Abstract
Fat mass is inversely associated with vitamin D status, and athletes with the most adipose tissue may have the greatest risk for insufficient (25(OH)D 20–32 ng mL−1) or deficient (25(OH)D < 20 ng ml−1) status. The effects of fat and lean mass on 25 (OH)D change in response to vitamin D supplementation have yet to be elucidated in athletes. In addition, vitamin D has a known role in bone health yet a link between short-term changes in 25(OH)D and bone turnover in indoor athletes have not yet been described. Thirty-two collegiate swimmers and divers (19 male, 13 female; 19 (1) years) participated in a 6-month randomized controlled trial and consumed either 4000 IU d−1 of vitamin D3 (n = 19) or placebo (PLA; n = 13). Anthropometry and blood collection of 25(OH)D, bone-specific alkaline phosphatase (B-ALP) and N-terminal telopeptide (NTx) occurred at three time points. Dual-energy X-ray absorptiometry measured body composition analysis at baseline and endpoint. In the vitamin D group, BMI was negatively correlated with 6-month 25(OH)D change (R =−0.496; P = .03) and a stronger predictor of 25(OH)D change (P = .04) than ultraviolet B exposure and fat mass change.Athletes in the high bone turnover group showed significantly greater losses of 25(OH)D over 6-months compared to athletes in the low bone turnover group (P = .03). These results suggest athletes within the normal BMI category experience a diminished response to 4000 IU d−1 of vitamin D3 supplementation, and periods of high bone turnover may be an additional risk factor for developing compromised vitamin D status in athletes.
Keywords: Bone turnover, fat mass, BMI, swimmer
Introduction
Vitamin D deficiency and insufficiency is prevalent among athletes (Ogan & Pritchett, 2013) and may negatively influence bone health, muscle function, and athletic performance (Cannell, Hollis, Sorenson, Taft, & Anderson, 2009; Larson-Meyer & Willis, 2010; Moran, McClung, Kohen, & Lieberman, 2013). Certain training regimens, environments and sports can place athletes at greater risk for insufficient status (25-hydroxy vitamin D (25(OH)D) 20–32 ng mL−1) where ultraviolet B (UVB) rays are not able to support vitamin D synthesis. Athletes living above or below the 37th parallel and indoor athletes seem to have the greatest risk of developing insufficient status (Farrokhyar et al., 2015). Body composition is an oftentimes overlooked predictor of 25(OH)D status in athletes that has recently been investigated (Fitzgerald, Peterson, Wilson, Rhodes, & Ingraham, 2015; Heller, Thomas, Hollis, & Larson-Meyer, 2015). With excess adiposity, there is a reduction in bioavailability with preferential storage of vitamin D presumably in adipose tissue. Other data also suggest that vitamin D may be stored in muscle tissue (Abboud et al., 2013, 2014; Jakobsen, Maribo, Bysted, Sommer, & Hels, 2007) and may play a role in mediating vitamin D status (Bischoff et al., 2001; Ceglia, 2009). Therefore it appears that the total fat and muscle content affects 25(OH)D change. More research is required to understand the extent to which body composition influences changes in 25(OH)D concentration in athletes during prolonged training and to determine if cheaper, more practical methods can be used to predict athlete risk of compromised vitamin D status.
Changes in vitamin D status that are influenced by soft tissue body changes during training have the potential to affect bone health (Holick, 1996). Vitamin D influences osteoblast differentiation and bone mineralization, and plays a role in osteoblast proliferation (van Driel & van Leeuwen, 2014) and osteoclast inhibition (Takahashi, Udagawa, & Suda, 2014). These processes directly contribute to bone turnover and bone mineral density (BMD). However, the effects of non-weight bearing swimming on bone turnover and BMD suggests higher rates of bone turnover without the BMD benefits seen in weight bearing sports (Gomez-Bruton, Gonzalez-Aguero, Gomez-Cabello, Casajus, & Vicente-Rodriguez, 2013; Karlsson, Nordqvist, & Karlsson, 2008). Further research is necessary to describe the relationship between vitamin D and bone turnover in competitive swimmers with changing body composition over the competitive season to examine implications on bone health.
The primary aim of this study was to examine the effect of body composition and BMI on 25(OH)D concentration in response to vitamin D supplementation over 6-months in collegiate swimmers and divers. A secondary aim was to investigate the relationship between changes in 25(OH)D and bone turnover.
Methods
Sample and study design
Division I NCAA collegiate swimmers and divers, located at latitude 38°N were recruited to participate in a 6-month, double-blinded, randomized trial. All participants were screened and were excluded if they were younger than 18 year of age or had a medical condition that could compromise their safety. More detailed methods are described elsewhere (Lewis, Redzic, & Thomas, 2013). This study was reviewed and approved by the Institutional Review Board and all study participants provided informed written consent prior to study enrolment. Study data were managed using REDCap (Harris et al., 2009).
Athletes were randomized to either 4000 IU d−1 of vitamin D3 or a placebo (PLA). Baseline measures were completed in August, with repeated measures at midpoint (December) and endpoint (March). Measurements included anthropometrics, blood measures, and a vitamin D lifestyle questionnaire (Halliday et al., 2011). The questionnaire was used to assess UVB exposure, food sources of vitamin D, menstrual status, and oral contraceptive (OC) use. Body composition measures using dual-energy X-ray absorptiometry (DXA;GE Lunar-Prodigy; software version 10.0), height (DETECTO 3P, Webb City, MO), and weight (Tanita 800S scale) were completed at baseline and endpoint. Training activities were documented by the team athletic trainer.
Blood collection and analysis
Vitamin D was measured as 25(OH)D by liquid chromatography/tandem mass spectrometry with an assay coefficient of variation of 10%. Bone turnover markers included bone-specific alkaline phosphatase (B-ALP; Quidel, Santa Clara, CA) and N-terminal telopeptide (NTx; Inverness Wampole, Princeton, NJ) measured by ELISA. Most blood draws occurred between late morning and early afternoon hours, but in order to accommodate changing athlete training and course schedules, some samples were collected between 8 am and 4 pm.
Statistical analysis
Data obtained from the study were analysed using Statistical Package for the Social Sciences (SPSS) Software (version 22.0). Descriptive characteristics are presented as means and standard deviations. Pearson correlation coefficients were used to assess the relationships between variables within the total sample and within both supplement and sex groups. Linear regression was used to evaluate predictors of 25(OH) D change in the vitamin D supplement group. Independent t tests were used to compare differences between supplement and sex groups when comparing 3- and 6-month change variable. An Analysis of Covariance (ANCOVA) was used to examine differences in 6-month 25(OH)D change between supplement and sex group. Since dietary intake of vitamin D contributes to 25(OH)D concentrations (Cashman & Mairead, 2015), averaged dietary intake over 6-months was added as a covariate when assessing changes in 25(OH)D. Significance was set at P ≤ .05.
Results
Forty-five athletes completed baseline measurements. Nineteen and 13 athletes were randomized to vitamin D or PLA, respectively. Thirteen athletes withdrew from the study before completing the intervention for personal reasons (n = 3) and scheduling conflicts (n = 10). A total of 32 athletes (19 males/13 females; 24 swimmers/8 divers) completed endpoint measurements. The majority of athletes were Caucasian (n = 29) with a mean age at baseline of 19 (1) years.
For the total sample, there were no significant differences in weight, height, BMI, bone turnover markers, total BMD, total bone mineral content (BMC), lean mass, or fat mass between supplement groups at baseline. Significant differences at baseline between males and females were observed for weight, total BMD, total BMC, lean mass, and fat mass (P < .01) and remained significantly different throughout the study (P < .01).
Vitamin D status by supplement and sex groups
Athletes in the vitamin D group started with a lower 25(OH)D concentration (52 (13) ng mL−1) than those in the PLA group (64 (17) ng mL−1) (P = .03). Changes in supplement group 25(OH)D concentration from baseline to midpoint and baseline to endpoint were significantly different (P < .01). The 3-month change at midpoint was 8 (14) ng mL−1 in the vitamin D group vs. −12 (16) ng mL−1 with PLA. The 6-month change at endpoint was 1 (10) ng mL−1 in the vitamin D group vs. −20 (12) ng mL−1 with PLA.
At baseline and endpoint, sex differences in 25 (OH)D concentrations were not significantly different. At midpoint females had significantly greater 25(OH)D than males (P = .01). When comparing 3-month 25(OH)D change from baseline to midpoint, females increased 25(OH)D concentrations by 10 ng mL−1 while males experienced a 7 ng mL−1 loss (P = .02). Six-month changes in 25(OH) D were significantly different by sex with males decreasing more (−11 (17) ng mL−1) than females (−1 (10) ng mL−1; P = .05).
Contributors to vitamin D status
It is well established that the most significant contributor to vitamin D status is total UVB exposure. In this trial, changes in total UVB exposure both at 3-months and 6-months were not significantly different between supplement groups. However, changes in total reported UVB exposure over the 6-month intervention was higher in males compared to females (P = .03). Dietary contribution to vitamin D status is thought to play a minimal role (Cashman & Mairead, 2015). Changes in 3- and 6-month consumption of vitamin D containing foods was not significantly different between supplement groups or sex groups (P > .05). Upon further analysis using ANCOVA, vitamin D intake was shown to have no effect on 6-month 25(OH)D change (P = .56).
Body composition and BMI by supplement and sex groups
On average, all athletes gained weight and lean mass over the course of the intervention. The change in fat mass was a trend for a negative correlation with 25 (OH)D change in the total sample (R = −0.343; P = .06). When comparing supplement groups, 6-month changes in body composition measures: total mass, lean mass, fat mass, BMD, and BMC were not different. Six-month changes in body composition variables by sex group are listed in Table I.
Table I.
Six-month changes in body composition, M (SD)
| Male (n = 19) | Female (n = 13) | P-value | Total Change (n = 32) | |
|---|---|---|---|---|
| Total mass (kg) | 2.5 (2.1) | 1.1 (2.4) | P = .11 | 1.9 (2.3) |
| Total lean mass (kg) | 1.8 (1.5) | 1.3 (8.8) | P = .18 | 1.6 (1.3) |
| Total fat mass (kg) | 0.6 (1.7) | 0.01 (2.3) | P = .42 | 3.5 (2.0) |
| Total BMD (g cm−2) | 0.02 (0.02) | 0.01 (0.02) | P = .28 | 0.01 (0.02) |
| Total BMC (g) | 52.5 (63.6) | 43.0 (80.9) | P = .73 | 48.7 (70) |
| Percent body fat | −0.3 (1.9) | 0.5 (2.6) | P = .33 | 0.0 (2.2) |
| BMI (kg m−2) | 0.8 (0.7) | 0.4 (0.9) | P = .18 | 0.6 (0.8) |
No significant differences between sexes.
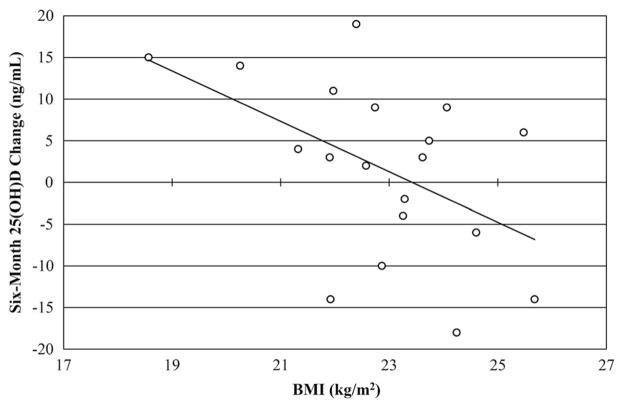
Six-month change in BMI was not significantly different between supplement or sex groups. In the vitamin D group there was a significant negative correlation between BMI and 6-month 25(OH)D change (R = −0.496; P = .03) (Figure 1). BMI was found to be a stronger predictor (P = .04) of vitamin D status than reported sunshine exposure and fat mass change.
Figure 1.
A negative linear relationship exists between 6-month 25 (OH)D change and BMI in the vitamin D group athletes (n = 19, R= −0.496, P < .05).
Bone turnover
In the total sample, 6-month B-ALP changes decreased by an average of 5.5 (6.5) μg L−1 and NTx increased by 3.8 (4.9) nM BCE. Bone turnover markers were not significantly different between supplement groups (P > .05) at any time point or by change over time. Six-month changes in bone turnover markers were not significantly correlated with DXA, total BMD, or BMC. Bone marker concentrations by sex and time point are listed in Table II.
Table II.
Bone turnover markers by time point and 6-month change, M (SD)
| Bone turnover marker | Baseline | Midpoint | Endpoint | 6-Month changes |
|---|---|---|---|---|
| B-ALP (μg L−1) male | 44.3 (15.4)* | 41.4 (15.8)* | 38.4 (14.7)* | −5.9 (7.4) |
| B-ALP (μg L−1) female | 31.1 (10.9)* | 26.6 (7)* | 26.2 (7.5)* | −5.0 (5.3) |
| NTx (nM BCE) male | 15.2 (7.3) | 19.8 (7.4) | 18.7 (5.2) | 3.5 (5.2) |
| NTx (nM BCE) female | 13.9 (5.3) | 19.4 (5.7) | 18.1 (5) | 4.2 (4.6) |
Significant difference between sex P < .05.
We also categorized athletes into low, moderate, or high bone turnover groups based on concentrations of bone resorption (NTx) and bone formation (B-ALP) at each study time point. NTx and B-ALP concentrations were categorized into either the top or bottom 50%. Athletes in the top 50% for both NTx and B-ALP concentrations were categorized into the high bone turnover group. Athletes in the bottom 50% for both NTx and B-ALP concentrations were categorized into the low bone turnover group. Athletes with one serum marker measured in the top 50% and the other serum marker in the bottom 50% were categorized into the moderate bone turnover group. Athletes who increased bone turnover group over 6-months changed from a low to a moderate grouping, moderate to high grouping, or low to high grouping. Athletes who decreased bone turnover group changed from high to moderate grouping, moderate to low grouping, or high to low grouping.
Forty-eight percent of vitamin D group athletes were categorized with low bone turnover at either midpoint or endpoint. Sixty-two percent of PLA athletes experienced high bone turnover at midpoint or endpoint. When categorized as high and low bone turnover at midpoint or endpoint, athletes with high bone turnover experienced a 25(OH)D mean change of −15 (18) ng mL−1 while those with low bone turnover had −1 (10) ng mL−1 change (P = .03). Vitamin D group athletes categorized with high bone turnover maintained 25(OH)D over 6-months (2 (16) ng mL−1) while those in PLA decreased (−24 (12) ng mL−1; P < .01). No athlete increased from a low to high bone turnover grouping or decreased from high to low in a 3-month period. Athletes that increased to a higher bone turnover group (n = 5) had a significantly greater change in 25(OH)D (−26 (12) ng mL−1) over 6-months (P < .01) compared to those that decreased to a lower bone turnover group (n = 6; 0 (8) ng mL−1).
Discussion
The primary aim of this study was to determine the effect of body composition and BMI on 25(OH)D response to vitamin D supplementation over 6-months. The change in 25(OH)D in swimmers and divers was a negative trend with change in fat mass towards statistical significance, highlighting the fat sequestration hypothesis (Wortsman, Matsuoka, Chen, Lu, & Holick, 2000) in which adipose tissue is a significant storage site of vitamin D (Holick, 2004). As fat mass decreases, stored vitamin D mobilizes and may promote an increase or maintenance of 25(OH)D concentration (Heller et al., 2015). BMI was negatively correlated with 25(OH) D change in the vitamin D group athletes (P = .03). This finding seems to support the volumetric dilution hypothesis (Drincic, Armas, Van Diest, & Heaney, 2012) and suggests that body size and fat mass are associated with 25(OH)D, but a linear regression analysis found that BMI was a stronger predictor of vitamin D status than changes in fat mass. There was no significant change in dietary vitamin D intake or total UVB exposure between groups throughout the study lending further support to the influence of BMI on 25(OH)D change. As BMI is a simple, non-invasive measurement, it may serve as a surrogate to assessing risk for vitamin D insufficiency over the competitive season in athletes without the costs of body composition analysis. The athletes in this study were all under 26 kg m−2, suggesting this method may be particularly applicable to athletes with a normal BMI range of 18.5–24.9 kg m−2.
The volumetric dilution model proposes that decreasing 25(OH)D concentrations in obesity is due to body size rather than fat mass alone and this association is hyperbolic rather than linear (Drincic et al., 2012). In this case, weight is a direct measure of body size and lean mass is an important contributor. Although we did not directly test the hyperbolic model (Drincic et al., 2012), lean mass, weight, and the changes in these variables were not significantly correlated with 25(OH)D in this study. More research examining the effects of body composition and body size on 25(OH)D change is necessary to delineate the role both muscle and adiposity may play in 25(OH)D storage and bioavailability.
In this study, no athlete was categorized as obese, yet an inverse correlation between BMI and 25(OH)D change in response to 4000 IU d−1 of vitamin D3 supplementation was observed. These data provide evidence that a diminished dose response extends into the normal BMI category despite receiving the upper tolerable limit of the Institute of Medicine recommended daily intake of vitamin D. Additionally, the study occurred above the 37th parallel further supporting the results as this prevented athletes from being able to synthesize vitamin D from the sun throughout the year. BMI values within the normal range may be a risk factor clinicians consider when recommending a vitamin D3 supplement dose to prevent insufficiency and maintain 25(OH)D status as an athlete with a BMI of 22 kg m−2 may respond more favourably than athletes who are in the high end of a normal to overweight BMI range (i.e. 25–27 kg m−2). A supplemental regimen consistent with the Endocrine Society Clinical Practice Guidelines (Holick et al., 2011) may be necessary to maintain sufficient 25(OH)D concentrations in not only athletes categorized with obese BMI but also overweight and normal categories.
The significant sex difference in 25(OH)D concentration at 3- and 6-months suggests that females were able to better maintain 25(OH)D status over the 6-month period. Dietary intake of vitamin D containing food and sunshine exposure did not contribute to 25 (OH)D differences observed between sexes. One explanation for this finding could be supported by OC use. Female athletes reported normal menstrual status at each time point with approximately 70% of females in our sample reported OC use, and OCs containing oestrogen have been shown to increase circulating concentrations of 25(OH)D (Moller et al., 2013).
The secondary aim of this study was to describe the relationship between 25(OH)D and bone turnover in swimmers and divers. Bone turnover was not correlated with total or changes in BMD or BMC. However, after participants were categorized by bone turnover marker concentrations into groups of high and low bone turnover, we observed that 48% of the vitamin D group athletes experienced low bone turnover over the course of the study, and a majority (62%) of PLA athletes experienced high bone turnover at mid- or end study. A significant difference in 6-month 25(OH)D change was noted between the high and low bone turnover groups suggesting the high bone turnover athletes are more at risk for decreasing 25(OH)D. Furthermore, the vitamin D supplemented athletes maintained more stable 25(OH)D concentrations throughout the study and experienced less bone turnover over 6-months compared to PLA. These observational data suggest that vitamin D supplementation may influence bone turnover by mediating or reducing the expected increase in exercise-induced bone turnover. To our knowledge, these are the first data demonstrating this difference in 25(OH)D changes in indoor athletes with sufficient 25(OH)D concentrations (>32 ng mL−1). Combined with previous literature documenting athletes with higher concentrations of bone turnover compared to sedentary individuals (Banfi, Lombardi, Colombini, & Lippi, 2010; Maimoun & Sultan, 2011), these data support an additional factor by which athletes are at risk for developing insufficient or deficient vitamin D status. However, the relationship between vitamin D supplementation and bone turnover needs further study to elucidate the role of supplementation in athlete bone turnover and if changes mediated by vitamin D are independent of the 25(OH)D bio-marker. Further analysis is required determine the role this may play in bone health as previous studies have shown no association between vitamin D and bone markers when adjusting for age and weight (Allison, Farooq, Hamilton, Close, & Wilson, 2015). The future use of high resolution peripheral quantitative computed tomography (HR-pQCT) may also be helpful to further elucidate the role bone turnover and vitamin D play in promoting beneficial bone changes.
This study provides valuable information regarding vitamin D, body composition, and bone turnover, but we acknowledge limitations. The athletes in this study participated in the same sport with similar body sizes potentially limiting the application of the results to all athletes. A more diverse sample that includes a wider range of body sizes from various sports would allow greater application in future studies. All athletes did not undergo the exact same training regimen as natural variations in training associated with swimmer type (sprint vs. endurance) and diving occurred equally, due to randomization, between supplement groups (vitamin D vs. placebo). Blood measures were not taken with consistent timing across time points due to athlete’s schedules, potentially impacting concentrations due to diurnal variation. Moreover, there was not a measure of bone microarchitecture to provide an additional estimate of bone strength, stability, and resistance to fracture. The inclusion of bioavailable vitamin D in future studies will provide an additional, and perhaps more accurate, method to examine the impact of vitamin D on bone health (Powe et al., 2011). Finally, athletes that had a 25(OH)D > 32 ng mL−1 were categorized with sufficient 25(OH)D status at baseline, but insufficient individuals may provide a better method of examining the effects of vitamin D repletion on bone markers and structure.
Conclusion
This study documents an inverse correlation between BMI and 25(OH)D change in athletes supplemented with 4000 IU d−1 of vitamin D3. Additionally, BMI serves as a significant predictor of 6-month 25(OH) D change in these athletes. Athletes within the normal BMI range may require vitamin D3 supplementation beyond the upper limit to maintain sufficient vitamin D status. Although BMI is not appropriate as a body composition surrogate in athletes BMI may serve as a simple, non-invasive alternative for predicting athletes at a greater risk for insufficient vitamin D status. This study documented significant loss in 25(OH)D in high bone turnover athletes compared to those with low bone turnover. Athletes with high bone turnover may be at greater risk for decreasing 25(OH)D than those with low bone turnover. As training is associated with higher bone turnover, athletes may have another risk factor for losing 25(OH)D concentration, supplementation should be considered as a method to maintain status while considering the body fat or size dose response. Future studies are needed to further delineate the body size dose response relationship to vitamin D3, the role muscle and adipose tissue play in vitamin D storage and circulation, and to further clarify the relationship between bone turnover and 25(OH)D.
Acknowledgments
All authors approved the final version of the paper. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors have no conflicting interests to declare. A sincere thanks to all the athletes who participated and to Nature Made® Pharmavite LLC (Northridge, CA) for providing vitamin D and placebo gel capsules.
Funding
This work was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences [UL1TR000117; T32DK007778-15].
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abboud M, Gordon-Thomson C, Hoy AJ, Balaban S, Rybchyn MS, Cole L, … Mason RS. Uptake of 25-hydroxyvitamin D by muscle and fat cells. Journal of Steroid Biochemistry and Molecular Biology. 2014;144(Pt A):232–236. doi: 10.1016/j.jsbmb.2013.10.020. [DOI] [PubMed] [Google Scholar]
- Abboud M, Puglisi DA, Davies BN, Rybchyn M, Whitehead NP, Brock KE, … Mason RS. Evidence for a specific uptake and retention mechanism for 25-hydroxyvitamin D (25OHD) in skeletal muscle cells. Endocrinology. 2013;154(9):3022–3030. doi: 10.1210/en.2012-2245. [DOI] [PubMed] [Google Scholar]
- Allison R, Farooq A, Hamilton B, Close G, Wilson M. No association between vitamin D deficiency and markers of bone health in athletes. Medicine and Science in Sports and Exercise. 2015;47(4):782–788. doi: 10.1249/MSS.0000000000000457. [DOI] [PubMed] [Google Scholar]
- Banfi G, Lombardi G, Colombini A, Lippi G. Bone metabolism markers in sports medicine. Sports Medicine. 2010;40(8):697–714. doi: 10.2165/11533090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Bischoff HA, Borchers M, Gudat F, Duermueller U, Theiler R, Stahelin HB, Dick W. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochemical Journal. 2001;33(1):19–24. doi: 10.1023/a:1017535728844. [DOI] [PubMed] [Google Scholar]
- Cannell JJ, Hollis BW, Sorenson MB, Taft TN, Anderson JJ. Athletic performance and vitamin D. Medicine and Science in Sports and Exercise. 2009;41(5):1102–1110. doi: 10.1249/MSS.0b013e3181930c2b. [DOI] [PubMed] [Google Scholar]
- Cashman KD, Mairead K. Tackling inadequate vitamin D intakes within the population: Fortification of dairy products with vitamin D may not be enough. Endocrine. 2015 doi: 10.1007/s12020-015-0711-x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Ceglia L. Vitamin D and its role in skeletal muscle. Current Opinion in Clinical Nutrition and Metabolic Care. 2009;12(6):628–633. doi: 10.1097/MCO.0b013e328331c707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Driel M, van Leeuwen JP. Vitamin D endocrine system and osteoblasts. Bonekey Reports. 2014;3:493. doi: 10.1038/bonekey.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring) 2012;20(7):1444–1448. doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]
- Farrokhyar F, Tabasinejad R, Dao D, Peterson D, Ayeni OR, Hadioonzadeh R, Bhandari M. Prevalence of vitamin D inadequacy in athletes: A systematic-review and meta-analysis. Sports Medicine. 2015;45(3):365–378. doi: 10.1007/s40279-014-0267-6. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J, Peterson B, Wilson P, Rhodes G, Ingraham S. Vitamin D status is associated with adiposity in male ice hockey players. Medicine and Science in Sports and Exercise. 2015;47(3):655–661. doi: 10.1249/MSS.0000000000000433. [DOI] [PubMed] [Google Scholar]
- Gomez-Bruton A, Gonzalez-Aguero A, Gomez-Cabello A, Casajus JA, Vicente-Rodriguez G. Is bone tissue really affected by swimming? A systematic review. PLoS One. 2013;8(8):e70119. doi: 10.1371/journal.pone.0070119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday TM, Peterson NJ, Thomas JJ, Kleppinger K, Hollis BW, Larson-Meyer DE. Vitamin D status relative to diet, lifestyle, injury, and illness in college athletes. Medicine and Science in Sports and Exercise. 2011;43(2):335–343. doi: 10.1249/MSS.0b013e3181eb9d4d. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller JE, Thomas JJ, Hollis BW, Larson-Meyer DE. Relation between vitamin D status and body composition in collegiate athletes. International Journal of Sport Nutrition and Exercise Metabolism. 2015;25(2):128–135. doi: 10.1123/ijsnem.2013-0250. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D and bone health. Journal of Nutrition. 1996;126(Suppl 4):1159S–1164S. doi: 10.1093/jn/126.suppl_4.1159S. [DOI] [PubMed] [Google Scholar]
- Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. American Journal of Clinical Nutrition. 2004;80(Suppl 6):1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, … Endocrine S. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- Jakobsen J, Maribo H, Bysted A, Sommer HM, Hels O. 25-hydroxyvitamin D3 affects vitamin D status similar to vitamin D3 in pigs – but the meat produced has a lower content of vitamin D. British Journal of Nutrition. 2007;98(5):908–913. doi: 10.1017/S0007114507756933. [DOI] [PubMed] [Google Scholar]
- Karlsson MK, Nordqvist A, Karlsson C. Physical activity increases bone mass during growth. Food and Nutrition Research. 2008;52 doi: 10.3402/fnr.v52i0.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Meyer DE, Willis KS. Vitamin D and athletes. Current Sports Medicine Reports. 2010;9(4):220–226. doi: 10.1249/JSR.0b013e3181e7dd45. [DOI] [PubMed] [Google Scholar]
- Lewis RM, Redzic M, Thomas DT. The effects of season-long vitamin D supplementation on collegiate swimmers and divers. International Journal of Sport Nutrition and Exercise Metabolism. 2013;23(5):431–440. doi: 10.1123/ijsnem.23.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimoun L, Sultan C. Effects of physical activity on bone remodeling. Metabolism. 2011;60(3):373–388. doi: 10.1016/j.metabol.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Moller UK, Streym S, Jensen LT, Mosekilde L, Schoenmakers I, Nigdikar S, Rejnmark L. Increased plasma concentrations of vitamin D metabolites and vitamin D binding protein in women using hormonal contraceptives: A cross-sectional study. Nutrients. 2013;5(9):3470–3480. doi: 10.3390/nu5093470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran DS, McClung JP, Kohen T, Lieberman HR. Vitamin D and physical performance. Sports Medicine. 2013;43(7):601–611. doi: 10.1007/s40279-013-0036-y. [DOI] [PubMed] [Google Scholar]
- Ogan D, Pritchett K. Vitamin D and the athlete: Risks, recommendations, and benefits. Nutrients. 2013;5(6):1856–1868. doi: 10.3390/nu5061856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, … Bhan I. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. Journal of Bone and Mineral Research. 2011;26(7):1609–1616. doi: 10.1002/jbmr.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Udagawa N, Suda T. Vitamin D endocrine system and osteoclasts. Bonekey Reports. 2014;3:495. doi: 10.1038/bonekey.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. American Journal of Clinical Nutrition. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]