Abstract
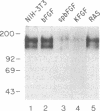
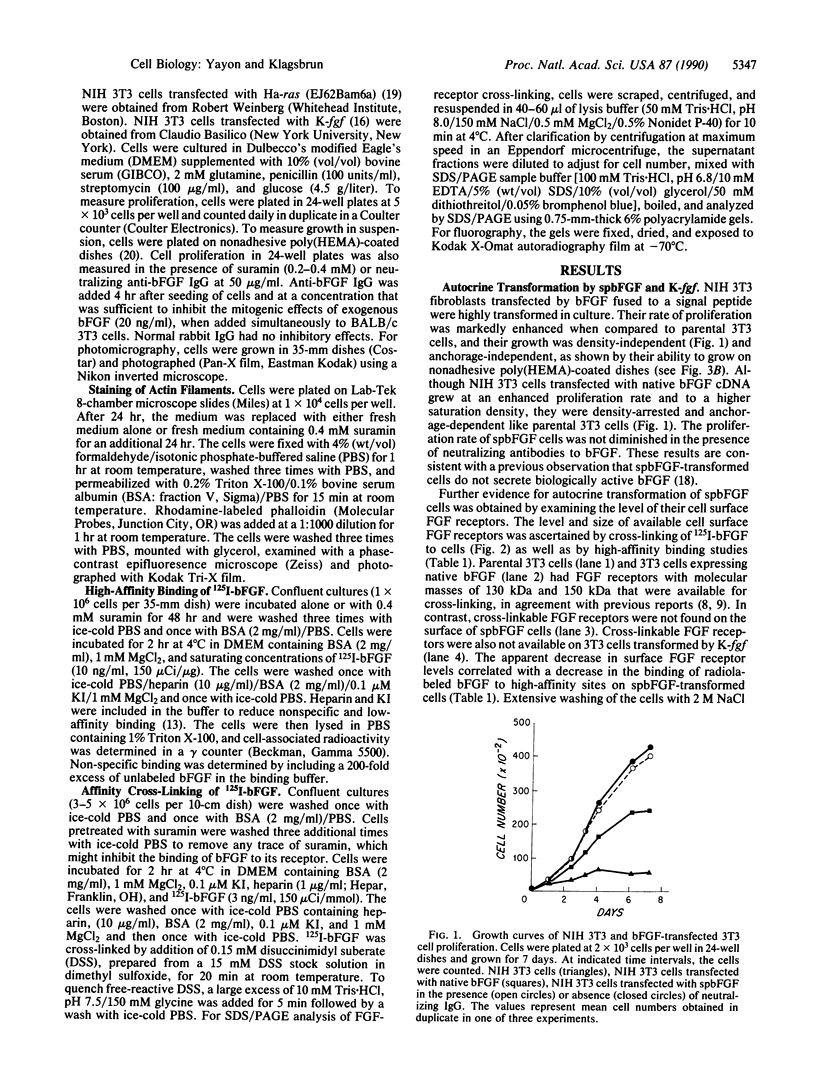
NIH 3T3 cells transfected with basic fibroblast growth factor (bFGF) fused to an immunoglobulin signal peptide sequence are transformed in vitro and tumorigenic in vivo. The transformed phenotype of chimeric signal peptide-bFGF (spbFGF) cells is characterized by an enhanced proliferation rate compared to parental NIH 3T3 cells, density- and anchorage-independent growth, a transformed morphology, and lack of cell adhesion. The rate of spbFGF cell proliferation is not diminished by anti-bFGF neutralizing antibodies. 125I-labeled bFGF receptor cross-linking and binding studies suggest that surface FGF receptors in spbFGF cells are unavailable and down-regulated. The FGF receptors are also down-regulated in K-fgf-transformed cells but not in parental 3T3, native bFGF-transfected, and ras-transformed NIH 3T3 cells. The addition of suramin to spbFGF and K-fgf cells rapidly promotes the up-regulation of FGF receptors. Suramin also induces lowering of the proliferation rate to that of parental cells, anchorage-dependent growth, assembly of cytoskeletal filaments, cellular adhesion, and spreading. These results suggest that spbFGF cells undergo autocrine transformation, possibly by an internal autocrine loop, in which there is constitutive activation of the FGF receptor. Suramin inhibits autocrine transformation, leading to a normal untransformed phenotype.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein S. C., Weinberg R. A. Expression of the metastatic phenotype in cells transfected with human metastatic tumor DNA. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1726–1730. doi: 10.1073/pnas.82.6.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B. Efficient reversion of simian sarcoma virus-transformation and inhibition of growth factor-induced mitogenesis by suramin. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6440–6444. doi: 10.1073/pnas.83.17.6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browder T. M., Abrams J. S., Wong P. M., Nienhuis A. W. Mechanism of autocrine stimulation in hematopoietic cells producing interleukin-3 after retrovirus-mediated gene transfer. Mol Cell Biol. 1989 Jan;9(1):204–213. doi: 10.1128/mcb.9.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browder T. M., Dunbar C. E., Nienhuis A. W. Private and public autocrine loops in neoplastic cells. Cancer Cells. 1989 Sep;1(1):9–17. [PubMed] [Google Scholar]
- Delli Bovi P., Curatola A. M., Kern F. G., Greco A., Ittmann M., Basilico C. An oncogene isolated by transfection of Kaposi's sarcoma DNA encodes a growth factor that is a member of the FGF family. Cell. 1987 Aug 28;50(5):729–737. doi: 10.1016/0092-8674(87)90331-x. [DOI] [PubMed] [Google Scholar]
- Dickson C., Peters G. Potential oncogene product related to growth factors. 1987 Apr 30-May 6Nature. 326(6116):833–833. doi: 10.1038/326833a0. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Moellmann G., Ghosh S., Edwards M., Halaban R. Transformation of murine melanocytes by basic fibroblast growth factor cDNA and oncogenes and selective suppression of the transformed phenotype in a reconstituted cutaneous environment. J Cell Biol. 1989 Dec;109(6 Pt 1):3115–3128. doi: 10.1083/jcb.109.6.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M., Sasse J., Wadzinski M., Ingber D., Vlodavsky I. A heparin-binding angiogenic protein--basic fibroblast growth factor--is stored within basement membrane. Am J Pathol. 1988 Feb;130(2):393–400. [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Garrett J. S., Coughlin S. R., Niman H. L., Tremble P. M., Giels G. M., Williams L. T. Blockade of autocrine stimulation in simian sarcoma virus-transformed cells reverses down-regulation of platelet-derived growth factor receptors. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7466–7470. doi: 10.1073/pnas.81.23.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannink M., Donoghue D. J. Requirement for a signal sequence in biological expression of the v-sis oncogene. Science. 1984 Dec 7;226(4679):1197–1199. doi: 10.1126/science.6095451. [DOI] [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Deuel T. F. Transforming protein of simian sarcoma virus stimulates autocrine growth of SSV-transformed cells through PDGF cell-surface receptors. Cell. 1984 Nov;39(1):79–87. doi: 10.1016/0092-8674(84)90193-4. [DOI] [PubMed] [Google Scholar]
- Huang S. S., Huang J. S. Rapid turnover of the platelet-derived growth factor receptor in sis-transformed cells and reversal by suramin. Implications for the mechanism of autocrine transformation. J Biol Chem. 1988 Sep 5;263(25):12608–12618. [PubMed] [Google Scholar]
- Iberg N., Rogelj S., Fanning P., Klagsbrun M. Purification of 18- and 22-kDa forms of basic fibroblast growth factor from rat cells transformed by the ras oncogene. J Biol Chem. 1989 Nov 25;264(33):19951–19955. [PubMed] [Google Scholar]
- Jaye M., Lyall R. M., Mudd R., Schlessinger J., Sarver N. Expression of acidic fibroblast growth factor cDNA confers growth advantage and tumorigenesis to Swiss 3T3 cells. EMBO J. 1988 Apr;7(4):963–969. doi: 10.1002/j.1460-2075.1988.tb02902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating M. T., Williams L. T. Autocrine stimulation of intracellular PDGF receptors in v-sis-transformed cells. Science. 1988 Feb 19;239(4842):914–916. doi: 10.1126/science.2829358. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M. The fibroblast growth factor family: structural and biological properties. Prog Growth Factor Res. 1989;1(4):207–235. doi: 10.1016/0955-2235(89)90012-4. [DOI] [PubMed] [Google Scholar]
- Kornbluth S., Paulson K. E., Hanafusa H. Novel tyrosine kinase identified by phosphotyrosine antibody screening of cDNA libraries. Mol Cell Biol. 1988 Dec;8(12):5541–5544. doi: 10.1128/mcb.8.12.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. L., Johnson D. E., Cousens L. S., Fried V. A., Williams L. T. Purification and complementary DNA cloning of a receptor for basic fibroblast growth factor. Science. 1989 Jul 7;245(4913):57–60. doi: 10.1126/science.2544996. [DOI] [PubMed] [Google Scholar]
- Moscatelli D. Metabolism of receptor-bound and matrix-bound basic fibroblast growth factor by bovine capillary endothelial cells. J Cell Biol. 1988 Aug;107(2):753–759. doi: 10.1083/jcb.107.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D., Quarto N. Transformation of NIH 3T3 cells with basic fibroblast growth factor or the hst/K-fgf oncogene causes downregulation of the fibroblast growth factor receptor: reversal of morphological transformation and restoration of receptor number by suramin. J Cell Biol. 1989 Nov;109(5):2519–2527. doi: 10.1083/jcb.109.5.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G., Gospodarowicz D. The identification and partial characterization of the fibroblast growth factor receptor of baby hamster kidney cells. J Biol Chem. 1985 Nov 5;260(25):13860–13868. [PubMed] [Google Scholar]
- Rifkin D. B., Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol. 1989 Jul;109(1):1–6. doi: 10.1083/jcb.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogelj S., Weinberg R. A., Fanning P., Klagsbrun M. Basic fibroblast growth factor fused to a signal peptide transforms cells. Nature. 1988 Jan 14;331(6152):173–175. doi: 10.1038/331173a0. [DOI] [PubMed] [Google Scholar]
- Rogelj S., Weinberg R. A., Fanning P., Klagsbrun M. Characterization of tumors produced by signal peptide-basic fibroblast growth factor-transformed cells. J Cell Biochem. 1989 Jan;39(1):13–23. doi: 10.1002/jcb.240390103. [DOI] [PubMed] [Google Scholar]
- Ruta M., Burgess W., Givol D., Epstein J., Neiger N., Kaplow J., Crumley G., Dionne C., Jaye M., Schlessinger J. Receptor for acidic fibroblast growth factor is related to the tyrosine kinase encoded by the fms-like gene (FLG). Proc Natl Acad Sci U S A. 1989 Nov;86(22):8722–8726. doi: 10.1073/pnas.86.22.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigerer L., Ferrara N., Haaparanta T., Neufeld G., Gospodarowicz D. Basic fibroblast growth factor: expression in cultured cells derived from corneal endothelium and lens epithelium. Exp Eye Res. 1988 Jan;46(1):71–80. doi: 10.1016/s0014-4835(88)80094-0. [DOI] [PubMed] [Google Scholar]
- Sommer A., Rifkin D. B. Interaction of heparin with human basic fibroblast growth factor: protection of the angiogenic protein from proteolytic degradation by a glycosaminoglycan. J Cell Physiol. 1989 Jan;138(1):215–220. doi: 10.1002/jcp.1041380129. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Sullivan R., Klagsbrun M. Purification of cartilage-derived growth factor by heparin affinity chromatography. J Biol Chem. 1985 Feb 25;260(4):2399–2403. [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E. J., Wormall A. Studies on suramin (Antrypol: Bayer 205). 7. Further observations on the combination of the drug with proteins. Biochem J. 1949;45(2):224–231. [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Bates B., Hu X. G., Goldfarb M. The human FGF-5 oncogene encodes a novel protein related to fibroblast growth factors. Mol Cell Biol. 1988 Aug;8(8):3487–3495. doi: 10.1128/mcb.8.8.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]