Abstract
We provide an integrative view of mechanisms that enable regeneration of the digestive tube in various animal models, including vertebrates, tunicates, echinoderms, insects, and flatworms. Two main strategies of regeneration of the endodermal luminal (mucosal) epithelium have evolved in metazoans. One of them involves proliferation of resident epithelial cells, while the other relies on recruitment of cells from extramucosal sources. In any of these two scenarios, either pluri-/multipotent stem cells or specialized differentiated cells can be used as the starting material. Posttraumatic visceral regeneration shares some common mechanisms with normal embryonic development as well as with organ homeostatic maintenance, but there are signaling pathways and/or cellular pools that are specific to the regenerative phenomena. Comparative analysis of the literature suggests that mammals share with spontaneously regenerating animals many of the regeneration-related adaptations and are able to efficiently repair components of their digestive tube at the level of individual tissues, but fail to do so at the whole-organ scale. We review what might cause this failure in the context of the current state of knowledge about various regenerative models.
If the latent capacity for regeneration persists […], then the restoration of its overt expression should be possible if the mechanisms of its inhibition could be discovered and eventually rendered ineffectual
1. INTRODUCTION
Comparative analysis of the mechanisms involved in gut regeneration is of considerable interest for two main reasons. First, the digestive tube as a whole is arguably one of the few organs whose homology throughout all major clades of the Eumetazoa raises few doubts. Therefore, it is interesting to see how mechanisms of tissue homeostasis and posttraumatic regeneration of the same structure have been evolving in animal phylogeny. Evolution of regenerative mechanisms is still one of the most obscure issues in developmental biology. There is no clear understanding in the field of why the regenerative potential varies so greatly from species to species. The more widely accepted explanation, advocated by Richard Goss (1992), assumes that regeneration is an intrinsic property of multicellular organisms, which has already been in place in all major ancestral groups and that could be lost in evolution, but not gained again. The second hypothesis is that regeneration has evolved independently de novo in some taxa as a result of secondary gains (Bely & Nyberg, 2010) and thus can depend on some locally evolved taxon-specific features, which have no direct counterparts in other organisms (Garza-Garcia, Driscoll, & Brockes, 2010). Studying regenerative mechanisms of the same conserved organ, such as the digestive tube, across animal phylogeny may therefore turn out very informative for understanding fundamental issues in evolutionary developmental biology.
The second reason attracting attention to comparative studies of gut regeneration is that digestive diseases are a serious burden on human society worldwide. For example, in the US alone, digestive diseases are diagnosed in about 35% of the population and account for ~10% of all deaths (Everhart & Ruhl, 2009). A better understanding of how structural and functional properties of the digestive tube are restored in spontaneously regenerating animals will eventually yield the necessary information to direct the design of new clinical approaches to cure bowel diseases in human patients.
The digestive tube shows stereotypical organization across phylogeny. As the name implies, it is generally a tubular structure with the central lumen communicating with the external environment through terminal openings. This inner cavity is lined with a single layer of endodermally derived luminal (mucosal) epithelium, whose functions and unique position within the body place it under a range of adverse conditions. First, since the gut lumen is technically an extension of the external environment, the luminal epithelium must act as an effective protective barrier to prevent pathogen invasion. Second, the very function of food digestion makes the gut lumen an extremely hostile environment for living cells. This implies that the luminal epithelium must be protected from chemical, enzymatic, and mechanical damage. On the other hand, this tissue cannot develop into a multilayered impermeable barrier, like the epidermis, because it has to perform its vitally important function of absorption of nutrients, ions, and water from the gut lumen. This multitude of functional requirements poses paradoxical constraints: the luminal epithelium must be thin and capable of efficient absorption, and, at the same time, it must be able to withstand a variety of harsh and potentially damaging conditions. Thus, across animal phylogeny, we see that the gut mucosal epithelium is a rapidly regenerating tissue with an extensive capacity to constantly replace its old and damaged cells.
Here, we provide an overview of what is known about normal physiological cell renewal and posttraumatic regeneration in the digestive system of various animal models with an attempt to develop an integrative view of the different strategies that have evolved to repair visceral damage and discuss what can be learned from comparative studies to improve the limited regenerative capacity in the human bowel.
2. MECHANISMS OF DIGESTIVE TRACT REGENERATION IN DIFFERENT ANIMAL MODELS
2.1. Mammals
2.1.1 Physiological self-renewal of the luminal epithelium
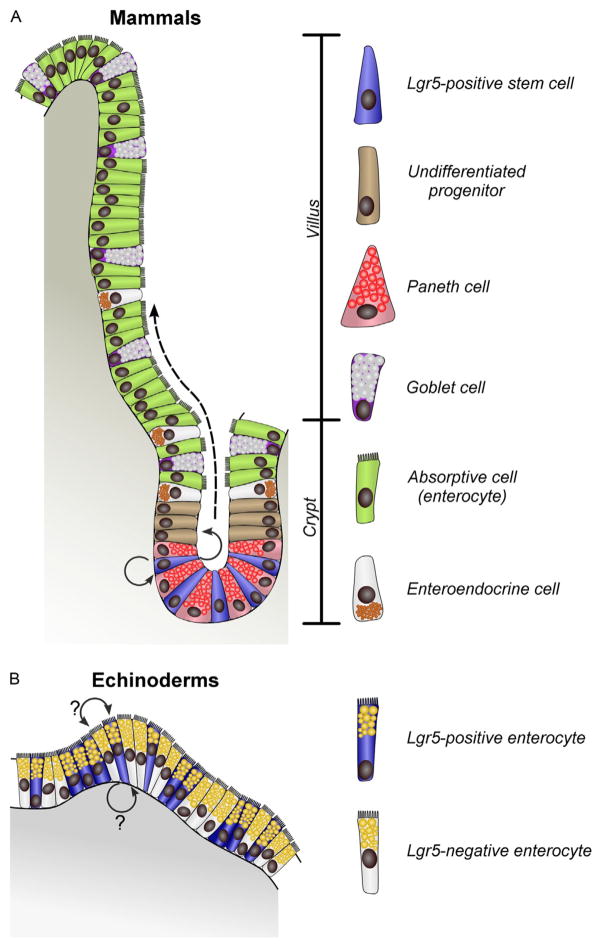
Physiological self-renewal of the intestinal epithelium has been extensively studied in the adult mammalian small bowel. In this organ, the mucosal epithelium is organized into a series of numerous projections, or villi, and depressions, called crypts of Lieberkühn (Fig. 7.1A). Not only does this organization increase the absorptive surface of the epithelium, but it also demarcates the differentiated (villi) and proliferative domains (crypts) within the tissue. Intestinal stem cells are localized to a well-defined niche at the base of the crypts, four to six cells per niche, interspersed between Paneth cells (Barker et al., 2007; Crosnier, Stamataki, & Lewis, 2006). Each crypt gives rise to ~300 new cells every day. The stem cells produce extensively dividing transit amplifying cells, whose progeny are displaced toward the crypt–villus junction where they differentiate into specialized cells (Figs. 7.1A and 7.2), such as enterocytes, goblet cells, and enteroendocrine cells, which then continue to gradually migrate toward the apex of the villus. After 2–7 days of performing their respective functions, the worn-out differentiated cells are shed into the bowel lumen (Barker et al., 2007; Crosnier et al., 2006). It has been recently shown that the intestinal crypt also harbors a second population of progenitor cells, which are relatively quiescent (slow-cycling) and, in normal physiological settings, give rise to only Paneth and enteroendocrine cells. In the regenerating intestinal mucosa, however, these cells acquire a much broader developmental potential, enter an extensively proliferative state, and give rise to all types of specialized epithelial cells (Buczacki et al., 2013). The separation of the differentiated and proliferative domains is a conserved feature of the intestinal luminal epithelium in vertebrates. In some species, such as zebrafish, crypts never develop, but stem cells are still maintained in the restricted zones of intervillus pockets (Crosnier et al., 2006).
Figure 7.1.
Diagram of organization of the mucosal epithelium in the mammalian small intestine (A) and in the digestive tube of a sea cucumber (B) highlighting the difference in cell renewal mechanisms. In mammals, Lgr5-positive intestinal stem cells are localized at the bottom of the crypt and both self-renew and produce rapidly dividing transit-amplifying progenitors (arrows), which gradually move apically (dashed arrow) and give rise to the specialized cells. Paneth cells are the only differentiated cell type that remains in the crypt and constitute a component of the stem cell niche. In the digestive epithelium of echinoderms, unlike in mammals, Lgr5-positive cells are not spatially segregated into distinct zones, but are instead interspersed among Lgr5-negative enterocytes. Lineage relationships still remain to be determined in this model (arrows with question marks).
Figure 7.2.
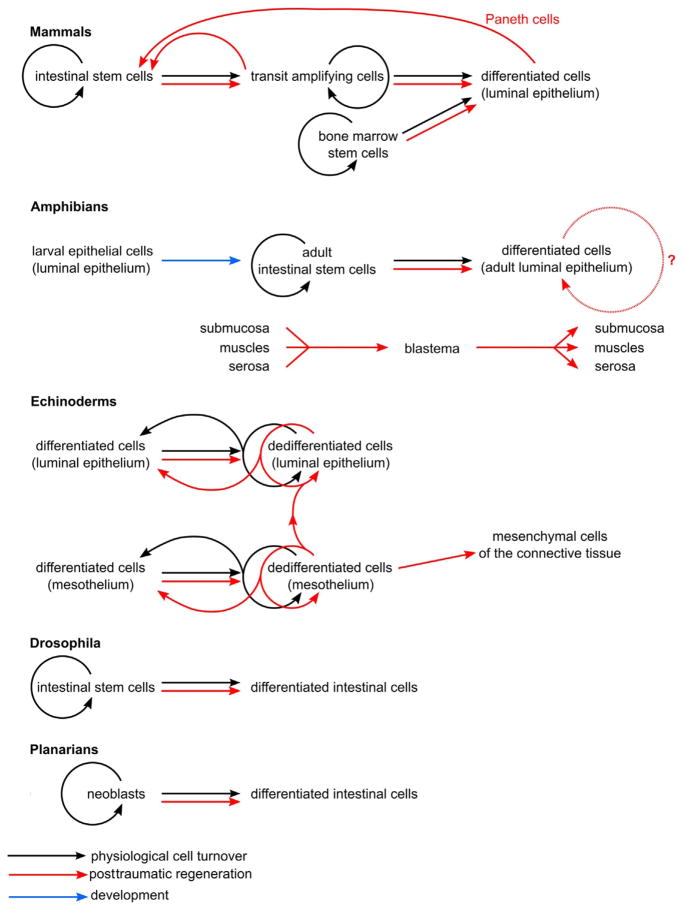
Diagram summarizing cell lineage relationships in normal (physiological) cell turnover and posttraumatic regeneration in the digestive tube of different bilaterian animal models. Straight arrows show cell lineage relationships in physiological cell turnover (black), posttraumatic regeneration (red), and development (blue). Circular arrows indicate self-renewal.
2.1.2 Molecular control of the stem cell niche
The maintenance of the stem cell niche and segregation of the proliferative and differentiated domains (crypt and villi, respectively) is controlled by an interplay between Wnt, Notch, Hedgehog, and Bmp signaling. The canonical Wnt pathway is essential for maintenance of the proliferative activity in the crypt and, thus, for physiological cell turnover in the luminal epithelium of the small intestine (Crosnier et al., 2006). The crucial role of Wnt allowed the use of perturbations of its signaling to identify specific genetic markers of intestinal stem cells among the Wnt target genes. One such gene, Lgr5 (leucine-rich repeat-containing G-protein coupled receptor 5), has proved to be of particular value, as its expression allows easy identification of the stem cells with high specificity (Barker et al., 2007). Other markers expressed in the intestinal stem cells include Bmi1, but its expression domain is broader as it also includes committed progenitors of specialized cells (Doupé & Jones, 2013; van Es et al., 2012).
2.1.3 Posttraumatic regeneration of the intestinal epithelium
Posttraumatic regenerative response of the mammalian digestive tube epithelium has been probed in a variety of injury paradigms and experimental settings, including surgical resection, gamma irradiation, transgenic ablation of stem cells, and exposure of the mucosal epithelium to burns and toxic chemicals. Although generally considered poor regenerators, mammals turned out to be capable of robust regeneration of their luminal epithelium after various types of injury. A number of studies have shown that there is far more developmental plasticity in the mucosal epithelium than was previously thought. For example, chemically burnt intestinal mucosa is readily healed, provided that subepithelial structures (the basal lamina and lamina propia) remain intact. The damaged necrotic cells are sloughed off into the gut lumen, while the subsequent reepithelialization proceeds very quickly. The surviving nongoblet epithelial cells surrounding the wound develop lamellipodia and migrate across the denuded basal lamina of the injured area. After just 2 h, 80–90% of the damaged mucosal surface is repaired (Feil et al., 1989). This early response is then followed by increased proliferation to compensate for the cell loss (Sturm & Dignass, 2008). Most importantly, the resident Lgr5+ intestinal stem cells are not the exclusive source of new cells in the regenerating intestinal epithelium (Fig. 7.2). After selective ablation of the stem cells, either by making them susceptible to diphtheria toxin or by radiation-induced damage, at least some of the committed transit-amplifying progenitors of both the absorptive and secretory lineages are capable of reverting back (dedifferentiating) to the Lgr5+ phenotype and thus replenishing the lost stem cell pool (Doupé & Jones, 2013; Tian et al., 2011; van Es et al., 2012). Notably, even differentiated cells are able to revert to the stem cell phenotype. For example, Paneth cells, the postmitotic differentiated cell component of the intestinal stem cell niche, have been recently shown to dedifferentiate, stop expressing their cell-type-specific markers, and assume stem cell identity after Lgr5+ stem cells had been ablated by sublethal doses of ionizing irradiation (Roth et al., 2012).
2.1.4 Contribution of nonintestinal cells to intestinal luminal epithelium repair
Some reports even suggest that differentiated cells of the digestive organs can originate from outside sources. Cell fate tracing in mice, as well as studies on human patients with transplanted sex-mismatched bone marrow or peripheral blood hematopoietic stem cells, have shown that the progeny of the adult stem cells from these grafted tissues can invade various organs and tissues of the recipient and differentiate into the respective specialized cell types, including mature epithelial cells of the gastrointestinal tract and liver hepatocytes (Fig. 7.2). Importantly, the number of bone marrow-derived epithelial cells increased after intestinal epithelium damage, suggesting that the bone marrow is recruited as an additional source of cells to spur tissue regeneration (Gupta, Dixit, Sales, Winslet, & Seifalian, 2006; Körbling et al., 2002; Krause et al., 2001; Okamoto, Matsumoto, & Watanabe, 2006; Okamoto et al., 2002). It is interesting to note here, however, that although these extraintestinal stem cells give rise to differentiated cells of the intestinal epithelium, they have never been reported to produce intestinal stem cells (Okamoto et al., 2006). This system of histogenetic relationships with bone marrow-derived stem cells localized outside of the digestive organs, whose cells they replenish, is reminiscent of the situation in planarians, whose neoblasts reside in the parenchyma and constitute the only proliferating universal pluripotent stem cell pool in the body (Reddien, 2013) (see below).
2.1.5 Molecular signaling in intestinal regeneration
As in normal cell turnover, Wnt signaling is one of the most essential signaling mechanisms required for regeneration of the intestinal epithelium. One of the key downstream genes activated by Wnt is c-Myc, whose elevated expression is required for regeneration to occur (Ashton et al., 2010; Bernal et al., 2005). It has been noted that not only the mere presence but also a significant increase in expression of the protein product of this gene is required for the biological effect. Forcing c-Myc expression to remain within the normal physiological range results in poor generation (Ashton et al., 2010). At the mechanistic level, it has been noted that Wnt signaling can affect cell fate plasticity, as exposure to Wnt3a can revert committed trans-amplifying secretory cell progenitors to the multipotent Lgr5+ stem cell phenotype (van Es et al., 2012).
2.1.6 Regeneration of nonmucosal intestinal tissues
The mucosal epithelium is not the only intestinal component that is able to regenerate. To different degrees, all tissue layers of the digestive tube show regenerative capacities. This is the reason why functional reconnection takes place after the intestine is transected and then sutured back in the course of surgery. However, regeneration of nonmucosal tissue layers of the mammalian gut has received much less attention than that of the luminal epithelium. Occasional studies have shown that various components of the submucosa are able to regenerate following injury. For example, smooth musculature grows and reestablishes the anatomical integrity across the lesion site after surgical transection followed by end-to-end reanastomosis (Galligan, Furness, & Costa, 1989). However, the origin of the new intestinal muscle tissue has not been determined in mammals. It has been shown that the mesothelium, the outermost tissue layer of the bowel wall, is also capable of developing a regenerative response. The mesothelial cells surrounding the injury change their morphology (from flattened to cuboidal), proliferate, and migrate into the wounded area (Nagai et al., 2013). Even the enteric nervous system has been shown to regenerate. Both newly produced and previously existing surviving neurons can grow axons that expand within the injured area and reinnervate their targets (Galligan et al., 1989; Hanani et al., 2003; Laranjeira et al., 2011).
2.1.7 Limits to adult intestinal organogenesis
In spite of their extensive capacity to renew and regenerate the intestinal epithelium, mammals do not regrow their digestive tube at the whole-organ level, as they are not able to spontaneously regenerate full-thickness wounds and entire ablated regions. In human patients, the intestinal tissue can be lost to various disorders, such as intestinal ischemia, cancer, traumatic injury, and inflammatory bowel disease. All these pathologies often require extensive surgery and removal of considerable lengths of the digestive tube, resulting in the short bowel syndrome, a life-threatening condition, in which the remnant of the digestive tube fails to support the needs of the organism (Chen & Beierle, 2004; Levin & Grikscheit, 2012). Among the most common therapeutic solutions to treat patients with the short bowel syndrome is organ transplantation. However, application of this procedure is limited by donor availability, high incidence of graft rejection, toxicity of immune suppression, and relatively low success rates (Howell & Wells, 2011). Although alternative approaches involving tissue engineering are being actively sought, their development is at its infancy as their progress heavily depends on still incomplete fundamental biological knowledge of how to stimulate visceral regeneration in mammals at the whole-organ level (Chen & Beierle, 2004; Gupta et al., 2006; Howell & Wells, 2011; Jwo et al., 2013; Levin & Grikscheit, 2012; Ross et al., 2013).
2.2. Amphibians
2.2.1 Intestinal regeneration following transection
Intestinal regeneration in amphibians has been studied in two models: (a) posttraumatic regeneration after complete surgical transection or removal of the intestine in larval and adult animals (O’Steen, 1959; O’Steen & Walker, 1962) and (b) intestinal remodeling during metamorphosis (Fujimoto, Matsuura, Hu-Wang, Lu, & Shi, 2012; Ishizuya-Oka, 2007). Unfortunately, the studies of posttraumatic regeneration date back to about half a century ago (O’Steen, 1959; O’Steen & Walker, 1962), that is, well before modern imaging and molecular biology techniques had come into existence. Nevertheless, those studies did document some interesting phenomena. It was established that, unlike in mammals, the rostral and caudal cut ends of the injured intestine were able to grow across the injury gap to reconnect spontaneously and restore the anatomical integrity and physiological function of the digestive tube. The luminal epithelium (mucosa) was the first tissue layer to reform and it regenerated from its own cells. The serosa, muscular layer, and submucosa contributed cells to the blastema, which then underwent growth, remodeling, and differentiation to regenerate these tissue layers across the site of the original transection (Fig. 7.2).
2.2.2 Intestinal remodeling during metamorphosis
The second model focuses on the extensive reorganization of the digestive tube, which accompanies the switch from larval to adult diet during amphibian metamorphosis. At the onset of this transformation, most of the larval luminal epithelium is eliminated by extensive programmed cell death. Only some of the specialized epithelial cells are spared. They dedifferentiate, transform into adult stem cells, and then give rise to the new adult mucosa via extensive proliferation (Ishizuya-Oka, 2007; Ishizuya-Oka et al., 2009). There is a clear difference in histogenetic relationships in the intestine of the larval and adult amphibians. Before metamorphosis, the larval luminal epithelium does not contain undifferentiated stem cells and is composed of specialized epithelial cells capable of reentering the cell cycle, whereas the adult intestine shows a clear separation between the proliferative and differentiated domains, very much like in mammals (Ishizuya-Oka et al., 2009). As to the molecular mechanisms underlying such a drastic tissue transformation, it has been shown that c-Myc plays a central role in gut remodeling during thyroid hormone-controlled metamorphosis in Xenopus. Its transcription is activated directly by the thyroid hormone receptor. In its turn, c-Myc regulates differential expression of a number of down-stream genes, including arginine methyltransferase 1, which is thought to play an important role in specification of adult intestinal stem cells (Fujimoto et al., 2012).
2.3. Tunicates
The highest regenerative capacities among the Tunicata, the subphylum of invertebrate chordates, are seen in those species that can reproduce asexually (Fujiwara, Isozaki, Mori, & Kawamura, 2011; Kaneko, Katsuyama, Kawamura, & Fujiwara, 2010; Kawamura, Sugino, Sunanaga, & Fujiwara, 2008). Depending on the species, one of several types of differentiated tissues, in budding or regenerating animals, is capable of undergoing dedifferentiation and giving rise to various organs, including the digestive tube. One of these tissues showing high developmental plasticity is the epicardium, an endodermally derived tubular or sac-like structure that surrounds the viscera (Kawamura et al., 2008). Another multipotent, but differentiated, tissue is the atrial epithelium, which constitutes the inner layer of the body wall in tunicates and has no homolog in vertebrates. The embryological origin of this structure is unclear, as conflicting results point to either the ectoderm or the endoderm as a possible source. In regenerating and asexually propagating animals, the atrial epithelium dedifferentiates to form a gut primordium. Interestingly, this process requires retinoic acid, since in the presence of inhibitors of retinoic acid synthesis, gut formation is suppressed(Kaneko et al., 2010). Gut formation from the atrial epithelium also depends on Myc expression, as injections of specific dsRNA caused defects in morphogenesis of the viscera (Fujiwara et al., 2011; Kawamura et al., 2008). The link between retinoic acid and Myc is not clearly established. Although investigators have proposed that while retinoic acid might contribute to the activation and maintenance of Myc transcription, it is not the factor that initiates the activation itself (Kaneko et al., 2010).
2.4. Echinoderms
The capacity of echinoderms to regenerate their visceral organs is among the highest among metazoan taxa. Most of what is known about organization and regeneration of the digestive tube in echinoderms has been learned from experiments on sea cucumbers (holothurians) (Figs. 7.3 and 7.4) (reviewed in García-Arrarás & Greenberg, 2001; Mashanov & García-Arrarás, 2011), although occasional studies were also done on members of other classes, such as brittle stars (ophiuroids) (Frolova & Dolmatov, 2010) and sea lilies (crinoids) (Mozzi, Dolmatov, Bonasoro, & Candia Carnevali, 2006).
Figure 7.3.
One of the best studied models of visceral regeneration: the sea cucumber Holothuria glaberrima Selenka. (A) Normal adult individual. (B) and (B′) An individual photographed early and late in the process of evisceration, respectively.
Figure 7.4.
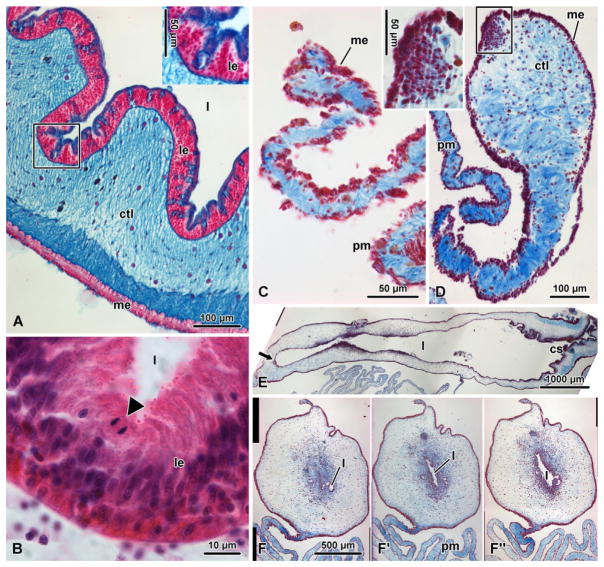
Histological organization of the digestive tube of the sea cucumber H. glaberrima in noneviscerated animals (A) and (B) and at different time points of regeneration (C–F″). (A) Cross-section of the intestine in a noneviscerated animal stained with alcian blue (pH 2.5) and nuclear fast red. The inset shows a higher magnification view of the luminal epithelium. (B) Dividing cell (anaphase, arrowhead) in the luminal epithelium of the normal intestine stained with hematoxylin and eosin. (C–F″) Regenerating digestive tube stained with Heindenhein’s azan. (C and D) Cross-sections through the early gut rudiment on days 3 and 7 postevisceration, respectively. The inset in D shows a detailed view of the mesothelial cells ingressing into the underlying connective tissue. (E) Longitudinal section through the growing gut rudiment on day 14 postevisceration. The growing tip (arrow) is on the left, whereas the cloacal stump is on the right. (F–F″) Day 14 postevisceration; successive cross-sections of a posterior gut rudiment taken through the growing tip (F) and at distances of 40 μm (F′) and 110 μm (F″). cs, cloacal stump; ctl, connective tissue layer of the gut wall; l, gut lumen; le, luminal (mucosal) epithelium; me, mesothelium; pm, proximal mesentery.
2.4.1 Anatomy of the adult sea cucumber intestine
The digestive tube of sea cucumbers consists of a number of specialized histologically distinct regions, including the pharynx, esophagus, long looped intestine, and the cloaca. It is attached to the inner surface of the body wall by the mesentery, a thin film made up of a central layer of connective tissue sandwiched between two layers of the contractile coelomic epithelium. The wall of the digestive tube is composed of an outer mesothelium, which is continuous with the coelomic epithelium of the mesentery, a middle connective tissue layer, and an inner luminal (digestive) epithelium (Fig. 7.4A). The luminal epithelium is made up mostly of vesicular enterocytes. Other less abundant cell types include granular enterocytes, goblet cells (mucocytes), and neurosecretory cells (Feral & Massin, 1982; Mashanov, Frolova, & Dolmatov, 2004). Like in many other metazoan animals, the luminal epithelium of echinoderms undergoes constant cell turnover under normal physiological conditions (Fig. 7.4B); however, unlike in vertebrates and insects, the proliferating cells are not localized to restricted specific locations, but rather seem to be scattered randomly throughout the luminal epithelium and mesothelium (Mashanov & García-Arrarás, 2011).
2.4.2 Stem cell status in the sea cucumber luminal epithelium
Morphological studies did not reveal any undifferentiated cells in the tissues of the gut wall, but instead identified mitotic cells of the luminal epithelium as vesicular enterocytes (Mashanov et al., 2004). Dividing enterocytes retained their secretory vacuoles and were thus capable of undergoing cell division while remaining in, at least, a partially differentiated state. It is currently unknown whether all enterocytes can divide or whether this capacity is restricted to only a subset of cells, thus separating the morphologically homogeneous population of enterocytes into proliferation-competent and permanently postmitotic cells, respectively. It is also unclear if the same cells can keep dividing in the long term and, thus, behave as bona fide stem cells or if the proliferative capacity is stochastically turned on and off in individual cells within the epithelium.
In order to clarify these issues, we have probed markers used to study intestinal stem cells in other animal models. One of the most basic techniques to mark putative stem cells in the tissue relies on their proposed ability to retain DNA labeling for extended periods of time after a pulse of a thymidine analog (e.g., BrdU) (Crosnier et al., 2006; Potten, 2004). After injecting holothurians with BrdU, randomly distributed, scattered, strongly immuno-reactive label-retaining cells were observed in both the luminal epithelium and mesothelium of the intestine for at least 2–5 weeks after the last injection, suggesting the presence of cells with stem cell-like behavior (Mashanov & García-Arrarás, 2011). Further experiments are, however, needed to directly establish whether the label-retaining enterocytes actually act as stem cells (i.e., are capable of renewing themselves and producing specialized progeny in further cell cycles) or merely represent terminally postmitotic cells, which originate via only a limited number of cell divisions, and thus remain strongly labeled for an extended duration of time. Such long-lived label-retaining differentiated cells are not uncommon. Mammalian Paneth cells, for example, are able to retain labeling for up to 100 days (Roth et al., 2012).
We also studied expression of pluripotency factors, such as homologs of mammalian Sox2, c-Myc, Lgr5, and BmiI (Mashanov et al., in preparation), which were identified as intestinal stem cell markers in vertebrates. These genes are widely expressed in the intestinal luminal epithelium of Holothuria glaberrima without preferential localization to any particular region. This expression is, however, not ubiquitous, as there are always luminal cells, which show no in situ hybridization signal (Fig. 7.1B). Thus, the morphologically uniform enterocyte population is obviously heterogeneous in terms of expression of stem cell/multipotency markers. However, it remains to be established how these differences affect proliferative potential in individual enterocytes.
2.4.3 Cellular events in intestinal regeneration
Many sea cucumber species are well known for their impressive ability to spontaneously autotomize (eviscerate) their alimentary canal, which is then completely regenerated over the time course of a few weeks (Byrne, 1986; García-Arrarás & Greenberg, 2001; Hyman, 1955; Mashanov & García-Arrarás, 2011; Wilkie, 2001) (Figs. 7.3 and 7.4C–F″). In regenerating sea cucumbers, the first morphogenetic process observed after autotomy is remodeling of the torn free edge of the mesentery. The connective tissue partition in this region swells to form the primordium of the new intestine (compare Fig. 7.4C and D). The mesothelium, which covers this connective tissue swelling, undergoes deep dedifferentiation, that is, the cells lose specialized phenotypic features, enhancing their ability to proliferate and migrate. Nonetheless, it retains its epithelial integrity except at the distal (antimesenterial) side where the cells undergo an epithelial-mesenchymal transition and ingress into the underlying extracellular matrix (Fig. 7.4D inset) (Dolmatov & Mashanov, 2007; García-Arrarás et al., 2011).
Development of the solid connective tissue rudiment is followed by lumen formation, which can be accomplished through one of two mechanisms. The first one involves invasion of the luminal epithelium from the cloacal and/or esophageal stumps (Fig. 7.4E–F″) (García-Arrarás et al., 1998). In this case, only partial dedifferentiation is seen in the luminal epithelium. In spite of extensive cell division, mitotic enterocytes remain connected to their neighbors by intercellular junctions and even retain microvilli and secretory vacuoles (Mashanov, Dolmatov, & Heinzeller, 2005; Odintsova, Dolmatov, & Mashanov, 2005). A second path to lumen formation involves infolding of the mesothelium with subsequent detachment of the invaginated epithelial folds from the coelomic surface of the gut rudiment and transdifferentiation into the luminal epithelium (Mashanov et al., 2005; Mosher, 1956). The latter mechanism exemplifies a relatively rare phenomenon of direct transformation of one type of differentiated tissue into another one across the germ layer boundaries, since in embryogenesis the gut mesothelium and luminal epithelium originate from the mesoderm and endoderm, respectively (Mashanov & Dolmatov, 2004).
2.4.4 Molecular hallmarks of intestinal regeneration
The molecular basis of tissue plasticity in the echinoderm digestive tube has recently started to be elucidated. One of the first hypotheses to be tested was whether extensive dedifferentiation of specialized cells is related to increased expression of transcription factors, such as homologs of the vertebrate Sox2, c-Myc, Klf4, and Oct3/4 genes, known to confer multipotency (Sridharan et al., 2009; Takahashi & Yamanaka, 2006). Of these, only Myc was found to be significantly upregulated in the regenerating tissues of the sea cucumber, and, importantly, this increase in expression correlated in time with commencing dedifferentiation. Surprisingly, SoxB1, the holothurian homolog of the mammalian Sox2, was almost completely absent from the dedifferentiating tissues, whereas the remaining two factors did not show any significant change in their expression values (Mashanov et al., in preparation). These observations suggest that the molecular signaling pathways that induce in vivo dedifferentiation of specialized cells in regenerating echinoderms might not be exactly the same as those employed to induce pluripotency in cultured mammalian cells. Nevertheless, the role of Myc in both processes seems to be conserved. Dedifferentiation in the gut mesothelium has also been shown to correlate with extensive expression of a number of genes known to be involved in both embryogenesis and cancer, including survivin, mortalin, TCTP, and Bmp1/Tll (Mashanov, Zueva, & García-Arrarás, 2012; Mashanov, Zueva, Rojas-Catagena, & García-Arrarás, 2010).
In echinoderms, Wnt signaling also potentially plays a role in intestinal regeneration, although its exact functions are not known yet. Expression of Wnt9 was studied in the noninjured and regenerating sea cucumber H. glaberrima (Mashanov et al., 2012). No expression of this gene was detected in the uninjured digestive tube, suggesting that this ligand is not involved in the normal physiological cell turnover. However, Wnt9 was extensively expressed in the mesothelium of the regenerating intestine. This contrasts with the expression pattern of Wnt9 in the mammalian digestive system, where Wnt9b, one of the two paralogs that constitute the vertebrate Wnt9 subfamily, is specifically restricted to the epithelium of the crypt (Crosnier et al., 2006; Gregorieff et al., 2005). Of interest, induction of Wnt9 expression in dedifferentiating tissues of the sea cucumber coincides with upregulation of Myc. As Myc homologs are known to be activated by Wnt signaling and are necessary to trigger intestinal regeneration in a number of phylogenetically distant species, including mammals and insects (Ashton et al., 2010; Bernal et al., 2005; Cordero, Stefanatos, Scopelliti, Vidal, & Sansom, 2012), overexpression of these genes might represent an evolutionary conserved signaling mechanism in visceral repair.
2.5. Insects
Most of what is known about insect intestinal regeneration has been learned from studies of Drosophila midgut. The midgut luminal epithelium consists of three cell types—columnar enterocytes, endocrine cells, and undifferentiated intestinal stem (regenerative) cells (ISCs). The Drosophila ISCs were discovered less than a decade ago by two groups (Micchelli & Perrimon, 2006; Ohlstein & Spradling, 2006), and were demonstrated to produce the other two (differentiated) intestinal cell types (Fig. 7.2). Similar results have shown an undifferentiated intestinal cell giving rise to different cell phenotypes in the midgut of another insect, the grasshopper Locusta migratoria (Illa-Bochaca & Montuenga, 2006). In Drosophila, mitotic division of the stem cell gives rise to another daughter stem cell and a diploid progenitor enteroblast. Unlike in mammals, the enteroblasts do not divide under normal conditions, but, instead, differentiate directly into one of the two cell types. In 90% of cases, they become absorptive enterocytes. The remaining 10% give rise to small enteroendocrine cells (Cordero et al., 2012). The Drosophila adult intestine shows a robust regenerative response to epithelial injuries. In particular, the ISCs respond by switching to a rapid proliferative state to compensate for the cell loss (Cordero et al., 2012; Shaw et al., 2010). However, an alternative hypothesis that stem cells responding to injury originate from a different pool from those associated with normal homeostasis has also been proposed (Biteau, Hochmuth, & Jasper, 2011; Hochmuth, Biteau, Bohmann, & Jasper, 2011).
The Drosophila model system has provided important information on the genetic basis of intestinal stem cell regulation both in normal homeostasis and after injury (Biteau et al., 2011). It has been shown that at least part of the gene regulatory machinery that controls intestinal development, growth, and regeneration is highly conserved between mammals and Drosophila (Apidianakis & Rahme, 2011; Shaw et al., 2010). For example, in both systems, pathways activated by the insulin receptor and the epidermal growth factor receptor are essential for the proliferation of intestinal stem cells during normal maintenance, while Jun-N-terminal Kinase (JNK), JAK/Stat, and Hippo/Yorkie pathways are required for stress-related responses (Biteau et al., 2011), Moreover, like in mammals, wingless (Wg) signaling acting through Myc is required for damage-induced proliferation in the intestinal epithelium (Cordero et al., 2012). The induction of Wg occurs via the activation of the JNK pathway and takes place parallel to the activation of JAK/Stat signaling, which suggests that activation of multiple pathways is necessary for midgut regeneration in Drosophila. Regulation of cell differentiation has also been well studied in this system. For example, stem cells with Delta-rich vesicles activate Notch and produce daughter cells with downregulated Delta that become enterocytes, while stem cells with weak Delta activity produce progeny that become enteroendocrine cells (Ohlstein & Spradling, 2007). Won-Jae Lee and Masayuki Miura expand on the cellular and molecular basis of Drosophila regeneration in an accompanying review in this volume.
2.6. Planarians
The planarian digestive system is formed by a single-opening tube (pharynx) with extensive blind ramifications (intestine). Unlike in many other organisms, the intestinal luminal epithelium of flatworms consists entirely of developmentally static, postmitotic differentiated cells. Both physiological cell renewal and posttraumatic regeneration of the digestive system is accomplished via the involvement of neoblasts, the only dividing cell population in the animal (Fig. 7.2), which reside in the surrounding mesenchymal parenchyma and give rise to all differentiated cell types in the body (Forsthoefel, Park, & Newmark, 2011; Wagner, Wang, & Reddien, 2011). Although the lineage relationship between neoblasts and terminally differentiated cells is clearly unidirectional (differentiation cannot be reversed), the details of histogenetic hierarchy within this progenitor cell population have not been worked out yet. The emerging data suggest that individual neoblasts may differ in their developmental potential (Reddien, 2013). On the one hand, at least some of the neoblasts are true pluripotent cells (Wagner et al., 2011); on the other hand, the existence of more committed proliferating progenitors has also been demonstrated. The bona fide pluripotent neoblasts can, thus, give rise to lineage-restricted neoblasts, which, in turn, generate differentiated progeny (Reddien, 2013).
Recent findings have shown some of the genes that might be involved in intestinal homeostasis and regeneration (Forsthoefel et al., 2012). Among them are genes that regulate intestinal branching and differentiation of phagocytes (the main cell type in the planarian gut and the one responsible for digestion). Moreover, results from the same group provide evidence for the regulation of neoblast proliferation dynamics suggesting the possibility that intestinal phagocytes play a niche-like role for somatic stem cells.
2.7. Coelenterates
Coelenterates possess a simple gastric cavity, where they digest their food. The gastric cavity communicates with the external environment through a single opening and is lined by an endodermal epithelial layer, or gastrodermis, composed mainly of epitheliomuscular cells, and also gland cells that secrete digestive enzymes and mucous-secreting cells. Most of what is known about regeneration in this animal group has been studied in Hydra (Galliot, 2012) and, to a smaller level, in Hydractinia (Plickert, Frnak, & Müller, 2012).
The central body region of coelenterates, or the gastric column, contains a larger proportion of interstitial undifferentiated (stem) cells in contrast to the head or foot regions made up of terminally differentiated cells (Galliot, Welschof, Schuckert, Hoffmeister, & Schaller, 1995). Stem cells in the gastric column divide continuously and their progeny are displaced toward the apical and foot ends of the body, where they are eventually discarded (Bode, 1996). In Hydra, three independent cell lineages (ectoderm, endoderm, and interstitial cells) arise during development. Each lineage has its own set of “stem cells” that constantly replenishes the cells of the corresponding lineage during the animal’s life and during regeneration (Hobmayer et al., 2012). The endodermal cell lineage consists of the epithelio-muscle cells that line the gastric cavity and, although differentiated, maintain the capability to divide (Plickert et al., 2012). Interstitial cells give rise to other cell types that are eventually incorporated into the endodermal epithelial layer. In addition to mucous and gland cells, they also produce other cell types such as nematocysts and neurons (David, 2012). The interstitial precursors undergo a limited number of divisions and differentiate into postmitotic cells (Bode, 1996). The differentiation of stem cells into nematocytes and neurons has been well studied, in comparison to the differentiation into mucous or gland cells (David, 2012). Nonetheless, it has been proposed that gland cells differentiate into mucous cells as they move from the body column and enter the head region (Bode, 1996; Siebert, Anton-Erxleben, & Bosch, 2008). The same authors also showed that mucous cells in the head region originate through direct differentiation of interstitial stem cells. Thus, in this simple digestive system, we find that many of the cellular processes are reminiscent of what takes place in intestinal regeneration of other metazoans. As in mammals, the interstitial stem cells within the body wall of Hydra show a compartmental separation between dividing and terminally differentiated cells. As in sea cucumbers, the differentiated cells of the endoderm (both epitheliomuscular and glands) retain their proliferative properties. Finally, similar to planarians, some of the cells within the gastrodermal layer (glands and mucous) originate from external stem cells.
Not only are the cellular processes reminiscent of what occurs in animals with a much more complex digestive tube, but similar molecules also appear to control these events. For example, Wnt signaling is associated with cell proliferation and maintenance of stemness during regeneration and budding (Hobmayer et al., 2012). Most interesting is the possible role of Myc family members, which, as in other metazoans, act downstream of Wnt. The expression of one of them (myc1) is specific to the proliferating interstitial stem cell derivatives and is absent in terminally differentiated cells (Hartl et al., 2010). This myc gene has been proposed to control proliferation of the rapidly dividing interstitial cellular lineage (Hobmayer et al., 2012).
3. EMERGING PRINCIPLES OF INTESTINAL REGENERATION ACROSS EUMETAZOANS
3.1. Regeneration strategies of the luminal epithelium
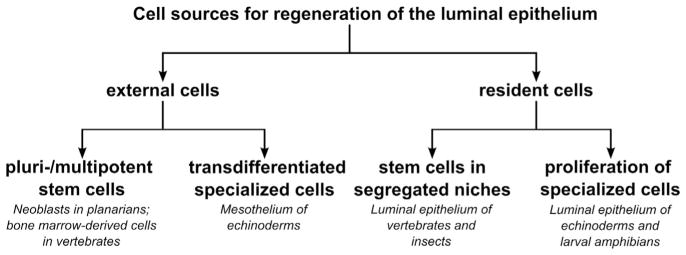
An overview of the literature shows that the luminal epithelium is the digestive system component whose regeneration has been best studied across animal phylogeny, while the connective tissue compartment, intestinal musculature and the outer mesothelium, has attracted much less attention. A comparative analysis suggests that two different strategies enable regeneration of the gut lining in metazoans (Fig. 7.5). The first strategy relies on resident epithelial cells, whose progeny give rise to differentiated cells that remain within the same tissue. The second strategy recruits cells from external sources located outside of the mucosal epithelium. Each strategy involves either stem cells or differentiated cells as the starting material. Unfortunately, the current state of knowledge precludes the drawing of any conclusions as to the evolution and maintenance of regeneration mechanisms.
Figure 7.5.
Diagram summarizing the different strategies employed by metazoan animals to regenerate their intestinal epithelium.
These strategies are widely distributed across animal phylogeny and do not show any obvious correlation with the taxonomic position or with the complexity of the histological organization of the gut wall. For example, generation of the luminal epithelium from tissue-specific intestinal stem cells is seen in both vertebrates and insects. On the other hand, the reverse lineage relationship, when the cell pool within the tissue is replenished through induction of proliferation in differentiated cells, has also been reported for representatives of different taxonomic groups, such as amphibian tadpoles and sea cucumbers. Likewise, regeneration of the luminal epithelium from external sources of stem cells has been documented in phylogenetically distant taxa, such as planarians (neoblasts) and mammals (bone marrow-derived stem cells). Hydra makes use of both strategies: the epitheliomuscular cells of the endoderm propagate through self-renewal, while the gland cells that are incorporated into the gastrodermis originate from interstitial stem cells (David, 2012; Hobmayer et al., 2012). The only mechanism of gut regeneration that seems to be unique to one animal group in view of the current state of knowledge is regeneration of the luminal epithelium through direct transdifferentiation of mesothelial cells in echinoderms (Mashanov et al., 2005).
It is important to note that different mechanisms of intestinal regeneration are not necessarily mutually exclusive and can be employed sequentially or simultaneously by the same organ in the same organism. Thus, in humans, the damaged mucosal epithelium regenerates predominantly through migration of surviving differentiated cells and proliferation of the resident stem cells, but it can also incorporate bone marrow-derived cells (Gupta et al., 2006; Körbling et al., 2002; Krause et al., 2001; Okamoto et al., 2006, 2002). Sea cucumbers provide even more drastic examples of how different regenerative mechanisms, in spite of being based on dissimilar developmental events and cell sources, lead to the same final result—rebuilding of the lost regions of the digestive tube. Upon induction of visceral autotomy, the holothurian Eupentacta fraudatrix eviscerates in such a way that the only remnant of the original digestive tube is the cloacal stump at the posterior end of the body, while no endodermal tissues are present at the anterior end (Mashanov et al., 2005). The new intestinal rudiments that develop at the posterior and anterior ends of the body employ two different mechanisms of lumen formation: outgrowth of the luminal epithelium of the cloacal stump and invagination of the mesothelium, respectively. These observations lead to interesting questions as to which signaling pathways underlie induction of different coexisting regenerative mechanisms. For example, what makes the dedifferentiated mesothelial cells produce only mesothelium in the posterior gut rudiment and both the mesothelium and luminal epithelium in the anterior gut rudiment?
3.2. Posttraumatic regeneration and physiological cell turnover
There are extensive parallels between posttraumatic regeneration and other modes of development, including embryonic/postembryonic development and cell turnover under normal conditions (physiological regeneration) (Carlson, 2007). It has been suggested that regeneration might have originally evolved as an extension of normal developmental programs (Bely & Nyberg, 2010). Here, we discuss relationships between visceral regeneration and other forms of development that take place in the digestive system.
Under normal physiological conditions, as new cells are being continuously born and induced to differentiate and gradually replace the worn out counterparts, there must be a tightly regulated equilibrium between the number of new cells being produced and the number being lost. In contrast, posttraumatic regeneration and wound healing require a burst of proliferative activity and production of a large cohort of new cells over short time spans. This increase in cell proliferation, however, has to be reversible and also cease once the required number of cells has been generated. Furthermore, the new cells must differentiate and organize into a patch of functional tissue continuous with the surrounding (noninjured) regions of the body. Moreover, posttraumatic regeneration, instead of acting locally within a single particular tissue, often requires some reorganization of multiple tissues at the whole-organ level. This is directly related to the question of why it is that regeneration in mammals, including humans, is so limited in spite of the high rate of homeostatic cell turnover in the mucosal epithelium (Radtke & Clevers, 2005).
When one considers the differences in requirements that must be met by physiological cell turnover and posttraumatic regeneration in the interest of the multicellular body as a whole, it is not surprising that the two processes may not be necessarily identical in terms of their cell sources and regulatory mechanisms. For example, studies on mammals and Drosophila showed that regeneration of the luminal epithelium depends on the expression of sets of genes that are not strictly required for physiological cell turnover (Ashton et al., 2010; Cordero et al., 2012). Thus, high level of physiological regeneration does not necessarily imply extensive capacities for posttraumatic regeneration. The reverse is also true: Relatively quiescent tissues, such as liver, can readily regenerate after injury (Gilgenkrantz & Collin de l’Hortet, 2011). Posttraumatic visceral regeneration, therefore, cannot be interpreted simply as an extension of tissue self-renewal under normal physiological conditions (Cordero et al., 2012).
3.3. Regeneration and embryonic development
In general terms, posttraumatic regeneration is known to incorporate certain elements of embryonic and/or postembryonic development (Liozner, 1982). This is also true in the context of visceral regeneration. For example, the molecular mechanisms underpinning intestinal adaptation in mice (compensatory growth following massive small bowel resection) show significant similarity in the differential gene expression signature to the developing and immature intestine (Erwin et al., 2006). In some cases, the relationships between regeneration and development are extremely intimate, such as when regeneration becomes an integral component of the developmental program. This kind of “development-through-regeneration” is seen, for instance, in the intestinal epithelium remodeling during amphibian metamorphosis (Ishizuya-Oka, 2007; Ishizuya-Oka et al., 2009) and the formation of viscera in asexually propagating tunicates (Kawamura et al., 2008).
On the other hand, regeneration differs from embryonic development in that it often involves changes in cell fate specification and lineage relationships, that is, cells in regenerating tissues do not necessarily arise from other cells of the same kind or from their immediate developmental progenitors or resident tissue stem cells, but can originate from sources that are fundamentally different from those in normal tissue turnover and embryogenesis (Brockes & Kumar, 2008). Sometimes, exact recapitulation of embryonic mechanisms is rendered impossible by the very nature of the injury, such as when all endodermal tissues are lost in the anterior body region of some sea cucumbers (Mashanov & García-Arrarás, 2011). In these cases, the ability to induce developmental plasticity in adult tissues and to divert from embryonic developmental trajectories is a necessary prerequisite for regeneration.
Certain levels of cell fate plasticity are seen in many visceral regeneration models and retained even in the adult mammalian intestine. For example, intestinal fibroblasts and myofibroblasts may derive from nonmesenchymal cells, such as epithelial and endothelial cells, through transdifferentiation (Speca, Giusti, Rieder, & Latella, 2012). Furthermore, as mentioned earlier, recent lineage tracing experiments have shown that cell fate changes also occur within the luminal epithelium itself. These examples include injury-induced reversal of normally unidirectional lineage relationships from the stem cell through committed transit-amplifying progenitors to differentiated cells, when the ablated stem cell pool can be restored from the trans-amplifying or even differentiated (Paneth) cells (Doupé & Jones, 2013; Roth et al., 2012; Tian et al., 2011; van Es et al., 2012). Finally, the endodermally derived luminal epithelium of the human gastrointestinal tract can regenerate from mesodermal sources (bone marrow-derived stem cells) (Gupta et al., 2006; Körbling et al., 2002; Krause et al., 2001; Okamoto et al., 2006, 2002). Thus, posttraumatic visceral regeneration can incorporate, but is not based entirely on programs of normal development.
3.4. What can we learn from model animals to improve bowel regeneration in mammals?
What underlies the ability to fully regenerate the digestive tube in such animals as sea cucumbers, and what prevents mammals from doing so? At the level of individual tissues, at least some of the mechanisms and adaptations required for successful regeneration are already in place in mammals. These have been best demonstrated in the mucosal epithelium and include proliferation of resident intestinal stem cells and induction of stem cell properties in transit-amplifying progenitors and differentiated cells, as well as recruitment of extramucosal cells, that is, the same set of strategies that are used by animals capable of spontaneous regeneration of the digestive tube at the whole-organ level. The paradox then is the following. If all individual tissues can regenerate, why cannot the organ itself regenerate following major injury or grow back after resection? There are several possible answers to this question. First, tissues themselves might have restricted regenerative capabilities and overall organ regeneration might be limited by the capacities of the least regenerating component. Thus, although the mucosal epithelium is a highly dynamic self-sustaining and self-organizing tissue, which can recreate its typical crypt-villus structure from as little as a single Lgr5+ stem cell (Merlos-Suárez et al., 2011; Sato et al., 2009), other tissue layers might have less prominent properties.
A second explanation for the low regenerative capacity of the mammalian bowel at the whole- organ level is the possible need for an additional factor that mediates intestinal lengthening. In this case, such a factor that would regulate the acquisition of the proper organ length might be lacking during the regenerative response. A number of signaling molecules affecting the lengthening of the intestine were identified in embryonic development, but have never been studied in the context of gut regeneration. They include epimorphin, FGF9, IGF-1, and Wnt 5a, which are factors that regulate cell proliferation and differentiation, particularly in the intestinal mesenchyme, as well as epithelial–mesenchymal interactions (Cervantes, Yamaguchi, & Hebrok, 2009; Geske, Zhang, Patel, Ornitz, & Stappenbeck, 2008; Wang et al., 2006; Williams, Fuller, Fagin, & Lund, 2002; Zhang et al., 2006).
Finally, the success of whole organ regeneration is determined not only by developmental processes at the level of discrete tissues, but also involves coordination and interaction between cellular and molecular events in individual components of the regenerating organ. For example, research on sea cucumbers has stressed the importance of timing and coordination of different events between tissue layers of the regenerating digestive tube (reviewed in García-Arrarás & Greenberg, 2001; Mashanov & García-Arrarás, 2011). The first structures to develop are the mesothelium and connective tissue of the new gut. These tissue layers initially form a solid rod-like structure, which only then is invaded by the luminal epithelium. Therefore, it is the outer, nonmucosal, layers of the gut wall that play the leading role during the early phases of regeneration in echinoderms. Depending on the organization and composition, the extracellular matrix (ECM) can either promote regeneration or suppress it. The importance of connective tissue remodeling for initializing the regrowth of the viscera was demonstrated by experimental inhibition of matrix metalloproteases, which either slowed down or completely abolished gut regeneration in holothurians, depending on the treatment protocol (Lamash & Dolmatov, 2013; Quiñones, Rosa, Ruiz, & García-Arrarás, 2002). On the other side of the spectrum is the observation that if acute posttraumatic inflammation of the mammalian bowel leads to formation of the fibrotic scar, regeneration is inhibited and the normal intestinal tissue architecture is permanently altered, resulting in inability of the affected region to resume normal organ function (Speca et al., 2012). Thus, since proper ECM remodeling is known to be required for the formation of various “new” functional organs (Daley, Peters, & Larsen, 2008), it is possible that regeneration-promoting ECM assemblage or degradation does not occur following resection or severe injury of the mammalian intestine.
The two contrasting properties of the mammalian digestive tube, namely, the lack of regeneration at the whole-organ level and high regenerative potential at the level of the mucosal epithelium, have long been taken into account by investigators in regenerative medicine and organ bioengineering. This has spurred the development of a variety of scaffolding materials to provide the neointestine with a three-dimensional supporting and organizing system to direct cell adhesion, proliferation, and proper differentiation. In addition, techniques have been developed that rely on seeding cells onto supporting scaffolds. Although it was possible to keep the bio-engineered tissue viable, it has proved difficult to completely reproduce the organization of the entire organ. The engineered constructs often lacked critical components of the normal gut wall, such as nervous elements, blood and lymphatic vessels, and the muscular layer (Ross et al., 2013).
The gaps in the current knowledge about interactions between the epithelial cells and the underlying connective tissue layer are possibly one of the major obstacles for designing new bioengineering technologies (Ross et al., 2013). Further studies of the properties of the supporting scaffold that is formed during spontaneous gut regeneration in different animal groups can provide valuable insight into how posttraumatic remodeling of the bowel wall can be better managed in mammals and which properties should be enhanced or developed in artificial grafting materials.
4. CONCLUSIONS
Regeneration of the digestive epithelium in eumetazoans is accomplished through recruitment of cells from two types of sources. The first primary source is multi- or pluripotent stem cells, while the second one is reactivation of specialized cells. In both cases, the source cells can either reside within the luminal epithelium itself or be recruited from other tissues through migration and transdifferentiation. There is no clear correlation between the employment of either of these strategies and the phylogenetic position. Moreover, different strategies can be utilized within the same animal. Likewise, the capacity to regenerate the digestive tube does not necessarily correlate with the level of anatomical and histological complexity.
Although mammals are unable to regenerate lost regions of their digestive tube at the whole-organ level, there are reasons to think that their regenerative capacities can be significantly improved if the right conditions are found. First, even though mammals do not regrow the ablated segments of their gastrointestinal system after surgical resection, they still show a rudimentary regenerative response in the form of intestinal adaptation, which involves widening of the absorptive surface in the remnant bowel through extensive proliferation of intestinal stem and transit-amplifying cells resulting in increase in intestinal diameter and villus height (Bernal et al., 2005; Erwin et al., 2006). Second, regeneration of full thickness injuries or entire tubular segments of the digestive tube has been documented in other closely related groups, such as lower vertebrates, tunicates, and echinoderms, and, thus, occurs both in the chordate and nonchordate branches of the deuterostomian lineage. There is, therefore, a possibility that at least some of the components of the regenerative machinery might be evolutionarily conserved and have been preserved in mammals, although in a dormant state, as has been suggested to be the case in the central nervous system (Mashanov, Zueva, & García-Arrarás, 2013). Third, although the mammalian digestive tube does not regenerate at the whole-organ level, other endodermally derived organs (embryological derivatives of the digestive tube) retain a high regenerative capacity. These include the thymus, thyroid, liver, and the exocrine pancreas (Baddour, Sousounis, & Tsonis, 2012; Desai et al., 2007; Gilgenkrantz & Collin de l’Hortet, 2011). Therefore, whole-organ regeneration is not ubiquitously suppressed in the entire endodermal lineage of the adult mammals and possibly can be improved in the digestive tube per se.
Although a wide range of cellular mechanisms of visceral regeneration have evolved in animal phylogeny, at least some of the key components of the underlying molecular signaling machinery appear to be highly conserved. More comparative studies on spontaneously regenerating species are needed in order to distinguish between phylogenetically conserved and locally evolved components of the signaling cascades involved in regeneration of the digestive tube. This knowledge will help us understand if and how visceral injuries in poorly regenerating organisms, including humans, can be better managed and healed with minimal intervention.
Acknowledgments
We thank Brigitte Galliot for her critical reading of the review, and members of the García-Arrarás lab for their contribution to understanding the phenomenon of intestinal regeneration in sea cucumbers. The work was supported by the NIH (Grants 1SC1GM084770-01, 1R03NS065275-01, 1R15NS081686-01), the NSF (Grant IOS-0842870), and the University of Puerto Rico.
References
- Apidianakis Y, Rahme LG. Drosophila melanogaster as a model for human intestinal infection and pathology. Disease Models and Mechanisms. 2011;4:21–30. doi: 10.1242/dmm.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton GH, Morton JP, Myant K, Phesse TJ, Ridgway RA, Marsh V, et al. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Developmental Cell. 2010;19:259–269. doi: 10.1016/j.devcel.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddour JA, Sousounis K, Tsonis PA. Organ repair and regeneration: An overview. Birth Defects Research Part C. 2012;96:1–29. doi: 10.1002/bdrc.21006. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Bely A, Nyberg K. Evolution of animal regeneration: Re-emergence of a field. Trends in Ecology & Evolution. 2010;25:161–170. doi: 10.1016/j.tree.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Bernal NP, Stehr W, Zhang Y, Profitt S, Erwin CR, Warner BW. Evidence for active Wnt signaling during postresection intestinal adaptation. Journal of Pediatric Surgeons. 2005;40:1025–1029. doi: 10.1016/j.jpedsurg.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, Jasper H. Maintaining tissue homeostasis: Dynamic control of somatic stem cell activity. Cell Stem Cell. 2011;9:402–411. doi: 10.1016/j.stem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode HR. The interstitial cell lineage of hydra: A stem cell system that arose early in evolution. Journal of Cell Science. 1996;109:1155–1164. doi: 10.1242/jcs.109.6.1155. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A. Comparative aspects of animal regeneration. Annual Review of Cell and Developmental Biology. 2008;24:525–549. doi: 10.1146/annurev.cellbio.24.110707.175336. [DOI] [PubMed] [Google Scholar]
- Buczacki SJA, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- Byrne M. Induction of evisceration in the holothurian Eupentacta quinquesemita and evidence for the existense of an endogenous evisceration factor. The Journal of Experimental Biology. 1986;120:25–39. [Google Scholar]
- Carlson BM. Principles of Regenerative Biology. Amsterdam: Elsevier; 2007. [Google Scholar]
- Cervantes S, Yamaguchi TP, Hebrok M. Wnt5a is essential for intestinal elongation in mice. Developmental Biology. 2009;326:285–294. doi: 10.1016/j.ydbio.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MK, Beierle EA. Animal models for intestinal tissue engineering. Bio-materials. 2004;25:1675–1681. doi: 10.1016/s0142-9612(03)00517-9. [DOI] [PubMed] [Google Scholar]
- Cordero JB, Stefanatos RK, Scopelliti A, Vidal M, Sansom OJ. Inducible progenitor-derived Wingless regulates adult midgut regeneration in Drosophila. The EMBO Journal. 2012;31:3901–3917. doi: 10.1038/emboj.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: Stem cells, signals and combinatorial control. Nature Reviews. Genetics. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. Journal of Cell Science. 2008;121:255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- David CN. Interstitial stem cells in Hydra: Multipotency and decision-making. The International Journal of Developmental Biology. 2012;56:489–497. doi: 10.1387/ijdb.113476cd. [DOI] [PubMed] [Google Scholar]
- Desai BM, Oliver-Krasinski J, De Leon DD, Farzad C, Hong N, Leach SD, et al. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. The Journal of Clinical Investigation. 2007;117:971–977. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmatov IY, Mashanov VS. Regeneration in holothurians. Vladivostok: Dalnauka; 2007. (in Russian) [Google Scholar]
- Doupé DP, Jones PH. Cycling progenitors maintain epithelia while diverse cell types contribute to repair. BioEssays. 2013;35:443–451. doi: 10.1002/bies.201200166. [DOI] [PubMed] [Google Scholar]
- Erwin CR, Jarboe MD, Sartor MA, Medvedovic M, Stringer KF, Warner BW, et al. Developmental characteristics of adapting mouse small intestine crypt cells. Gastroenterology. 2006;130:1324–1332. doi: 10.1053/j.gastro.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: Overall and upper gastrointestinal diseases. Gastroenterology. 2009;136:376–386. doi: 10.1053/j.gastro.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Feil W, Lacy ER, Wong YM, Burger D, Wenzl E, Starlinger M, et al. Rapid epithelial restitution of human and rabbit colonic mucosa. Gastroenterology. 1989;97:685–701. doi: 10.1016/0016-5085(89)90640-9. [DOI] [PubMed] [Google Scholar]
- Feral J, Massin C. Digestive system: Holothuroidea. In: Jangoux M, Lawrence J, editors. Echinoderm nutrition. Rotterdam: Balkema; 1982. pp. 192–212. [Google Scholar]
- Forsthoefel DJ, James NP, Escobar DJ, Stary JM, Vieira AP, Waters FA, et al. An RNAi screen reveals intestinal regulators of branching morphogenesis, differentiation, and stem cell proliferation in planarians. Developmental Cell. 2012;23:691–704. doi: 10.1016/j.devcel.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsthoefel DJ, Park AE, Newmark PA. Stem cell-based growth, regeneration, and remodeling of the planarian intestine. Developmental Biology. 2011;356:445–459. doi: 10.1016/j.ydbio.2011.05.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolova LT, Dolmatov IY. Microscopic anatomy of the digestive system in normal and regenerating specimens of the brittlestar Amphipholis kochii. The Biological Bulletin. 2010;218:303–316. doi: 10.1086/BBLv218n3p303. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Matsuura K, Hu-Wang E, Lu R, Shi Y. Thyroid hormone activates protein arginine methyltransferase 1 expression by directly inducing c-Myc transcription during Xenopus intestinal stem cell development. The Journal of Biological Chemistry. 2012;287:10039–10050. doi: 10.1074/jbc.M111.335661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S, Isozaki T, Mori K, Kawamura K. Expression and function of myc during asexual reproduction of the budding ascidian Polyandrocarpa misakiensis. Development, Growth and Differentiation. 2011;53:1004–1014. doi: 10.1111/j.1440-169X.2011.01312.x. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, Furness JB, Costa M. Migration of the myoelectric complex after interruption of the myenteric plexus: Intestinal transection and regeneration of enteric nerves in the guinea pig. Gastroenterology. 1989;97:1135–1146. doi: 10.1016/0016-5085(89)91683-1. [DOI] [PubMed] [Google Scholar]
- Galliot B. Hydra, a fruitful model system for 270 years. The International Journal of Developmental Biology. 2012;56:411–423. doi: 10.1387/ijdb.120086bg. [DOI] [PubMed] [Google Scholar]
- Galliot B, Welschof M, Schuckert O, Hoffmeister S, Schaller HC. The cAMP response element binding protein is involved in hydra regeneration. Development. 1995;121:1205–1216. doi: 10.1242/dev.121.4.1205. [DOI] [PubMed] [Google Scholar]
- García-Arrarás JE, Estrada-Rodgers L, Santiago R, Torres II, Díaz-Miranda L, Torres-Avillán I. Cellular mechanisms of intestine regeneration in the sea cucumber, Holothuria glaberrima Selenka (Holothuroidea:Echinodermata) The Journal of Experimental Zoology. 1998;281:288–304. doi: 10.1002/(sici)1097-010x(19980701)281:4<288::aid-jez5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- García-Arrarás JE, Greenberg MJ. Visceral regeneration in holothurians. Microscopy Research and Technique. 2001;55:438–451. doi: 10.1002/jemt.1189. [DOI] [PubMed] [Google Scholar]
- García-Arrarás JE, Valentiín-Tirado G, Flores JE, Rosa RJ, Rivera-Cruz A, San Miguel-Ruiz JE, et al. Cell dedifferentiation and epithelial to mesenchymal transitions during intestinal regeneration in H. glaberrima. BMC Developmental Biology. 2011;11:61. doi: 10.1186/1471-213X-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza-Garcia AA, Driscoll PC, Brockes JP. Evidence for the local evolution of mechanisms underlying limb regeneration in salamanders. Integrative and Comparative Biology. 2010;50:528–535. doi: 10.1093/icb/icq022. [DOI] [PubMed] [Google Scholar]
- Geske MJ, Zhang X, Patel KK, Ornitz DM, Stappenbeck TS. Fgf9 signaling regulates small intestinal elongation and mesenchymal development. Development. 2008;135:2959–2968. doi: 10.1242/dev.020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilgenkrantz H, Collin de l’Hortet A. New insights into liver regeneration. Clinics and Research in Hepatology Gastroenterology. 2011;35:623–629. doi: 10.1016/j.clinre.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Goss RJ. The evolution of regeneration: Adaptive or inherent? Journal of Theoretical Biology. 1992;159:241–260. doi: 10.1016/s0022-5193(05)80704-0. [DOI] [PubMed] [Google Scholar]
- Gregorieff A, Pinto D, Begthel H, Destrée O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Gupta A, Dixit A, Sales KM, Winslet MC, Seifalian AM. Tissue engineering of small intestine—Current status. Biomacromolecules. 2006;7:2701–2709. doi: 10.1021/bm060383e. [DOI] [PubMed] [Google Scholar]
- Hanani M, Ledder O, Yutkin V, Abu-Dalu R, Huang T, Härtig W, et al. Regeneration of myenteric plexus in the mouse colon after experimental denervation with benzalkonium chloride. The Journal of Comparative Neurology. 2003;462:315–327. doi: 10.1002/cne.10721. [DOI] [PubMed] [Google Scholar]
- Hartl M, Mitterstiller AM, Valoka T, Breuker K, Hobmayer B, Bister K. Stem cell-specific activation of an ancestral myc proto- oncogene with conserved basic functions in the early metazoan Hydra. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4051–4056. doi: 10.1073/pnas.0911060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobmayer B, Jenewein M, Eder D, Eder MK, Glasauer S, Gufler S, et al. Stemness in Hydra—A current perspective. The International Journal of Developmental Biology. 2012;56:509–517. doi: 10.1387/ijdb.113426bh. [DOI] [PubMed] [Google Scholar]
- Hochmuth CE, Biteau B, Bohmann D, Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell. 2011;8:188–199. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell JC, Wells JM. Generating intestinal tissue from stem cells: Potential for research and therapy. Regenerative Medicine. 2011;6:743–755. doi: 10.2217/rme.11.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman L. The invertebrates. IV. Echinodermata. The celomate bilateria. New York: McGraw-Hill Book Co. Inc; 1955. [Google Scholar]
- Illa-Bochaca I, Montuenga LM. The regenerative nidi of the locust midgut as a model to study epithelial cell differentiation from stem cells. The Journal of Experimental Biology. 2006;209:2215–2223. doi: 10.1242/jeb.02249. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A. Regeneration of the amphibian intestinal epithelium under the control of stem cell niche. Development, Growth and Differentiation. 2007;49:99–107. doi: 10.1111/j.1440-169X.2007.00913.x. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Hasebe T, Buchholz DR, Kajita M, Fu L, Shi Y. Origin of the adult intestinal stem cells induced by thyroid hormone in Xenopus laevis. The FASEB Journal. 2009;23:2568–2575. doi: 10.1096/fj.08-128124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jwo SC, Tang SJ, Chen JR, Chiang KC, Huang TS, Chen HY. A novel model for simultaneous study of neointestinal regeneration and intestinal adaptation. Wound Repair and Regeneration. 2013;21:309–319. doi: 10.1111/wrr.12026. [DOI] [PubMed] [Google Scholar]
- Kaneko N, Katsuyama Y, Kawamura K, Fujiwara S. Regeneration of the gut requires retinoic acid in the budding ascidian Polyandrocarpa misakiensis. Development, Growth and Differentiation. 2010;52:457–468. doi: 10.1111/j.1440-169X.2010.01184.x. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Sugino Y, Sunanaga T, Fujiwara S. Multipotent epithelial cells in the process of regeneration and asexual reproduction in colonial tunicates. Development, Growth and Differentiation. 2008;50:1–11. doi: 10.1111/j.1440-169X.2007.00972.x. [DOI] [PubMed] [Google Scholar]
- Körbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, et al. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. The New England Journal of Medicine. 2002;346:738–746. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Lamash NE, Dolmatov IY. Proteases from the regenerating gut of the holothurian Eupentacta fraudatrix. PLoS One. 2013;8:e58433. doi: 10.1371/journal.pone.0058433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laranjeira C, Sandgren K, Kessaris N, Richardson W, Potocnik A, Vanden Berghe P, et al. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. The Journal of Clinical Investigation. 2011;121:3412–3424. doi: 10.1172/JCI58200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DE, Grikscheit TC. Tissue-engineering of the gastrointestinal tract. Current Opinion in Pediatrics. 2012;24:365–370. doi: 10.1097/MOP.0b013e328352ec19. [DOI] [PubMed] [Google Scholar]
- Liozner LD. Regeneration and development. Moscow: Nauka; 1982. (in Russian) [Google Scholar]
- Mashanov VS, Dolmatov IY. Functional morphology of the developing alimentary canal in the holothurian Eupentacta fraudatrix (Holothuroidea, Dendrochirota) Acta Zoologica. 2004;85:29–39. [Google Scholar]
- Mashanov VS, Dolmatov IY, Heinzeller T. Transdifferentiation in holothurian gut regeneration. The Biological Bulletin. 2005;209:184–193. doi: 10.2307/3593108. [DOI] [PubMed] [Google Scholar]
- Mashanov V, Frolova L, Dolmatov I. Structure of the digestive tube in the holothurian Eupentacta fraudatrix (Holothuroidea:Dendrochirota) Russian Journal of Marine Biology. 2004;30:314–322. [Google Scholar]
- Mashanov VS, García-Arrarás JE. Gut regeneration in holothurians: A snapshot of recent developments. The Biological Bulletin. 2011;221:93–109. doi: 10.1086/BBLv221n1p93. [DOI] [PubMed] [Google Scholar]
- Mashanov VS, Zueva OR, García-Arrarás JE. Expression of Wnt9, TCTP, and Bmp1/Tll in sea cucumber visceral regeneration. Gene Expression Patterns. 2012;12:24–35. doi: 10.1016/j.gep.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashanov VS, Zueva OR, García-Arrarás JE. Radial glial cells play a key role in echinoderm neural regeneration. BMC Biology. 2013;11:49. doi: 10.1186/1741-7007-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashanov VS, Zueva OR, Rojas-Catagena C, García-Arrarás JE. Visceral regeneration in a sea cucumber involves extensive expression of survivin and mortalin homologs in the mesothelium. BMC Developmental Biology. 2010;10:117. doi: 10.1186/1471-213X-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlos-Suárez A, Barriga FM, Jung P, Iglesias M, Céspedes MV, Rossell D, et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8:511–524. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Mosher C. Observation on evisceration and visceral regeneration in the sea cucumber Actinopyga agassizi Selenka. Zoologica NY. 1956;41:17–26. [Google Scholar]
- Mozzi D, Dolmatov I, Bonasoro F, Candia Carnevali MD. Visceral regeneration in the crinoid Antedon mediterranea: Basic mechanisms, tissues and cells involved in gut regrowth. Central European Journal of Biology. 2006;1:609–635. [Google Scholar]
- Nagai H, Chew SH, Okazaki Y, Funahashi S, Namba T, Kato T, et al. Metamorphosis of mesothelial cells with active horizontal motility in tissue culture. Scientific Reports. 2013;3:1144. doi: 10.1038/srep01144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odintsova N, Dolmatov I, Mashanov V. Regenerating holothurian tissues as a source of cells for long-time cell culture. Marine Biology. 2005;146:915–921. [Google Scholar]
- Ohlstein B, Spradling A. The aduot Drosophila posterior midgut is maintained by pluripotent cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Okamoto R, Matsumoto T, Watanabe M. Regeneration of the intestinal epithelia: Regulation of bone marrow-derived epithelial cell differentiation towards secretory lineage cells. Human Cell. 2006;19:71–75. doi: 10.1111/j.1749-0774.2006.00010.x. [DOI] [PubMed] [Google Scholar]
- Okamoto R, Yajima T, Yamazaki M, Kanai T, Mukai M, Okamoto S, et al. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nature Medicine. 2002;8:1011–1017. doi: 10.1038/nm755. [DOI] [PubMed] [Google Scholar]
- O’Steen WK. Regeneration and repair of the intestine in Rana clamitans larvae. The Journal of Experimental Zoology. 1959;141:449–475. doi: 10.1002/jez.1401410303. [DOI] [PubMed] [Google Scholar]
- O’Steen WK, Walker BE. Radioautographic studies or regeneration in the common newt. III. Regeneration and repair of the intestine. The Anatomical Record. 1962;142:179–187. doi: 10.1002/ar.1091420210. [DOI] [PubMed] [Google Scholar]
- Plickert G, Frnak U, Müller WA. Hydractinia, a pioneering model for stem cell biology and reprogramming somatic cells to pluripotency. The International Journal of Developmental Biology. 2012;56:519–534. doi: 10.1387/ijdb.123502gp. [DOI] [PubMed] [Google Scholar]
- Potten CS. Keratinocyte stem cells, label-retaining cells and possible genome protection mechanisms. The Journal of Investigative Dermatology. 2004;9:183–195. doi: 10.1111/j.1087-0024.2004.09305.x. [DOI] [PubMed] [Google Scholar]
- Quiñones JL, Rosa R, Ruiz DL, García-Arrarás JE. Extracellular matrix remodeling and metalloproteinase involvement during intestine regeneration in the sea cucumber Holothuria glaberrima. Developmental Biology. 2002;250:181–197. doi: 10.1006/dbio.2002.0778. [DOI] [PubMed] [Google Scholar]
- Radtke F, Clevers H. Self-renewal and cancer of the gut: Two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- Reddien PW. Specialized progenitors and regeneration. Development. 2013;140:951–957. doi: 10.1242/dev.080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CL, Booth C, Sanders B, Babbar P, Bergman C, Soker T, et al. Regeneration and bioengineering of transplantable abdominal organs: Current status and future challenges. Expert Opinion on Biological Therapy. 2013;13:103–113. doi: 10.1517/14712598.2013.732063. [DOI] [PubMed] [Google Scholar]
- Roth S, Franken P, Sacchetti A, Kremer A, Anderson K, Sansom O, et al. Paneth cells in intestinal homeostasis and tissue injury. PLoS One. 2012;7:e38965. doi: 10.1371/journal.pone.0038965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, Tapon N. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development. 2010;137:4147–4158. doi: 10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert S, Anton-Erxleben F, Bosch TC. Cell type complexity in the basal metazoan Hydra is maintained by both stem cell based mechanisms and trans-differentiation. Developmental Biology. 2008;313:13–24. doi: 10.1016/j.ydbio.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Speca S, Giusti I, Rieder F, Latella G. Cellular and molecular mechanisms of intestinal fibrosis. World Journal of Gastroenterology. 2012;18:3635–3661. doi: 10.3748/wjg.v18.i28.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan R, Tchieu J, Mason MJ, Yachechko R, Kuoy E, Horvath S, et al. Role of the murine reprogramming factors in the induction of pluripotency. Cell. 2009;136:364–377. doi: 10.1016/j.cell.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World Journal of Gastroenterology. 2008;14:348–353. doi: 10.3748/wjg.14.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, Sato T, van de Wetering M, Lyubimova A, Nee ANY, Gregorieff A, et al. Dll1 + secretory progenitor cells revert to stem cells upon crypt damage. Nature Cell Biology. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DE, Wang IE, Reddien PW. Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science. 2011;332:811–816. doi: 10.1126/science.1203983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang L, Iordanov H, Swietlicki EA, Zheng Q, Jiang S, et al. Epimorphin(−/−) mice have increased intestinal growth, decreased susceptibility to dextran sodium sulfate colitis, and impaired spermatogenesis. The Journal of Clinical Investigation. 2006;116:1535–1546. doi: 10.1172/JCI25442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie IC. Autotomy as a prelude to regeneration in echinoderms. Microscopy Research and Technique. 2001;55:369–396. doi: 10.1002/jemt.1185. [DOI] [PubMed] [Google Scholar]
- Williams KL, Fuller CR, Fagin J, Lund PK. Mesenchymal IGF-I over-expression: Paracrine effects in the intestine, distinct from endocrine actions. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2002;283:G875–G885. doi: 10.1152/ajpgi.00089.2002. [DOI] [PubMed] [Google Scholar]
- Zhang X, Stappenbeck TS, White AC, Lavine KJ, Gordon JI, Ornitz DM. Reciprocal epithelial-mesenchymal FGF signaling is required for cecal development. Development. 2006;133:173–180. doi: 10.1242/dev.02175. [DOI] [PMC free article] [PubMed] [Google Scholar]