells (Fig. 3B). These results suggest that TAMs might suppress the immune response to OSCC through increased production of IL-10 and PD-L1.
Figure 3.
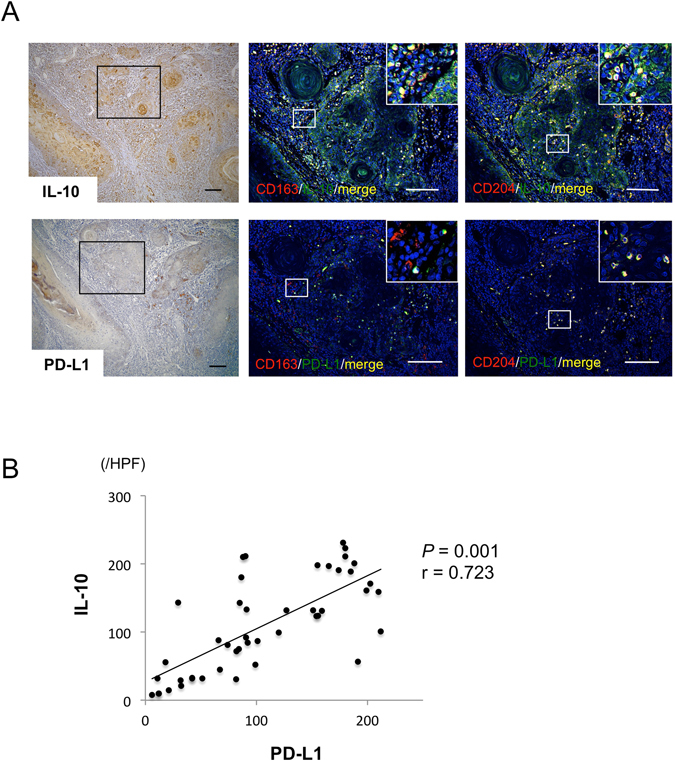
Co-localization of TAM markers and immunosuppressive molecules in OSCC. Representative images of paraffin sections in/around tumor stained with IL-10 (a) and PD-L1 (d). Counterstaining with Mayer’s hematoxylin is shown in blue. Double immunofluorescence staining performed with TAM markers (red) such as CD163 (b,e) and CD204 (c,f), and immunosuppressive molecules (green) such as IL-10 (b,c) and PD-L1 (e,f), and DAPI for staining nuclei (blue). (b,c,e,f) Merged CD163 and CD204 images (yellow). Scale bars, 50 μm.
Expression levels of immunosuppressive molecules produced by TAMs
As IL-10 and PD-L1 were expressed by both CD163- and CD204-positive cells, we next compared the expression levels of these molecules produced by each TAM subset (CD163+CD204−, CD163−CD204+, and CD163+CD204+ cells) in PBMCs from OSCC patients as described in the Materials and Methods. As shown in Fig. 4 and Supp. Fig. 2, we found that CD163+CD204+ cells expressed higher levels and numbers of IL-10 and PD-L1 on the cell surface in comparison with CD163+CD204− and CD163−CD204+cells, indicating a difference in the expression of immunosuppressive molecules among the TAM subsets.
Figure 4.
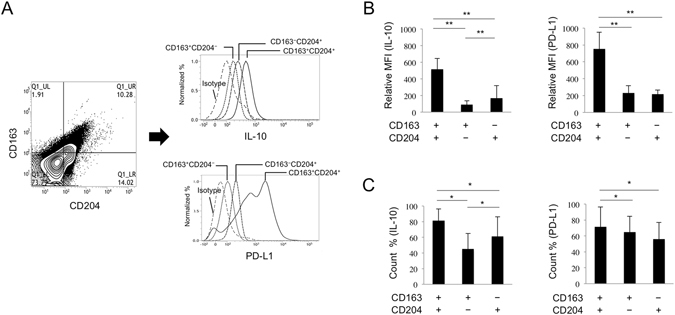
IL-10 and PD-L1 expression on TAM subsets in OSSC patients. (A) Flow cytometric analysis of IL-10 and PD-L1 expression on cultured TAM subsets. The detailed methods for cultivating cells are described in the Materials and Methods section. (B) IL-10 and PD-L1 expression (MFI; mean fluorescent intensity) on TAM subsets and (C) the number of IL-10+ and PD-L1+ cells were tested using flow cytometry (n = 3 for each subset). Statistically significant differences between groups were determined by Wilcoxon signed-rank test (**P < 0.01, *P < 0.05).
Effects of apoptotic and activation status on CD3+ T cells by co-culture with TAMs
To quantitatively confirm the immune suppression inducing effect on TAMs, we performed flow cytometric analysis for purified CD3+ T cells co-cultured with purified CD163−CD204+ or CD163+CD204+ cells as described in the Materials and Methods (Fig. 5A). The number of purified CD163+CD204− cells was too small to measure the appropriate cell count by flow cytometry. The dot plots allowed us to distinguish dead CD3+ T cells from viable cells and TAMs. The ability of TAMs to cause apoptosis of CD3+ T cells was further substantiated by measuring 7-AAD+CD3+ T cells following their co-culture with purified CD163−CD204+ or CD163+CD204+ cells. The number of dead CD3+ T cells after co-culture with CD163+CD204+ was significantly higher than that after co-culture with CD163−CD204+ cells and without TAMs (Fig. 5B). On the other hand, the ability of TAMs to inhibit activation of CD3+ T cells was further substantiated by measuring CD69+CD3+ T cells following their co-culture with purified CD163−CD204+ or CD163+CD204+ cells. The number of activated CD3+ T cells after co-culture with CD163+CD204+ was significantly lower than that after co-culture with CD163−CD204+ cells and without TAMs (Fig. 5C).
Figure 5.
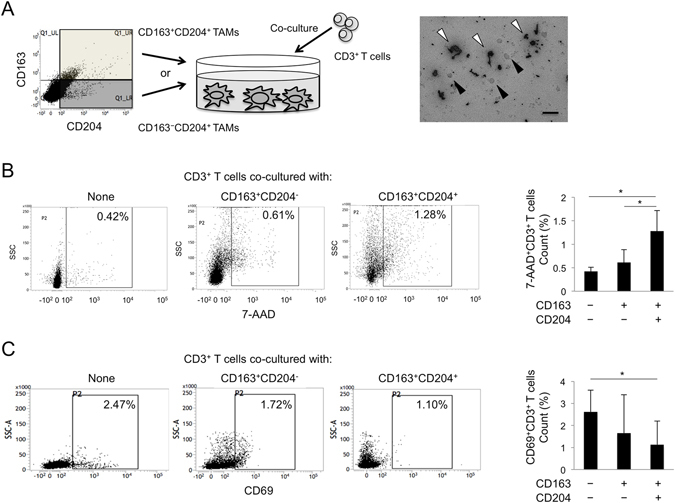
T cell regulation and apoptosis by co-culture with TAM subsets. (A) Scheme and representative image for the co-culture of TAM subsets (white arrowhead) and CD3+ T cells (black arrowhead) for 5 days. The detailed methods for cultivating cells are described in the Materials and methods section. Scale bars, 10 μm. (B) The population of 7-AAD+CD3+ T cells co-cultured with TAM subsets from a representative OSCC patient. The number of 7-AAD+CD3+ T cells co-cultured with TAM subsets was analyzed using flow cytometry (n = 3 for each subset). Statistically significant differences between groups were determined by Wilcoxon signed-rank test (*P < 0.05). (C) The population of CD69+CD3+ T cells co-cultured with TAM subsets from a representative OSCC patient. The number of CD69+CD3+ T cells co-cultured with TAM subsets was analyzed using flow cytometry (n = 3 for each subset). Statistically significant differences between groups were determined by Wilcoxon signed-rank test (*P < 0.05).
Associations of TAMs with clinical and pathological findings of OSCC patients
We next examined the associations of TAMs with the clinicopathologic factors of OSCC patients. TAMs were counted by the following three methods: (1) CD163+ cells detected by single staining, (2) CD204+ cells detected by single staining, and (3) CD163+CD204+ cells detected by double staining. As shown in Table 1, the number of CD163+ cells did not show significant differences among all of the clinicopathologic factors, while that the numbers of CD204+ and CD163+CD204+ cells were positively correlated with clinical T classification, mode of invasion, and the prevalence of distant metastasis. Interestingly, the OSCC patients with cervical nodal metastasis showed a significant increase only in the number of CD163+CD204+ cells. On the other hand, the number of CD25+ cells was negatively correlated with clinical stage, clinical T classification, and the prevalence of distant metastasis.
Associations of TAMs with clinical outcomes and prognosis among the groups
To evaluate the correlation between TAMs and the clinical outcomes of OSCC patients, the survival rates were calculated by the Kaplan-Meier method. The OSCC patients were divided into two groups according to the mean number of TAMs (CD163+, CD204+, and CD163+CD204+ cells): low and high expression groups. In the progression-free survival curves, the patients in the high CD163+CD204+ expression group had a significantly more unfavorable outcome than those in the low expression group, while had no significant difference among the groups of CD163+and CD204+ cells. In the disease-specific survival curves, the patients had no significant difference among the groups of CD163+, CD204+, and CD163+CD204+ cells (Fig. 6). Univariate analysis revealed that progression-free survival was associated with advanced age, YK criteria, number of CD163+CD204+ cells (Table 2). Multivariate analysis identified number of CD163+CD204+ cells as a marginally significant prognostic factor for progression-free survival (hazard ratio, 1.97; P = 0.0722) (Table 2).
Figure 6.
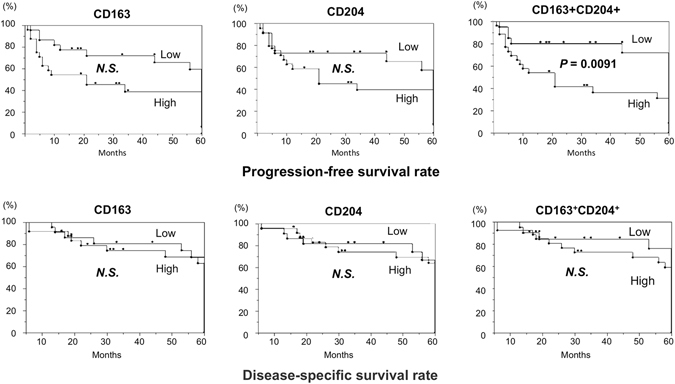
Survival curves according to the expression of TAM subsets in OSCC. The survival rates were calculated by the Kaplan-Meier method with high versus low expression of CD163+, CD204+, or CD163+CD204+ TAMs. The classifications are described in the Materials and Methods section. Statistically significant differences between groups were determined by log-rank test.
Table 2.
Univariate and multivariate analysis of progression-free survival and disease-specific survival in advanced stage.
| Progression-free survival | |||||
|---|---|---|---|---|---|
| Variables | Categories | Univariate analysis | Multivariate analysis | ||
| Progression-free survival | Progression-free survival | ||||
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age (y) | <65 vs z 65 | 3.46 (1.01–13.35) | 0.05* | ||
| Pathologic tumor status | pT1 +pT2 vs pT3 +pT4 | 2.00 (0.57–7.07) | 0.27 | ||
| Pathologic node status | pN− vs pN+ | 0.37 (0.05–1.81) | 0.23 | ||
| Pathologic metastasis status | pM0 vs pM1 | 2.60 (0.39–21.47) | 0.32 | ||
| Clinical stage | 1 vs 2 + 3 + 4 | 1.04 (0.28–4.09) | 0.95 | ||
| Histological grade | 1 vs 2 | 1.59 (0.45–5.65) | 0.47 | ||
| YK criteria | 1 +2 vs 3 + 4 | 8.05 (1.31–156.4) | 0.02* | ||
| CD163 positive cells | Low vs high | 1.53 (0.77 – 3.02) | 0.21 | 1.18 (0.57 – 2.42) | 0.64 |
| CD204 positive cells | Low vs high | 1.79 (0.92 – 3.52) | 0.08 | 1.77 (0.89 – 3.52) | 0.10 |
| CD163+CD204+ cells | Low vs high | 2.07 (1.04–4.32) | 0.03* | 1.97 (0.94 – 4.30) | 0.07 |
| Disease-specific survival | |||||
| Variables | Categories | Univariate analysis | Multivariate analysis | ||
| Disease-specific survival | Disease-specific survival | ||||
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age (y) | <65 vs z65 | 1.39 (0.69–2.86) | 0.34 | ||
| Pathologic tumor status | pT1 +pT2 vs pT3 +pT4 | 1.79 (0.84–3.63) | 0.12 | ||
| Pathologic node status | pN− vs pN+ | 0.99 (0.39–2.17) | 0.98 | ||
| Pathologic metastasis status | pM0 vs pM1 | 5.58 (1.17–21.3) | 0.03 | ||
| Clinical stage | 1 vs 2 + 3 + 4 | 1.18 (0.57–2.54) | 0.65 | ||
| Histological grade | 1 vs 2 | 1.20 (0.58–2.41) | 0.60 | ||
| YK criteria | 1 +2 vs 3 + 4 | 1.23 (0.54–3.32) | 0.62 | ||
| CD163 positive cells | Low vs high | 1.22 (0.57–2.50) | 0.58 | 1.20 (0.56–2.46) | 0.61 |
| CD204 positive cells | Low vs high | 1.46 (0.71–2.94) | 0.28 | 1.16 (0.41–4.14) | 0.79 |
| CD163+CD204+ cells | Low vs high | 1.45 (0.72–2.97) | 0.29 | 1.22 (0.34–3.41) | 0.72 |
HR, hazard ratio; CI, confidence interval, *Significant.
These results suggest that CD163+CD204+ cells play a critical role in the suppression of tumor immunity and are involved in the invasion and metastasis in OSCC.
Discussion
In 1908, Mechnikov et al. first described that macrophages were professional phagocytes and play key roles in inflammation and natural cellular immunity16. In 1970’s, many researchers thought that activated macrophages were important effector cells in cytotoxic killing of tumor cells17. Macrophages are classified into two major subsets: classically activated (M1) macrophages stimulated by Th1-type responses and alternatively activated (M2) macrophages stimulated by Th2-type responses6, 7. M1 macrophages secrete pro-inflammatory cytokines and contribute to microbicidal and tumoricidal immunity, whereas M2 macrophages scavenge debris and contribute to angiogenesis, suppression of adaptive immunity, wound healing and fibrosis by producing IL-10 and CCL18. Pollard et al.18 reported that macrophages infiltrating cancer tissues polarized to the M2 phenotype and were involved in the development of the tumor microenvironment by inducing angiogenesis and immune suppression. Recent studies have referred to these M2-polarized macrophages as TAMs, which express specific markers such as CD163 and CD20419, 20.
CD163 is a member of the scavenger receptor cysteine-rich family class B and is expressed on most subpopulations of mature tissue macrophages21. The best characterized function of CD163 is essentially a homeostatic one and is related to the binding of hemoglobin:haptoglobin complexes. Furthermore, CD163-positive macrophages play a role in the resolution of inflammation, as they are found in high numbers in inflamed tissue22, 23. We found the strong infiltration of CD163-positive cells but not CD204-positive cells in the lesions of non-specific ulcer (Supp. Fig. 3). Komohara et al. indicated that CD163 antigen might be a better marker of the M2 anti-inflammatory phenotype in clear cell renal cell carcinoma tissues compared with CD204.
CD204 is a prototypic member of a family of structurally diverse transmembrane receptors collectively termed scavenger receptors24. CD204 is preferentially expressed in dendritic cells and macrophages. CD204 functions as a pattern recognition receptor capable of binding a broad range of ligands, including chemically modified or altered molecules, bacterial surface components, and apoptotic cells, and plays roles in lipid metabolism, atherogenesis, and metabolic processes25, 26. Several studies have shown that CD204 deficiency resulted in impaired protection against pathogen infection27, 28, which has been partially attributed to the increased susceptibility of CD204-deficient animals to the overproduction of pro-inflammatory cytokines during endotoxic shock29. Emerging evidence also implicates CD204 as a suppressor in the inflammatory response30, 31. Furthermore, in esophageal squamous cell carcinomas, CD204 was demonstrated as a better marker for the TAM populations associating with tumor progression compared with CD16332. Thus, there is not yet a consensus regarding which of the two markers are more suitable for TAMs.
In the present study, we examined the expression and immunosuppression of TAMs in OSCC using both CD163 and CD204. There were clear differences in the localization of CD163- and CD204-positive cells in OSCC sections. Interestingly, the double immunofluorescent staining data revealed that CD163+CD204+TAMs mainly infiltrated around tumors and expressed higher levels of IL-10 and PD-L1 compared with other TAM subsets. Moreover, there is a positive correlation between number of IL-10 and PD-L1. IL-10 leads to the phosphorylation of STAT3 and then IL-10/STAT3 signaling induces PD-L1 overexpression33. We previously reported that phosphorylated-STAT3 expression was localized in the nucleus of the cancer cells and scattered widely in the cancer nests from patients with OSCC at advanced clinical stage34. In addition, CD163+CD204+ TAMs were also found to activate the function of T cell regulation and apoptosis. These findings indicate that CD163+CD204+ TAMs might play an important role in immune suppression and tumor progression via IL-10-Stat3-PD-L1 signaling.
Recently, PD-1 ligand 2 (PD-L2) was identified as a second ligand for PD-1. PD-L1 constitutively expressed by various immune cells including T cells, B cells, macrophages, DCs, and tumor cells. On the other hands, PD-L2 expression is limited to macrophages and DCs35. In malignant tumor, TAMs suppressed anti-tumor immune responses by overexpression of PD-L1/236. Therefore, further examinations are required to elucidate the expression of PD-L2 in each TAM subsets in OSCC.
We next examined the association of the TAMs with the prognosis of OSCC patients. The number of CD204+ and CD163+CD204+ TAMs in OSCCs was positively correlated with clinical T classification and distant metastasis. Moreover, the numbers of CD163+CD204+ TAMs also significantly associated with the progression-free survival curves. However, the association between the expression of TAM markers and clinical prognosis for cancer patients remains controversial. Hirayama et al.37 reported that a high number of CD204+ TAMs in lung SCCs was significantly correlated with advanced clinicopathological parameters and poor prognosis. Another study showed that there was no significant difference in clinicopathological factors and clinical prognosis between high and low expression groups of CD163+ TAMs in OSCC38. These results were consistent with our results in the present study. On the contrary, in gliomas, both CD204+ and CD163+ TAMs were positively correlated with the histological malignancy39. This contradictory conclusion may be due to different tumor types or methodologies.
In conclusion, we have confirmed that CD163+CD204+ TAMs promote T-cell apoptosis and immunosuppression via IL-10 and PD-L1 and predict unfavorable prognosis in OSCC patients. We are currently examining which of the TAM subsets contribute to the tumor proliferation and angiogenesis in OSCC. A more thorough understanding of the role of each TAM subset could lead to the development of novel pharmacological strategies aimed at disrupting TAMs or their products and inhibiting the progression and/or metastasis of OSCC.
Electronic supplementary material
Acknowledgements
We appreciate the technical assistance from the Research Support Center, Research Center for Human Disease Modeling, Kyushu University Graduate School of Medical Sciences. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (24249091, 26293430, 16K20584), and the “QR program Wakaba research”, a matching fund subsidy from Kyushu University (FB79229743).
Author Contributions
Study conception and design: K.K., M.M., S.F., and S.N. Acquisition of data: K.K., H.A.S.M.R., Y.M., T.J., A.T., M.O., N.I., M.Y., M.S., T.M., and J.-N.H. Analysis and interpretation of data: M.M., S.K., T.K., and S.N.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Keigo Kubota and Masafumi Moriyama contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01661-z
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Josephs DH, Bax HJ, Karagiannis SN. Tumour-associated macrophage polarisation and re-education with immunotherapy. Front Biosci (Elite Ed) 2015;7:293–308. doi: 10.2741/E735. [DOI] [PubMed] [Google Scholar]
- 2.Weber M, et al. Macrophage polarisation changes within the time between diagnostic biopsy and tumour resection in oral squamous cell carcinomas–an immunohistochemical study. Br J Cancer. 2015;113(3):510–9. doi: 10.1038/bjc.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li XS, Ke MR, Zhang MF, Tang QQ, Zheng BY, Huang JD. A non-aggregated and tumourassociated macrophage-targeted photosensitiser for photodynamic therapy: a novel zinc(II) phthalocyanine containing octa-sulphonates. Chem Commun (Camb) 2015;51(22):4704–7. doi: 10.1039/C4CC09934F. [DOI] [PubMed] [Google Scholar]
- 4.Sica A, et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18(5):349–55. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Jacques A, Bleau C, Turbide C, Beauchemin N, Lamontagne L. Macrophage interleukin-6 and tumour necrosis factor-alpha are induced by coronavirus fixation to Toll-like receptor 2/heparan sulphate receptors but not carcinoembryonic cell adhesion antigen 1a. Immunology. 2009;128:e181–92. doi: 10.1111/j.1365-2567.2008.02946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varney ML, Johansson SL, Singh RK. Tumour-associated macrophage infiltration, neovascularization and aggressiveness in malignant melanoma: role of monocyte chemotactic protein-1 and vascular endothelial growth factor-A. Melanoma Res. 2005;15(5):417–25. doi: 10.1097/00008390-200510000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 8.Gordon S, Martinez FO. Alternative Activation of Macrophages: Mechanism and Functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Qian BZ, Pollard JW. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014;105(1):1–8. doi: 10.1111/cas.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shigeoka M, et al. Cyr61 promotes CD204 expression and the migration of macrophages via MEK/ERK pathway in esophageal squamous cell carcinoma. Cancer Med-Us. 2015;4(3):437–46. doi: 10.1002/cam4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komohara Y, et al. Clinical significance of CD163(+) tumor-associated macrophages in patients with adult T-cell leukemia/lymphoma. Cancer Sci. 2013;104(7):945–51. doi: 10.1111/cas.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikami M, Sadahira Y, Suetsugu Y, Wada H, Sugihara T. Monocyte/macrophage-specific marker CD163(+) histiocytic sarcoma: Case report with clinical, morphologic, immunohistochemical, and molecular genetic studies. Int J Hematol. 2004;80(4):365–9. doi: 10.1532/IJH97.04064. [DOI] [PubMed] [Google Scholar]
- 14.Yi HF, et al. Pattern recognition scavenger receptor SRA/CD204 down-regulates Toll-like receptor 4 signaling-dependent CD8 T-cell activation. Blood. 2009;113(23):5819–28. doi: 10.1182/blood-2008-11-190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Annna, K. et al. CD274 (PD-L1)/PDCD1 (PD-1) expression in de novo and transformed diffuse large B-cell lymphoma. Br J Hematol, doi:10.1111/bjh.14432 (2016).
- 16.Lokaj J, John C. [Ilya Ilich Metchnikov and Paul Ehrlich: 1908 Nobel Prize winners for their research on immunity] Epidemiol Mikrobiol Imunol. 2008;57(4):119–24. [PubMed] [Google Scholar]
- 17.Zembala M, Ptak W, Hanczakowska M. The role of macrophages in the cytotoxic killing of tumour cells in vitro. I. Primary immunization of lymphocytes in vitro for target cell killing and the mechanism of lymphocytemacrophage cooperation. Immunology. 1973;25(4):631–44. [PMC free article] [PubMed] [Google Scholar]
- 18.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 19.Wang B, et al. High CD204+ tumor-infiltrating macrophage density predicts a poor prognosis in patients with urothelial cell carcinoma of the bladder. Oncotarget. 2015;6(24):20204–14. doi: 10.18632/oncotarget.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komohara Y, Niino D, Ohnishi K, Ohshima K, Takeya M. Role of tumor-associated macrophages in hematological malignancies. Pathol Int. 2015;65:170–6. doi: 10.1111/pin.12259. [DOI] [PubMed] [Google Scholar]
- 21.Zwadlo-Klarwasser G, Neubert R, Stahlmann R, Schmutzler W. Influence of dexamethasone on the RM 3/1-positive macrophages in the peripheral blood and tissues of a New World monkey (the marmoset Callithrix jacchus) Int Arch Allergy Immunol. 1992;97:178–80. doi: 10.1159/000236115. [DOI] [PubMed] [Google Scholar]
- 22.Fabriek BO, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163. Immunobiology. 2005;210:153–60. doi: 10.1016/j.imbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Tentillier N, et al. Anti-inflammatory therapy via CD163-macrophages in the 6-OHDA Parkinson's disease model. J Neurosci. 2016;36(36):9375–90. doi: 10.1523/JNEUROSCI.1636-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Platt N, Gordon S. Is the class A macrophage scavenger receptor (SR-A) multifunctional? The mouse’s tale. J Clin Invest. 2001;108:649–654. doi: 10.1172/JCI200113903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krieger M. The other side of scavenger receptors: pattern recognition for host defense. Curr Opin Lipidol. 1997;8:275–280. doi: 10.1097/00041433-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Berwin B, et al. Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigenpresenting cells. EMBO J. 2003;22:6127–36. doi: 10.1093/emboj/cdg572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki H, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–6. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 28.Peiser L, et al. The class A macrophage scavenger receptor is a major pattern recognition receptor for Neisseria meningitidis which is independent of lipopolysaccharide and not required for secretory responses. Infect Immun. 2002;70:5346–54. doi: 10.1128/IAI.70.10.5346-5354.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haworth R, et al. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J Exp Med. 1997;186:1431–9. doi: 10.1084/jem.186.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jozefowski S, Arredouani M, Sulahian T, Kobzik L. Disparate regulation and function of the class A scavenger receptors SR-AI/II and MARCO. J Immunol. 2005;175:8032–41. doi: 10.4049/jimmunol.175.12.8032. [DOI] [PubMed] [Google Scholar]
- 31.Becker M, Cotena A, Gordon S, Platt N. Expression of the class A macrophage scavenger receptor on specific subpopulations of murine dendritic cells limits their endotoxin response. Eur J Immunol. 2006;36:950–60. doi: 10.1002/eji.200535660. [DOI] [PubMed] [Google Scholar]
- 32.Shigeoka M, et al. Tumor associated macrophage expressing CD204 is associated with tumor aggressiveness of esophageal squamous cell carcinoma. Cancer Sci. 2013;104(8):1112–9. doi: 10.1111/cas.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wölfle SJ, et al. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur J Immunol. 2011;41(2):413–24. doi: 10.1002/eji.201040979. [DOI] [PubMed] [Google Scholar]
- 34.Jinno TM, et al. Increased expression of interleukin-6 predicts poor response to chemoradiotherapy and unfavorable. Oncol Rep. 2015;33(5):2161–8. doi: 10.3892/or.2015.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghiotto M, et al. PD-L1 and PD-L2 differ in their molecular mechanisms of interaction with PD-1. Int Immunol. 2010;22(8):651–60. doi: 10.1093/intimm/dxq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horlad H, et al. An IL-27/Stat3 axis induces expression of programmed cell death 1 ligands (PD-L1/2) on infiltrating macrophages in lymphoma. Cancer Sci. 2016;107(11):1696–704. doi: 10.1111/cas.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirayama S, et al. Prognostic impact of CD204-positive macrophages in lung squamous cell carcinoma: possible contribution of CD204-positive macrophages to the tumor-promoting microenvironment. J Thorac Oncol. 2012;7:1790–7. doi: 10.1097/JTO.0b013e3182745968. [DOI] [PubMed] [Google Scholar]
- 38.Fujii N, et al. Cancer-associated fibroblasts and CD163-positive macrophages in oral squamous cell carcinoma: clinicopathological and prognostic significance. J Oral Pathol Med. 2012;41:444–51. doi: 10.1111/j.1600-0714.2012.01127.x. [DOI] [PubMed] [Google Scholar]
- 39.Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216:15–24. doi: 10.1002/path.2370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


