Abstract
We sought to describe the characteristics of catheter-associated urinary tract infections (CAUTIs) in a children’s hospital, while demonstrating efficacy of electronic identification of CAUTIs. 25 CAUTIs were identified over 24 months, with most (88%) occurring in the ICUs. The incidence of ICU CAUTIs decreased during the study period (p=0.04). Concordance between electronic identification and validation by infection control staff was 83% and increased to 100% with correction of nursing documentation.
Keywords: CAUTI, pediatric, hospital-associated infection, indwelling urinary catheter, electronic surveillance
Introduction
Catheter-associated urinary tract infections (CAUTIs) are a focus of quality improvement in the United States, but there are limited data on pediatric CAUTIs. Several small studies outside the U.S. have described CAUTIs, but it is uncertain if they are pertinent to the American pediatric patient population1–5. One quality improvement study conducted in an American children’s hospital demonstrated a decrease in CAUTIs from 5.41 to 2.49 per 1000 catheter-days6.
Given the reporting requirements for healthcare-associated infections (HAIs), there is increasing interest in automated electronic surveillance methodologies for HAIs. Few studies have described experience with electronic surveillance for HAIs in children. In this project, we aim to describe [1] the patient characteristics and pathogens associated with CAUTIs during 24 months of prospective surveillance and [2] the accuracy of electronic surveillance methods for CAUTIs in our children’s hospital.
Methods
We studied the clinical characteristics of pediatric patients with CAUTIs diagnosed from May 2012 – April 2014 and described the process for identifying CAUTIs. The study was performed at our 200-bed academically affiliated children’s hospital, which includes a Pediatric ICU (22 beds) and Pediatric Cardiac ICU (14 beds). The Institutional Review Board at Columbia University Medical Center approved the study with a waiver of informed consent.
CAUTIs were identified prospectively in all inpatient units, excluding the Neonatal ICU. Rates were calculated using the Centers for Disease Control and Prevention National Healthcare Safety Network (NHSN) case definition [http://www.cdc.gov/nhsn]. The definition used during the study period differs from the current CAUTI definition. For example, the former definition included use of urinalysis for cultures with lower colony counts. CAUTI rates are reported to the New York State Department of Health and publically.
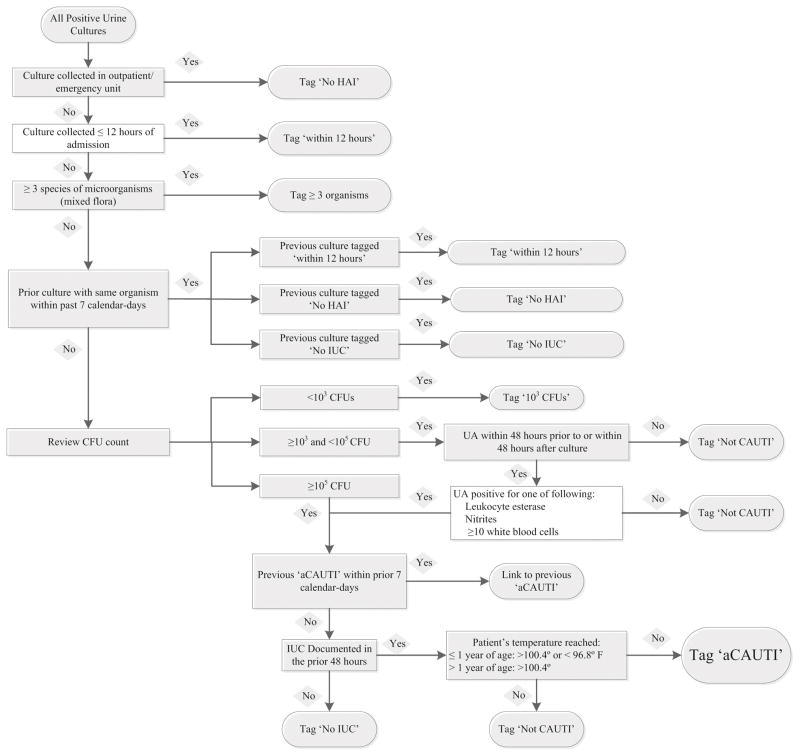
CAUTIs were identified using a two-step process. Initial detection of CAUTIs occurred electronically (aCAUTIs) using the NewYork-Presbyterian Hospital Department of Infection Prevention and Control (IP&C) EpiPortal system.7 EpiPortal is an electronic surveillance system that integrates demographic, clinical and microbiologic data from the electronic medical record (EMR), including relevant signs (i.e. fever) and laboratory data (i.e. positive urine culture and/or pyuria) to detect HAIs among hospitalized patients, as shown in the Figure. EpiPortal also calculates the number of indwelling urinary catheter (IUC)-days per unit.
Figure.
Epiportal Algorithm. The diagnostic algorithm for identifying aCAUTIs is detailed. Abbreviations used in the figure include colony forming units (CFU), indwelling urinary catheter (IUC), hospital-associated infection (HAI) and urinalysis (UA).
IP&C staff reviewed all aCAUTIs to confirm that they fulfilled the NHSN definition, in addition to reviewing daily every positive urine cultures to ensure that no patient had been missed by EpiPortal. Confirmed CAUTIs were adjudicated by hospital epidemiologists and shared with the patients’ clinicians for quality improvement. The demographic and clinical characteristics of CAUTI patients, outcomes, and pathogens were abstracted from the EMR.
Temporal trends in CAUTI rates were modeled using Poisson regression with the monthly number of CAUTIs as the outcome variable, time (expressed as quarter of the year) as the predictor and catheter-days as the exposure variable. The chi-squared goodness of fit test was used to determine model fit. Incidence rate ratios were calculated and an alpha of 0.05 was significant. Analysis used STATA 13.1 (StataCorp, College Station, TX).
Results
Most CAUTIs identified during the study period (22/25, 88%) occurred in ICU patients. The mean and median duration of IUC use prior to CAUTI was 7.2 and 6 days, respectively, and three (12%) IUCs were placed ≤ 48 hours prior to the CAUTI. Most IUCs were placed in the ICU (n=16, 64%) or operating room (n=6, 24%).
The characteristics of CAUTI patients are shown (Table). Such patients had longer median ICU length of stay (LOS) when compared to the median LOS of all ICU patients (26.0 days vs. 2.1 days, respectively). None of the patients with CAUTIs had a prior central line-associated blood stream infection or ventilator-associated pneumonia during that hospitalization.
Table.
Demographic and Clinical Characteristics of Pediatric Patients Diagnosed with CAUTIs
| Characteristics | |
|---|---|
|
| |
| Mean age [range] | 93 months [1–225 months] |
|
| |
| Median age | 78 months |
|
| |
| Male (n, %) | 8 (32) |
|
| |
| Comorbid Conditions (n, %) | |
| Cardiac disease | 10 (40) |
| Bone marrow transplant | 1 (4) |
| Oncologic (non-bone marrow transplant) | 2 (8) |
| Solid organ transplant | 2 (8) |
| Neurosurgical | 3 (12) |
| History of prematurity | 3 (12) |
| Tracheostomy | 5 (20) |
|
| |
| Hospital location (n, %) | |
| Pediatric ICU | 14 (56) |
| Pediatric Cardiac ICU | 8 (32) |
| Inpatient floor | 3 (12) |
|
| |
| Surgery during hospitalization prior to CAUTI (n, %) | 14 (56) |
|
| |
| Immunosuppressive medications1 | 8 (32) |
|
| |
| Mechanical Ventilation1 | 18 (72) |
|
| |
| Any inotropic medications1 | 8 (32) |
| Milrinone only1 | 4 (16) |
|
| |
| Antibiotics1 | 13 (52) |
|
| |
| Length of hospital stay prior to CAUTI | |
| Median [Range] | 9 [1–326] days |
|
| |
| Length of ICU stay prior to CAUTI2 | |
| Median [Range] | 9 [1–324] days |
|
| |
| Length of hospital stay total | |
| Median [Range] | 37 [3–635] days |
|
| |
| Length of ICU stay | |
| Median [Range] | 26 [2–633] days |
|
| |
| Death during hospitalization | 2 (8) |
≤48 hours prior to CAUTI
Patients diagnosed with CAUTI while in ICU
During the study period, 105 urine cultures were selected by EpiPortal for review and 30 (29%) were identified as “aCAUTI.” IP&C review found that 25/30 (83%) fulfilled NHSN criteria. In the remaining five cases, incorrect documentation in the EMR indicated the presence of an IUC in patients undergoing intermittent catheterization. Manual review of positive urine cultures by IP&C staff did not detect additional CAUTIs.
During the study period, the total number of catheter days decreased from 296/1000 patient-days in the first quarter to 264/1000 patient-days in the last quarter (p=0.18).
In the regression model for CAUTI rates, CAUTI rates decreased from 10.4/1000 catheter days to 0 in the last quarter of the study period (IRR 0.9, 95% CI 0.6–0.9, p=0.04).
CAUTIs were caused by Gram-negative organisms (n=18, 72%), yeast (n=5, 20%) and Gram-positive organisms (n=2, 8%). Urinalyses were performed for 20 (80%) of 25 CAUTIs. Seventeen (85%) were positive for leukocyte esterase and five (25%) were positive for nitrite. Five (28%), three (17%) and ten (56%) of the 18 with microscopic analyses had <5, 5–10, and >10 leukocytes, respectively.
Discussion
This study describes the salient characteristics of patients with CAUTIs at an academic children’s hospital. The majority of CAUTIs occurred in the ICU as IUCs are frequently used in critically ill children. During the study, the incidence of CAUTIs significantly declined, which was not coincident with a significant decline in IUC-days. We attribute this to ongoing educational strategies with clinical staff, instituting CAUTI bundles, and administrative support. We found that electronic surveillance for CAUTIs was very accurate. The discordant diagnoses were explained by EMR documentation errors that represent an opportunity for quality improvement.
This study had limitations. This is a single center study at a site that performs a large number of cardiac surgical procedures. Thus, our findings may not be generalizable. Although CAUTIs were identified prospectively, the patient characteristics were gathered retrospectively. We could not perform an assessment of risk factors for CAUTIs due to limitations in available data, and thus reported the data descriptively.
In conclusion, we described the characteristics of 25 pediatric patients with CAUTIs over a 24-month period. Most CAUTIs occurred in children in the ICUs. Electronic surveillance for CAUTIs was highly accurate in this population.
Future efforts to reduce CAUTIs will focus on root cause analysis to identify potential risk factors and continual reduction of IUC-days.
Highlights.
25 CAUTIs were identified over 24 months in a large urban children’s hospital.
Most CAUTIs (88%) occurred in the intensive care units.
Electronic surveillance for CAUTIs in children proved accurate.
Acknowledgments
The authors thank our hospital’s nurses for their dedication to CAUTI prevention efforts. Philip Zachariah is supported by the training grant “Training in Pediatric Infectious Diseases” (NIAID T32AI007531).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bi XC, Zhang B, Ye YK, et al. Pathogen incidence and antibiotic resistance patterns of catheter-associated urinary tract infection in children. J Chemother. 2009;21(6):661–5. doi: 10.1179/joc.2009.21.6.661. [DOI] [PubMed] [Google Scholar]
- 2.Duenas L, Bran de Casares A, Rosenthal VD, Jesus Machuca L. Device-associated infections rates in pediatrics and neonatal intensive care units in El Salvador: findings of the INICC. J Infect Dev Ctries. 2011;5(6):445–51. doi: 10.3855/jidc.1319. [DOI] [PubMed] [Google Scholar]
- 3.Esteban E, Ferrer R, Urrea M, et al. The impact of a quality improvement intervention to reduce nosocomial infections in a PICU. Pediatr Crit Care Med. 2013;14(5):525–32. doi: 10.1097/PCC.0b013e31828a87cc. [DOI] [PubMed] [Google Scholar]
- 4.Rasslan O, Seliem ZS, Ghazi IA, et al. Device-associated infection rates in adult and pediatric intensive care units of hospitals in Egypt. International Nosocomial Infection Control Consortium (INICC) findings. J Infect Public Health. 2012;5(6):394–402. doi: 10.1016/j.jiph.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal VD, Ramachandran B, Duenas L, et al. Findings of the International Nosocomial Infection Control Consortium (INICC), Part I: Effectiveness of a multidimensional infection control approach on catheter-associated urinary tract infection rates in pediatric intensive care units of 6 developing countries. Infect Control Hosp Epid. 2012;33(7):696–703. doi: 10.1086/666341. [DOI] [PubMed] [Google Scholar]
- 6.Davis KF, Colebaugh AM, Eithun BL, et al. Reducing catheter-associated urinary tract infections: a quality-improvement initiative. Pediatrics. 2014;134(3):e857–64. doi: 10.1542/peds.2013-3470. [DOI] [PubMed] [Google Scholar]
- 7.Behta M, Ross B, Chaudhry R. A comprehensive decision support system for the identification, monitoring and management of patients with multi-drug resistant organisms (MDRO) AMIA Annu Symp Proc. 2008:1218. [PubMed] [Google Scholar]