Abstract
Objectives
Cisplatin ototoxicity affects 42-88% of treated children. COMT, TPMT, and AYCP2 genetic variants have been associated with ototoxicity, but the findings have been contradictory. The aims of the study were to (a) investigate these associations in a carefully phenotyped cohort of UK children, and (b) to undertake a systematic review and meta-analysis.
Methods
We recruited 149 children from seven UK centres using a retrospective cohort study design. All participants were carefully clinically phenotyped. Genotyping was undertaken for one ACYP2 (rs1872328), three TPMT (rs12201199, rs1142345 and rs1800460), two COMT (rs4646316 and rs9332377) variants.
Results
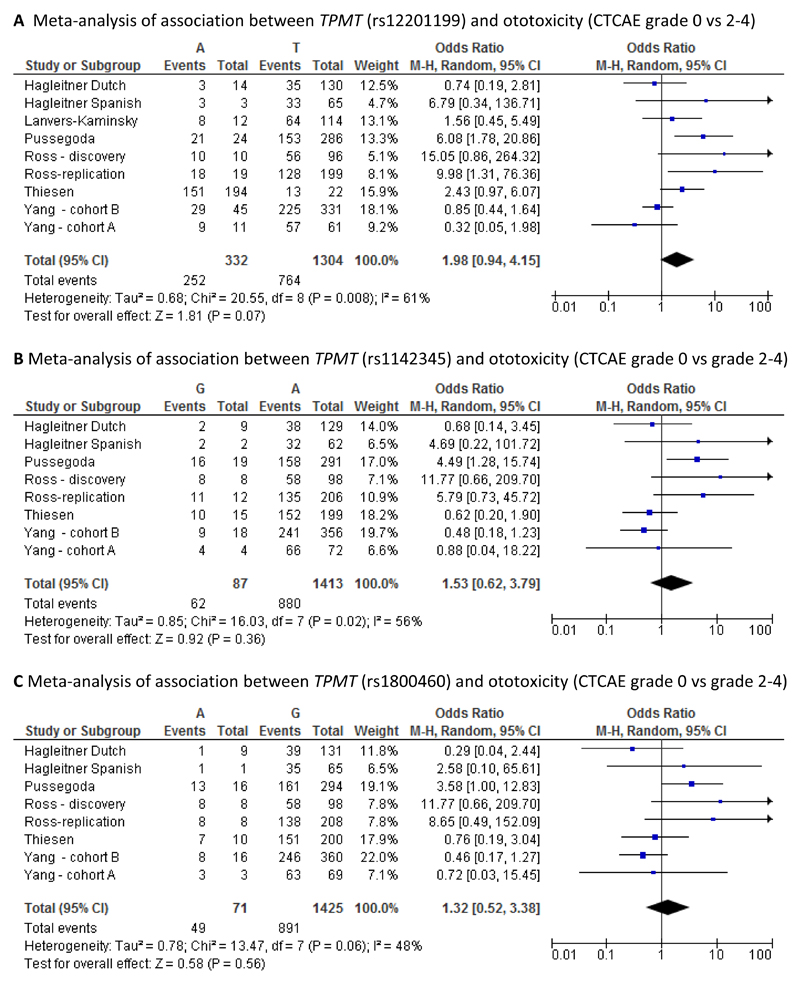
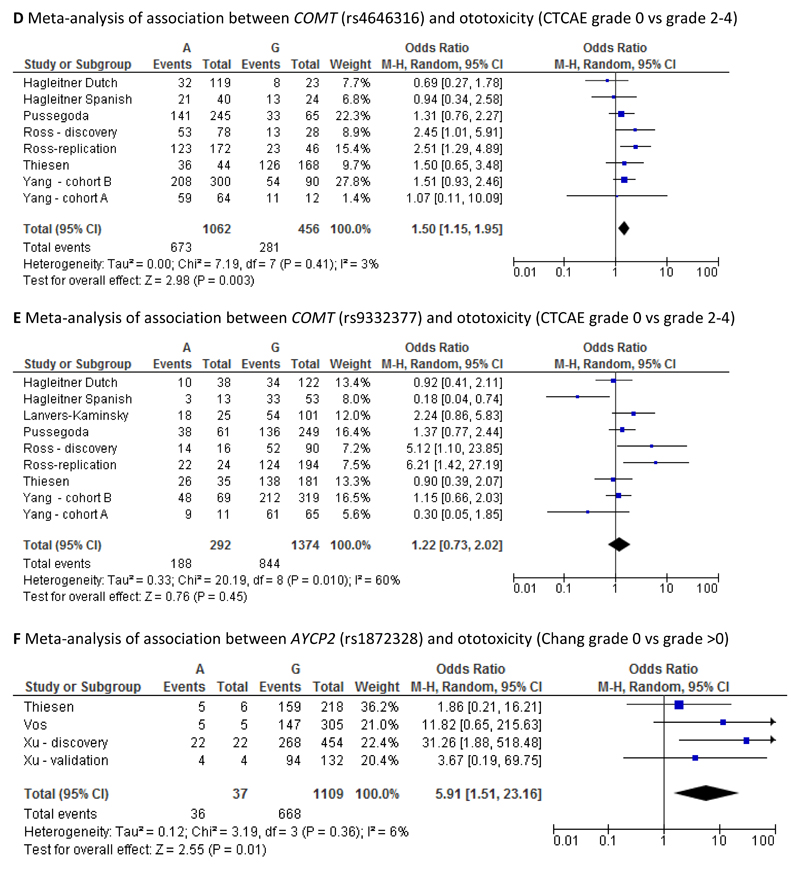
For CTCAE grading, hearing loss was present in 91/120 (75.8%; worst ear) and 79/120 (65.8%; better ear). Using Chang grading, hearing loss was diagnosed in 85/119 (71.4 %; worst ear) vs 75/119 (63.0%; better ear). No TPMT or COMT SNPs were associated with ototoxicity. ACYP2 SNP rs1872328 was associated with ototoxicity (p=0.027; worst ear). Meta-analysis of our data with that reported in previous studies showed the pooled odds ratio (OR) to be statistically significant for both the COMT SNP rs4646316 (odds ratio 1.50, 95% CI: 1.15-1.95) and the ACYP2 SNP rs1872328 (odds ratio 5.91, 95% CI: 1.51-23.16).
Conclusions
In conclusion, we showed an association between the ACYP2 polymorphism and cisplatin-induced ototoxicity, but not with the TPMT and COMT. A meta-analysis was statistically significant for both the COMT rs4646316 and the ACYP2 rs1872328 SNPs. Grading the hearing of children with asymmetric hearing loss requires additional clarification.
Keywords: Cisplatin, Paediatric cancer, Cancer, Pharmacogenetics, Ototoxicity, ACYP2, TPMT, COMT
Introduction
Cisplatin is a chemotherapeutic agent used to treat solid malignancies in childhood, including in the central nervous system (CNS). Although highly effective, its therapeutic index is narrow. Adverse effects include irreversible bilateral sensorineural hearing loss [1].
Hearing loss in childhood affects crucial areas of development. Even minimal sensorineural hearing loss can affect both academic ability and overall level of function (behaviour, energy, stress, social support, and self-esteem) [2]. It may be of particular importance for children with brain tumours where a variety of neuro-toxicities can occur and significantly combine to impair recovery and lead to worsening disability.
There are established risk factors for the development of cisplatin related hearing loss including cumulative dose of cisplatin, younger age (especially <5 years), concomitant (or preceding) radiotherapy to the CNS [3], and exposure to carboplatin in myelo-ablative doses [1,4–6].
The reported incidence rates for cisplatin induced hearing loss in children range between 42-88% [1,5,7–18]. Study populations have often been small (median 67 patients; range 22-238), and heterogeneous with regard to diagnoses, age, dose of cisplatin, treatment schedules, hearing grading and co-administration of concurrent ototoxic agents and cranial radiotherapy. In addition, there is no consensus about how to define hearing loss, leading to variability in the assessment and grading of ototoxicity.
Despite this, there does also appear to be significant inter-individual variability in cisplatin induced hearing loss. Irreversible hearing loss can occur after a single dose of cisplatin in some individuals, whereas other children do not develop hearing loss even after multiple and high doses of cisplatin [9]. Several studies have identified potential predisposing genetic variants influencing cisplatin induced hearing loss. Thiopurine Methyltransferase (TPMT) and Catechol-O-Methyltransferase (COMT) [12] (n=162) risk genotypes were first described in 2009, with TPMT (but not COMT) being replicated in a subsequent cohort [15] (n=155). However neither gene was replicated in three additional studies [16,17] (n=213, n=110, and n=38). A meta-analysis of all of the above studies did associate COMT (but not TPMT) with cisplatin induced ototoxicity [17]. Subsequently, another group have failed to find an association with either TPMT or COMT [18] (n=63). There is therefore considerable uncertainty about whether TPMT and/or COMT polymorphisms represent genuine risk factors for cisplatin-induced ototoxicity.
More recently, another genetic variant (ACYP2 (rs1872328)) was identified using a genome-wide association study in 238 children with brain tumours [14], which was replicated within the original study. An additional study has also recently replicated the association with ACYP2 in patients (n=156) with osteosarcoma [19]. In the present study, our aim was two-fold: first, to test for an association between variants in the COMT, TPMT and ACYP2 genes and cisplatin induced hearing loss, in a carefully phenotyped cohort of UK children; and second, to undertake a systematic review and meta-analysis to determine the association with TPMT, COMT and ACYP2 data.
Materials and Methods
Study design and criteria
Participants were recruited to the Molecular Genetics of Adverse Drug Reactions in Childhood (MAGIC) study. Ethical approval for this study was granted by North West 3 Research Ethics Committee (10/H1002/57). For cisplatin, a target recruitment of 400 has been established for a genome wide association study. This sub-study sample size was determined by recruitment achieved at the time the candidate gene analysis was undertaken.
For inclusion into the study, the patients needed to have started cisplatin on or after 1st January 2001 and had at least one evaluable audiogram following the last dose of cisplatin (post treatment audiogram). To be considered evaluable the audiogram had to fulfil the following criteria: (1) Either pure tone audiogram (PTA) or visual response audiogram (VRA) in db HL and (2) tested at 1, 2 and 4 kHz and either 6 or 8 kHz. The exclusion criteria were as follows:
-
1)
Parent/guardian unwilling to take part (if participant <16 years at recruitment).
-
2)
Participant unwilling to consent (if ≥16 years at recruitment).
-
3)
Competent paediatric participant unwilling to assent (competence assessed on a case by case basis).
-
4)
Hearing impairment prior to cisplatin treatment.
-
5)
No evaluable post treatment audiogram
-
6)
Patient is, in the opinion of the investigator or the clinical team, not suitable to participate in the study.
Consent and data collection
All parents (patient <16 years) or patients (age ≥16 years) provided written informed consent prior to recruitment to this study. Hearing impairment was assessed on the post-treatment audiogram.
DNA collection and extraction
Patient samples for DNA were collected as whole blood ethylenediaminetetraacetic acid (EDTA) samples or salivary samples. DNA collection and extraction for saliva samples has been described previously [20]. EDTA blood samples were stored at -80°C and following defrosting, genomic DNA was extracted using the Chemagen whole-blood DNA extraction kit on the Chemagic Magnetic Separation Module I according to the manufacturer's protocol (PerkinElmer chemagen Technologie GmbH, Baesweiler, Germany; www.chemagen.com).
Genotyping
Genotyping was undertaken for three TPMT variants (rs12201199, rs1142345 and rs1800460) and two COMT variants (rs4646316 and rs9332377) [12], and the SNP rs1872328 for ACYP2 [14], as described previously [20]. The following Taqman Drug metabolism genotyping assays were used: C__31923406_10 (for rs12201199), C__19567_20 (for rs1142345), , C__30634116_20 (for rs1088460), C__29193982_10 (for rs4646316), C__29614343_10 (for rs9332377) and C__11643398_10 (for rs1872328).
Phenotype Definition
We graded all audiograms according to CTCAE for COMT and TPMT [12,21] and assigned Chang grades for ACYP2 [14,22] (Supplementary Table S1). If several post treatment audiograms were available, the highest-grade audiogram was used in the analysis. For patients with asymmetric hearing loss, we graded both ears separately and the results of both grades analysed as outlined below.
Statistical Analysis
Quality control procedures were applied to the genotype data and individuals or SNPs included in the analysis based on the following criteria: sample call rate (samples missing ≥2 SNPs excluded), SNP call rate (only SNPs with call rate >95% included), minor allele frequency (only SNPs with minor allele frequency (MAF) > 0.01 included) and Hardy-Weinberg (HW) test (only SNPs with Hardy-Weinberg test p-value > 0.05 included). An additive mode of inheritance was assumed with SNPs coded 0, 1 or 2 to represent wild-type homozygotes, heterozygotes and mutant-homozygotes respectively.
For the purpose of our primary analyses, our phenotype was treated as ordinal. First, a univariate multinomial logistic regression model was fitted for each non-genetic factor in turn, to identify which non-genetic factors to adjust for in the SNP association analyses. Next, multivariable multinomial logistic regression models were fitted for each SNP in turn. For each SNP, two models were fitted. The first model included covariates to represent all non-genetic factors with p<0.25 univariately. Stepwise variable selection was applied to this baseline model to remove any covariates no longer significant in the multivariable model. The final model following variable selection was called the ‘baseline model’. The second model (‘the genetic model’) was the same as the baseline model but also included a covariate to represent the SNP. The likelihood ratio test was applied to compare the two models and thus assess for statistical significance of the SNP. Since 5 SNPs were tested for association with the outcome of CTCAE grade, a Bonferroni adjustment for 5 tests was applied to these analyses of association. No adjustment was applied to the test for association between the ACYP2 SNP and Chang grade. In cases of asymmetric hearing loss, the worse ear grade was used as final ototoxicity grade [14,16,22]. In order to avoid bias arising from this approach, a sensitivity analysis was carried out using the ototoxicity grade of the better ear as final grade. Further sensitivity analyses of ototoxicity grades were performed by dichotomising outcomes in three different ways: CTCAE grade 0 vs. 1-4; CTCAE grade 0 vs. 2-4; CTCAE grade 0 vs. 3-4. The statistical approach to the sensitivity analyses was the same as for the ordinal outcome but logistic regression models were used instead of multinomial logistic regression models.
Systematic Review and Meta-Analysis
A search was undertaken on 17th March 2016 in EMBASE and MEDLINE databases using the search strategy and exclusion criteria detailed in Supplementary Table S2. The paediatric search terms were based on published examples [23].
Two reviewers (CB and ALJ) independently screened all papers identified for inclusion. Any conflicts were resolved by discussion (CB and ALJ). Methodological quality of the papers was assessed using a published quality assessment checklist [24]. The following data were extracted from each study: year of publication, ethnicity of participants, SNPs investigated, outcomes investigated including their definition, sample size, and study design. If >1 study investigated associations between the same SNP and outcome combination, data required to undertake a meta-analysis was extracted, including: numbers in each genotype group, number of cases and controls per genotype group, odds ratio, standard error of odds ratio, confidence interval for odds ratio.
These data were synthesised using the software package Review Manager 5.3 (The Cochrane Collaboration, 2014). Since different papers had undertaken analyses assuming different modes of inheritance, and due to the variability between studies in how data were reported, it was only possible to conduct a meta-analysis where the allelic odds ratio was calculated (i.e. the odds ratio of developing ototoxicity for the mutant allele vs wild-type allele). The statistical method used to estimate a pooled odds ratio across studies was the Mantel-Haenszel random-effects method [25] and heterogeneity was assessed by referring to the I2 statistic [26,27]. No formal adjustment for study quality was made in the meta-analyses; however results of assessing methodological quality were considered when exploring potential sources of heterogeneity.
Results
One hundred and forty-nine patients were consented, but data was not available for six. In addition, 23 did not have the required audiograms to allow any grading of ototoxicity, and a single patient’s data were insufficient to distinguish between Chang grade 1b and 2a. There were 120 evaluable patients for CTCAE and 119 patients for the Chang criteria (Supplementary data Figure S3).
The distribution of underlying diagnoses was: Medulloblastoma (30.0%; 36/120), Hepatoblastoma (12.5% 15/120), Osteosarcoma (24.2%; 29/120), Neuroblastoma (12.5%; 15/120), other CNS tumours (15.8%; 19/120), and other non-CNS tumours (5.0% 6/120). The self-reported ethnicity for 88.3% (106/120) patients was Caucasian, for 5% Asian (6/120), for 3.3% (4/120) African, for one child Chinese and for three children, the ethnicity was not known.
Clinically, using CTCAE grading, 91/120 patients (75.8%) experienced hearing loss (analysis of worst ear) vs 79/120 patients (65.8%) (better ear). Using Chang grading and considering the worse ear in cases of asymmetrical hearing loss, 85/119 patients (71.4 %) experienced hearing loss vs 75/119 (63.0%) considering the better ear. In our study, the number of patients with asymmetric hearing loss, leading to differential grading in each ear was therefore 12/120 (10.0 %) for CTCAE grading and 10/119 (8.4%) for Chang grading.
Genomic Quality control results
Four patients were removed from the analysis after quality control as results for two or more SNPs were missing. All variants had minor allele frequency >5% and all passed the HW test (p values > 0.05). Table 1 shows the demographic details of the 116 children included in the genetic analysis.
Table 1. Patient characteristics and results of univariate analysis using CTCAE & Chang grading as ordinal outcomes.
| CTCAE | P value** | Chang | P value** | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 (n =26) |
1 (n=8) |
2 (n=41) |
3 (n=35) |
4 (n=6) |
0 (n=31) |
1a (n=17) |
1b (n=15) |
2a (n=5) |
2b (n=10) |
3 (n=32) |
4 (n=5) |
|||
| Age (years) | ||||||||||||||
| Median | 7.73 | 12.78 | 8.80 | 4.94 | 3.95 | 0.010 | 8.49 | 9.66 | 8.80 | 4.41 | 6.43 | 5.24 | 4.04 | 0.040 |
| Range | 0.59-17.67 | 0.83-18.60 | 0.80, 18.18 | 0.62, 17.15 | 1.17, 10.05 | 0.69, 18.03 | 1.98, 18.18 | 0.80, 18.60 | 1.22, 14.02 | 1.20, 17.15 | 0.62, 15.98 | 1.17, 10.05 | ||
| Ethnicity | ||||||||||||||
| Caucasian | 23(22.8%) | 7 (6.9%) | 33(32.7%) | 33(32.7%) | 5 (5.0%) | 0.45 | 27(27.0%) | 13(13.0%) | 12(12.0%) | 4 (4.0%) | 9 (9.0%) | 31(31.0%) | 4 (3.0%) | 0.62 |
| Other | 3 (25.0%) | 0 (0.0%) | 6 (50.0%) | 2 (16.7%) | 1 (8.3%) | 3 (25%) | 3 (25%) | 2 (16.7%) | 1 (8.3%) | 1 (8.3%) | 1 (8.3 %) | 1 (8.3%) | ||
| Gender | ||||||||||||||
| Male | 17 (23.0%) | 6 (8.1%) | 32(43.2%) | 15(20.3%) | 4 (5.4%) | 0.030 | 22(30.1%) | 10(13.7%) | 13(17.8%) | 4 (5.5%) | 7 (9.6%) | 14(19.2%) | 3 (4.1%) | 0.089 |
| Female | 9 (21.4%) | 2 (4.8%) | 9 (21.4%) | 20(47.6%) | 2 (4.8%) | 9 (21.4%) | 7 (16.7%) | 2 (4.8%) | 1 (2.4%) | 3 (7.1%) | 18(42.9%) | 2 (4.8%) | ||
| Cumulative cisplatin dose in mg/m2 | ||||||||||||||
| Median | 344 | 317.0 | 480 | 320 | 260 | 0.023 | 350 | 480 | 480 | 400 | 440 | 280 | 280 | 0.012 |
| Range | 60-600 | 240-560 | 208-560 | 180-560 | 100-800 | 60-600 | 208-560 | 240-560 | 280-480 | 216-516 | 180-560 | 100-800 | ||
| Cranial Irradiation | ||||||||||||||
| YES | 3 (7.5%) | 2 (5.0%) | 16(40.0%) | 17(42.5%) | 2 (5.0%) | 0.028 | 4 (10.0%) | 5 (12.5%) | 6 (15.0%) | 3 (7.5%) | 4(10.0%) | 16(40.0%) | 2 (5.0%) | 0.048 |
| NO | 23 (30.3%) | 6 (7.9%) | 25(32.9%) | 18(23.7%) | 24(5.3%) | 27(35.5%) | 12(15.8%) | 9 (11.8%) | 2 (2.6%) | 6 (7.9%) | 16(21.0%) | 3 (4.0%) | ||
| Vincristine | ||||||||||||||
| YES | 9 (14.3%) | 2(3.17%) | 22(34.9%) | 25(39.7%) | 5(7.94%) | 0.0091 | 10(15.9%) | 8 (12.7%) | 7 (11.1%) | 3 (4.8%) | 5 (7.9%) | 25(39.7%) | 4 (6.4%) | 0.012 |
| NO | 17 (32.1%) | 6(11.3%) | 19(35.8%) | 10(18.9%) | 1(1.89%) | 21(39.6%) | 9 (17.0%) | 8 (15.1%) | 2 (3.8%) | 5 (9.4%) | 7(13.2%) | 1 (1.9%) | ||
| Carboplatin* | ||||||||||||||
| YES | 2 (9.52%) | 0 (0%) | 8 (38.1%) | 10(47.6%) | 1(4.76%) | 0.097 | 2 (9.52%) | 2 (9.52%) | 3 (14.3%) | 0 (0%) | 1(4.76%) | 11(52.4%) | 1(4.76%) | 0.076 |
| NO | 24 (25.3%) | 8(8.42%) | 33(34.7%) | 25(26.3%) | 5(5.26%) | 29(30.5%) | 15(15.8%) | 12(12.6%) | 5 (5.26%) | 9(9.47%) | 21(22.1%) | 4(4.21%) | ||
Data are presented as number (%) of patients, or median with range (minimum, maximum), unless otherwise indicated. Analysis is of the worse ear if asymmetric hearing loss present. Cumulative cisplatin dose is measured in mg/m2.
Carboplatin YES refers to patients who cisplatin and carboplatin as part of the same treatment protocol. NO refers to patients who were not exposed to carboplatin whilst they were also treated with cisplatin. Patients in the latter group may have been changed from cisplatin to carboplatin therapy during their treatment.
p-value from multinomial logistic regression model.
Results of univariate analysis
Results of univariate analyses of association with each non-genetic factor are also shown in Table 1. Of the 98 children in this study who did not receive combined cisplatin and carboplatin therapy, 27 received carboplatin after cisplatin therapy, likely as alternative therapy due to cisplatin induced nephro- or ototoxicity. All children in this group also had grade CTCAE 1-4 hearing loss (worse ear), whereas 1/27 did not have hearing loss using Chang criteria (worse ear) and two children did not have hearing loss on the better ear (Chang and CTCAE criteria).
Analysis of COMT and TPMT variants
The summary statistics detailing frequency of hearing loss for each genetic variant investigated are shown in Table 2. Clinical factors included in the multivariable analysis (p<0.25) were: patient age at diagnosis, gender, cranial irradiation, cumulative dose of cisplatin, exposure to vincristine and carboplatin (Table 1). On applying variable selection to the multivariable model including all these factors, vincristine was removed due to correlation with cranial irradiation (correlation = 0.52). The Bonferroni corrected likelihood ratio test (LRT) p-values for the 5 SNPs are shown in Table 3. None of the TPMT or COMT SNPs were associated with cisplatin induced hearing loss (Table 3). In sensitivity analyses, there were still no significant associations with cisplatin induced hearing loss for any of the variants using dichotomised outcomes (Supplementary tables S4+S5), better ear grade for ordinal outcomes (Supplementary table S6) or when restricting the analysis to individuals of Caucasian ancestry (Supplementary tables S4, S5, S6).
Table 2. Summary statistics for genetic data CTCAE grading for COMT, TPMT and ACYP2 CTCAE criteria using worse ear in cases of asymmetric hearing loss.
|
COMT |
Grade 0 (n=26) |
Grade 1 (n=8) |
Grade 2 (n=41) |
Grade 3 (n=35) |
Grade 4 (n=6) |
|||
|---|---|---|---|---|---|---|---|---|
| rs9332377 | CC | 18 (22.5%) | 4 (5%) | 28 (35%) | 27 (33.8%) | 3 (3.8%) | ||
| CT | 7 (21.1%) | 3 (9.4%) | 12 (37.5%) | 7 (21.9%) | 3 (9.4%) | |||
| TT | 1 (25%) | 1 (25%) | 1 (25%) | 1 (25%) | 0 (0%) | |||
| rs4646316 | CC | 18 (26.1%) | 4 (5.8%) | 24 (34.8%) | 19 (27.5%) | 4 (5.8%) | ||
| CT | 6 (14.3%) | 4 (9.5%) | 14 (33.3%) | 16 (38.1%) | 2 (4.8%) | |||
| TT | 1 (33.3%) | 0 (0%) | 2 (66.7%) | 0 (0%) | 0 (0%) | |||
| TPMT | ||||||||
| rs12201199 | AA | 18 (19.1%) | 7 (7.4%) | 33 (35.1%) | 30 (31.9%) | 6 (6.4%) | ||
| AT | 7 (33.3%) | 1 (4.8%) | 8 (38.1%) | 5 (23.8%) | 0 (0%) | |||
| TT | 1(100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| rs1142345 | TT | 22 (22%) | 7 (7%) | 34 (34%) | 31 (31%) | 6 (6%) | ||
| TC | 3 (21.4%) | 1 (7.1%) | 6 (42.9%) | 4 (28.6%) | 0 (0%) | |||
| CC | 1(100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| rs1800460 | CC | 24 (22.6%) | 7 (6.6%) | 37 (34.9%) | 32 (30.2%) | 6 (5.7%) | ||
| CT | 1 (11.1%) | 1 (11.1%) | 4 (44.4%) | 3 (33.3%) | 0 (0%) | |||
| TT | 1(100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| ACYP2 | ||||||||
|
Grade 0 (n=31) |
Grade 1a (n=17) |
Grade 1b (n=15) |
Grade 2a (n=5) |
Grade2b (n=10) |
Grade 3 (n=32) |
Grade 4 (n=5) |
||
| rs1872328 | GG | 29 (27.4%) | 16 (15.1%) | 10 (9.4%) | 5 (4.7%) | 10 (9.4%) | 31 (29.2%) | 5 (4.7%) |
| GA | 1 (16.7%) | 1 (16.7%) | 4 (66.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| AA | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
Table 3. Multivariable regression analysis of the association between COMT and TPMT polymorphisms and cisplatin-induced ototoxicity using CTCAE grading as an ordinal outcome.
| Estimate (SE) | Overall P-value | Bonferroni corrected P- value | |
|---|---|---|---|
| COMT rs9332377 (CC) | |||
| Grade 1 vs Grade 0: | 1.03 (0.72) | ||
| Grade 2 vs Grade 0: | 0.19 (0.53) | 0.30 | 1.00 |
| Grade 3 vs Grade 0: | -0.54 (0.60) | ||
| Grade 4 vs Grade 0: | 0.47 (0.76) | ||
| COMT rs4646316 (CC) | |||
| Grade 1 vs Grade 0: | 0.52 (0.77) | ||
| Grade 2 vs Grade 0: | -0.10 (0.56) | 0.93 | 1.00 |
| Grade 3 vs Grade 0: | 0.079 (0.59) | ||
| Grade 4 vs Grade 0: | -0.24 (0.94) | ||
| TPMT rs12201199 (AA) | |||
| Grade 1 vs Grade 0: | -1.07 (1.12) | ||
| Grade 2 vs Grade 0: | -0.92 (0.65) | 0.068 | 0.34 |
| Grade 3 vs Grade 0: | -1.66 (0.78) | ||
| Grade 4 vs Grade 0: | -17.6 (2940) | ||
| TPMT rs1142345 (TT) | |||
| Grade 1 vs Grade 0: | -0.47 (1.10) | ||
| Grade 2 vs Grade 0: | -0.43 (0.70) | 0.55 | 1.00 |
| Grade 3 vs Grade 0: | -0.72 (0.83) | ||
| Grade 4 vs Grade 0: | -16.9 (3450) | ||
| TPMT rs1800460 (CC) | |||
| Grade 1 vs Grade 0: | -0.041 (1.10) | ||
| Grade 2 vs Grade 0: | -0.13 (0.78) | 0.83 | 1.00 |
| Grade 3 vs Grade 0: | -0.23 (0.96) | ||
| Grade 4 vs Grade 0: | -16.4 (3990) | ||
Analysis undertaken using worse ear grade (in cases of asymmetric hearing loss)
Analysis of ACYP2 variant
The summary statistics detailing frequency of hearing loss for each genetic variant investigated are shown in table 2. Clinical factors included in the multivariable analysis (p<0.25) were: patient age at diagnosis, gender, cranial irradiation, cumulative dose of cisplatin, exposure to vincristine and carboplatin (Table 1). ACYP2 SNP rs1872328 was associated with ototoxicity (p=0.027), where ototoxicity grade was modelled as an ordinal variable with reference to the worse ear grade in cases of asymmetric hearing loss (Table 4). However, in sensitivity analyses using the better ear grade and ordinal as well as dichotomised outcomes, there was no significant association between the investigated ACYP2 variant and cisplatin induced hearing loss (Supplementary data tables S7, S8, S9). When restricting the analysis to individuals of Caucasian origin, the association was no longer significant (Table 5).
Table 4. Multivariable regression analysis of the association with the ACYP2 genetic variant using worse ear grade (Chang classification) and ordinal outcomes.
| ACYP2 rs1872328 (GG) | Estimate (SE) | Overall P-value |
|---|---|---|
| Grade 1a vs Grade 0: | 0.70 (1.55) | |
| Grade 1b vs Grade 0: | 2.60 (1.32) | 0.027 |
| Grade 2a vs Grade 0: | -15.5 (6790) | |
| Grade 2b vs Grade 0: | -16.8 (7650) | |
| Grade 3 vs Grade 0: | -17.6 (4950) | |
| Grade 4 vs Grade 0: | -16.4 (7360) | |
Table 5. Multivariable regression analysis of the association with the ACYP2 genetic variant in Caucasians using worse ear grade (Chang classification) and ordinal outcomes.
| ACYP2 rs1872328 (GG) | Estimate (SE) | Overall P-value |
|---|---|---|
| Grade 1a vs Grade 0: | -18.1 (10086) | |
| Grade 1b vs Grade 0: | 1.50 (1.59) | 0.29 |
| Grade 2a vs Grade 0: | -17.3 (9704) | |
| Grade 2b vs Grade 0: | -17.8 (10123) | |
| Grade 3 vs Grade 0: | -18.3 (9122) | |
| Grade 4 vs Grade 0: | -16.9 (9971) | |
Systematic Review and Meta-Analysis
The search strategy identified 256 possibly relevant papers, but after screening for inclusion/exclusion criteria 7 studies were included [12,14–19]. The Quorum flow chart detailing the studies identified and included is shown in Supplementary Figure S10, with key study information summarised in Table 6. Results of the methodological quality assessment of included studies are in Supplementary Table S11, with discussion of these results in Supplementary Appendix S12.
Table 6. Key information about the papers included in the systematic review.
| Number of patients | Study design | Ethnicity | SNP(s) investigated | Outcomes and Definitions | |
|---|---|---|---|---|---|
| Hagleitner et al 2014 | |||||
| Dutch cohort | 110 | Retrospective | Dutch | TPMT rs12201199, rs1800460 and rs1142345; COMT rs4646316 and rs9332377 | Hearing loss, CTCAE version 3.0 (Grade 0 vs 2-4) and SIOP Boston ototoxicity scale (Grade 0 vs 2-4). Unclear whether worse or better ear. |
| Spanish cohort | 38 | Retrospective | European | ||
| Lanvers-Kaminsky et al 2014 | |||||
| 63 | Retrospective | unclear | TPMT rs12201199 and COMT rs9332377 | Hearing loss, Muenster classification (Grade 0 vs 2-4). | |
| Pussegoda et al 2013 | |||||
| 155 | Case-control | 80% Caucasian 20% other | TPMT rs12201199, rs1800460 and rs1142345; COMT rs4646316 and rs9332377 | Hearing loss, CTCAE version 3 criteria (Grade 0 vs 2-4). Unclear whether worse or better ear. | |
| Ross et al 2009 | |||||
| Discovery cohort | 53 | Case-control | 85% European 15% Non-European | Genotyped for 1,949 SNPs using customized genotyping assay designed to capture the genetic variation of 220 key drug metabolism genes | Ototoxicity, CTCAE version 3 criteria (Grade 2-4 vs 0). Unclear whether worse or better ear. |
| Replication cohort | 109 | Case-control | |||
| Yang et al 2013 | |||||
| Cohort A | 213 | Retrospective | 79% White 21% non-White | TPMT rs1800462, rs12201199, rs1800460 and rs1142345; COMT rs4818, rs4646316 and rs9332377 | Hearing loss, worse ear, CTCAE version 3 criteria (Grade 0 vs >0, Grade 0 vs 2-4 and analysed as ordinal variable) and Chang criteria, (Grade 0 vs Grade>0; Grade <2a vs ≥2a and analysed as ordinal variable) |
| Cohort B | 14 | Retrospective | 61% White 39% non-White | ||
| Xu et al 2015 | |||||
| Discovery cohort | 238 | Retrospective | 66% European American, 34% Other. | GWAS 2,602,667 SNPs on Illumina HumanOmni2.5+HumanExome BeadChip. | Time to hearing loss (worse ear), classified as Chang score>0 and analysed as ordinal variable. |
| Validation cohort | 68 | Retrospective | Unclear | ACYP2 rs1872328; rs7604464. | Time to hearing loss (worse ear), classified as Chang score>0 |
| Vos et al 2016 | |||||
| 156 | Retrospective | 99% European | ACYP2 SNP rs1872328 | Hearing loss, Chang score, Grade 0 vs Grade>0 | |
It was possible to undertake six different meta-analyses, one each for SNPs rs12201199, rs1800460 and rs1142345 in the TMPT gene, one each for SNPs rs4646316 and rs9332377 in the COMT gene and one for SNP rs1872328 in the ACYP2 gene. For the first five meta-analyses, cases were defined as those with CTCAE grade 2-4 whilst controls were defined as those with CTCAE grade 0. For the sixth meta-analysis, cases were defined as those with Chang grade >0 whilst controls were defined as those with Chang grade 0. Although the study by Lanvers-Kaminsky, investigating the rs12201199 and rs9332377 SNPs, used a different method for defining ototoxicity (Muenster classification), it was included in the meta-analyses. However, sensitivity meta-analyses excluding the study by Lanvers-Kaminsky were also conducted for these two SNPs. Forest plots illustrating the results of the meta-analyses are provided in Figures 1 A-F.
Figure 1.
A-F Meta-analyses of associations between genetic variants of TMPT, COMT and ACYP2 and ototoxicity
The pooled odds ratio was statistically significant for the associations with the COMT SNP rs4646316 (odds ratio 1.50, 95% CI: 1.15-1.95) and with the ACYP2 SNP rs1872328 (odds ratio 5.91, 95% CI: 1.51-23.16). In both cases, the extent of heterogeneity between studies was low (3% and 6% respectively). For the rs12201199 SNP in the TPMT gene, the pooled odds ratio was not statistically significant (odds ratio 1.83, 95% CI: 0.89-3.76) but there was relatively high heterogeneity between studies (I2: 59%). The same was true for the other two TPMT SNPs, rs1142345 (odds ratio 1.59, 95% CI: 0.67-3.77, I2: 53%) and rs1800460 (odds ratio 1.34, 95% CI: 0.54-3.35, I2: 47%). The pooled odds ratio for COMT SNP rs9332377 was also non-significant (odds ratio 1.15, 95% CI: 0.67-1.95) but again the level of heterogeneity between studies was relatively high (I2:66%). When excluding the study by Lanvers-Kaminsky from the meta-analyses for SNPs rs12201199 and rs9332377 the conclusions remained the same (data not shown), but the level of heterogeneity increased in both cases.
Discussion
Our study highlights known risk factors for cisplatin induced ototoxicity in children, including increasing cumulative dose of cisplatin [1,5,9,11,28], younger age [5,11,16], cranial radiotherapy [4,13,29] and exposure to other ototoxic agents such as vincristine [15]. Our study did not detect ethnic origin as a risk factor for hearing loss in patients receiving cisplatin therapy, but this was not our intention as nearly 90% of our study population were Caucasian.
Our data did not replicate previous findings that COMT and TPMT variants are risk factors for cisplatin-induced ototoxicity, consistent with several other studies [16–18]. Heterogeneity between study populations with regards to size, age range, tumour type, cranial radiotherapy, ethnicity, use of potentially otoprotectant therapy (amifostine) and retrospective versus prospective nature of studies are likely to be confounding variables [16,17]. We addressed these through additional sensitivity analyses, and by using multivariable models, but the conclusions remained the same. Sample size is perhaps the most limiting factor of our study. However, combining our data with previous studies, the pooled odds ratio was statistically significant for the associations with the COMT SNP rs464316 (odds ratio 1.50, 95% CI:1.15-1.95, I2:3%), which supports the findings from a previous meta-analysis [17].
In addition, our study replicated the association between the ACYP2 polymorphism and cisplatin induced ototoxicity, first identified through a GWAS [14] using ordinal outcome measures (Table 4). However, the association was lost when restricting our analysis to individuals of Caucasian ancestry. This is likely due to the “loss” of three cases of ototoxicity in GA carriers (data not shown). We were also not able to demonstrate the association with dichotomised outcomes comparing Chang 0 vs > 0 (Supplementary data section), which was the approach used by Xu et al. [14] to define their discovery cohort. This is likely to be due to several differences between our population and that used by Xu et al [14], including a higher rate of cranial irradiation, higher rate of vincristine use (100% vs 52.2%), a more homogeneous patient group in terms of tumour type, and a lower rate of ototoxicity (73% v 61% Chang grade >0, and 45.2% v 37% Chang ≥2a) in the latter. Furthermore, more than 50% of the patients in the Xu et al cohort received the otoprotectant amifostine compared with none of our patients.
Despite these confounders, we followed the analysis of our primary data by undertaking a meta-analysis comparing Chang 0 vs >0 in rs1872328 ‘AG’ or ‘AA’ genotype carriers vs ‘GG’ carriers as reported in Vos et al [19] as presented in Figure 1. Our pooled odds ratio showed a significant association with this ACYP2 SNP (odds ratio 5.91, 95% CI: 1.51-23.16).) with no heterogeneity observed (I2: 0%). Taken collectively, our results suggest that there is a true association between ACYP2 and cisplatin induced ototoxicity, although further studies are still needed to understand how confounding factors affect this association. Furthermore, functional studies should be undertaken to understand the biological plausibility underlying the association, as the explanations to date have been speculative.
Patients in this study who received carboplatin and cisplatin combined, were not at a higher risk of experiencing hearing loss (Table 2). This is not an unexpected result. The majority of children who experience hearing loss after carboplatin therapy have also received cisplatin and/or have received high-dose carboplatin regimens prior to stem cell transplant [6,30]. Children who receive standard dose carboplatin alone experience no, or only mild hearing loss [31]; indeed carboplatin is often used as alternative to cisplatin once significant ototoxicity has been confirmed.
One of the limitations of this study is that the retrospective study design did not make it feasible to collect detailed dosage data of concomitant medications. In common with many of the previous studies, we did not investigate exposure to aminoglycosides, or furosemide in our study. Although ototoxicity is listed in the adverse drug reaction profile in the Summary of Product Characteristics (SPC) for these medicines independent of cisplatin use, studies that did include some or all of them, did not find an association with cisplatin-induced hearing loss [6,12,15,28].
The level of heterogeneity noted in the systematic review between studies examining all of the TPMT SNPs, and COMT rs9332377 was high (I2 47%-66%). There are several potential sources for this, including different follow up periods between studies, differing study designs, different ethnic groups represented in the study populations, different treatment regimens, and different ages of children recruited. In addition, a factor that has not previously been discussed, but which will impact on all studies investigating ototoxicity, is the grading of asymmetric hearing loss, i.e. worse ear vs. better ear. UK clinical practice has been to use Brock ototoxicity grading, using the better ear to assign the overall grade. Chang, CTCAE and the new SIOP Boston scale [8] do not stipulate which ear to use. Both Xu et al. [14] and Yang et al. [16] used the worse ear to grade ototoxicity but it is not clear in other study populations. This variability in how the hearing loss is recorded may partially account for the difficulties in replicating studies.
In conclusion, we have found an association between the ACYP2 polymorphism and cisplatin induced ototoxicity, although we could not replicate the association with TPMT and COMT variants. Cisplatin is used in a wide variety of tumours, and patient heterogeneity is thus likely to be a confounding factor. However, we were able to show in a meta-analysis that there was an association with the COMT rs464316 SNP and the ACYP2 SNP. Further studies in larger populations would still be worthwhile in order to define factors that modulate this association, and importantly, we also need to understand the biological basis of the genetic associations.
Supplementary Material
Acknowledgements
We would like to thank the paediatric oncology units who recruited patients to the MAGIC study for the cisplatin cohort: Alder Hey Children’s Hospital, Leeds General Infirmary, Great North Children’s Hospital, Great Ormond Street Hospital for Children, Nottingham Children’s Hospital, Royal Manchester Children’s Hospital and Leicester Royal Infirmary.
Sources of Funding/Research support: Data collection and recruitment was part funded by the Liverpool Oncology Fund/Image appeal. DH is part funded by the NIHR Alder Hey Clinical Research Facility. MP is an NIHR senior investigator. We thank the NHS Chair of Pharmacogenetics, the Wolfson Centre for Personalised Medicine and the MRC Centre for Drug Safety Science (CDSS), University of Liverpool for funding.
Footnotes
Conflict of Interest: The authors have not competing interests to disclose.
References
- 1.McHaney V, Thibadoux G, Hayes F, Green A. Hearing loss in children receiving cisplatin chemotherapy. The Journal of pediatrics. 1983;102(2):314–317. doi: 10.1016/s0022-3476(83)80551-4. [DOI] [PubMed] [Google Scholar]
- 2.Bess FH, Dodd-Murphy J, Parker RA. Children with minimal sensorineural hearing loss: prevalence, educational performance, and functional status. Ear and hearing. 1998;19(5):339–354. doi: 10.1097/00003446-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Walker D, Pillow J, Waters K, Keir E. Enhanced cis-platinum ototoxicity in children with brain tumours who have received simultaneous or prior cranial irradiation. Medical and pediatric oncology. 1989;17(1):48–52. doi: 10.1002/mpo.2950170110. [DOI] [PubMed] [Google Scholar]
- 4.Schell MJ, McHaney VA, Green AA, Kun LE, Hayes FA, Horowitz M, et al. Hearing loss in children and young adults receiving cisplatin with or without prior cranial irradiation. Journal of Clinical Oncology. 1989;7(6):754–760. doi: 10.1200/JCO.1989.7.6.754. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Womer R, Silber J. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. European Journal of Cancer. 2004;40(16):2445–2451. doi: 10.1016/j.ejca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Punnett A, Bliss B, Dupuis LL, Abdolell M, Doyle J, Sung L. Ototoxicity following pediatric hematopoietic stem cell transplantation: a prospective cohort study. Pediatric blood & cancer. 2004;42(7):598–603. doi: 10.1002/pbc.20036. [DOI] [PubMed] [Google Scholar]
- 7.Knight KRG, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. Journal of Clinical Oncology. 2005;23(34):8588–8596. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- 8.Brock PR, Knight KR, Freyer DR, Campbell KC, Steyger PS, Blakley BW, et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. Journal of Clinical Oncology. 2012;30(19):2408–2417. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brock PR, Bellman SC, Yeomans EC, Pinkerton CR, Pritchard J. Cisplatin ototoxicity in children: a practical grading system. Medical and pediatric oncology. 1991;19(4):295–300. doi: 10.1002/mpo.2950190415. [DOI] [PubMed] [Google Scholar]
- 10.Skinner R, Pearson A, Amineddine H, Mathias D, Craft A. Ototoxicity of cisplatinum in children and adolescents. British journal of cancer. 1990;61(6):927. doi: 10.1038/bjc.1990.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coradini PP, Cigana L, Selistre SG, Rosito LS, Brunetto AL. Ototoxicity from cisplatin therapy in childhood cancer. Journal of pediatric hematology/oncology. 2007;29(6):355–360. doi: 10.1097/MPH.0b013e318059c220. [DOI] [PubMed] [Google Scholar]
- 12.Ross CJ, Katzov-Eckert H, Dubé M-P, Brooks B, Rassekh SR, Barhdadi A, et al. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nature genetics. 2009;41(12):1345–1349. doi: 10.1038/ng.478. [DOI] [PubMed] [Google Scholar]
- 13.Paulino AC, Lobo M, Teh BS, Okcu MF, South M, Butler EB, et al. Ototoxicity after intensity-modulated radiation therapy and cisplatin-based chemotherapy in children with medulloblastoma. International Journal of Radiation Oncology* Biology* Physics. 2010;78(5):1445–1450. doi: 10.1016/j.ijrobp.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 14.Xu H, Robinson GW, Huang J, Lim JY-S, Zhang H, Bass JK, et al. Common variants in ACYP2 influence susceptibility to cisplatin-induced hearing loss. Nature genetics. 2015 doi: 10.1038/ng.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pussegoda K, Ross CJ, Visscher H, Yazdanpanah M, Brooks B, Rassekh SR, et al. Replication of TPMT and ABCC3 Genetic Variants Highly Associated With Cisplatin-Induced Hearing Loss in Children. Clinical Pharmacology & Therapeutics. 2013;94(2):243–251. doi: 10.1038/clpt.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang JJ, Lim JY-S, Huang J, Bass J, Wu J, Wang C, et al. The Role of Inherited TPMT and COMT Genetic Variation in Cisplatin-Induced Ototoxicity in Children With Cancer. Clinical Pharmacology & Therapeutics. 2013;94(2):252–259. doi: 10.1038/clpt.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagleitner MM, Coenen MJ, Patino-Garcia A, de Bont ES, Gonzalez-Neira A, Vos HI, et al. Influence of Genetic Variants in TPMT and COMT Associated with Cisplatin Induced Hearing Loss in Patients with Cancer: Two New Cohorts and a Meta-Analysis Reveal Significant Heterogeneity between Cohorts. PloS one. 2014;9(12):e115869. doi: 10.1371/journal.pone.0115869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanvers-Kaminsky C, Malath I, Deuster D, Ciarimboli G, Boos J, Zehnhoff-Dinnesen A. Evaluation of Pharmacogenetic Markers to Predict the Risk of Cisplatin-Induced Ototoxicity. Clinical Pharmacology & Therapeutics. 2014;96(2):156–157. doi: 10.1038/clpt.2014.67. [DOI] [PubMed] [Google Scholar]
- 19.Vos HI, Guchelaar H-J, Gelderblom H, de Bont ES, Kremer LC, Naber AM, et al. Replication of a genetic variant in ACYP2 associated with cisplatin-induced hearing loss in patients with osteosarcoma. Pharmacogenetics and genomics. 2016;26(5):243–247. doi: 10.1097/FPC.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 20.Hawcutt D, Ghani A, Sutton L, Jorgensen A, Zhang E, Murray M, et al. Pharmacogenetics of warfarin in a paediatric population: time in therapeutic range, initial and stable dosing and adverse effects. The pharmacogenomics journal. 2014 doi: 10.1038/tpj.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Health UDo, Services H. Common terminology criteria for adverse events (CTCAE) version 4.0. National Institutes of Health, National Cancer Institute; 2009. 4 (03) [Google Scholar]
- 22.Chang KW, Chinosornvatana N. Practical grading system for evaluating cisplatin ototoxicity in children. Journal of Clinical Oncology. 2010;28(10):1788–1795. doi: 10.1200/JCO.2009.24.4228. [DOI] [PubMed] [Google Scholar]
- 23.Boluyt N, Tjosvold L, Lefebvre C, Klassen TP, Offringa M. Usefulness of systematic review search strategies in finding child health systematic reviews in MEDLINE. Archives of pediatrics & adolescent medicine. 2008;162(2):111–116. doi: 10.1001/archpediatrics.2007.40. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen AL, Williamson PR. Methodological quality of pharmacogenetic studies: issues of concern. Statistics in medicine. 2008;27(30):6547–6569. doi: 10.1002/sim.3420. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Wiley Online Library; 2008. [Google Scholar]
- 26.Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yancey A, Harris MS, Egbelakin A, Gilbert J, Pisoni DB, Renbarger J. Risk factors for cisplatin-associated ototoxicity in pediatric oncology patients. Pediatric blood & cancer. 2012;59(1):144–148. doi: 10.1002/pbc.24138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hua C, Bass JK, Khan R, Kun LE, Merchant TE. Hearing loss after radiotherapy for pediatric brain tumors: effect of cochlear dose. International Journal of Radiation Oncology* Biology* Physics. 2008;72(3):892–899. doi: 10.1016/j.ijrobp.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 30.Parsons S, Neault M, Lehmann L, Brennan L, Eickhoff C, Kretschmar C, et al. Severe ototoxicity following carboplatin-containing conditioning regimen for autologous marrow transplantation for neuroblastoma. Bone marrow transplantation. 1998;22(7) doi: 10.1038/sj.bmt.1701391. [DOI] [PubMed] [Google Scholar]
- 31.Bertolini P, Lassalle M, Mercier G, Raquin MA, Izzi G, Corradini N, et al. Platinum compound-related ototoxicity in children: long-term follow-up reveals continuous worsening of hearing loss. Journal of pediatric hematology/oncology. 2004;26(10):649–655. doi: 10.1097/01.mph.0000141348.62532.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.