Abstract
Psoriasis is a chronic inflammatory skin disease mediated by dysregulated auto-reactive immune system. In this study, in order to confirm and further extend the pharmacological basis of topical steroids in psoriasis therapy, we investigated the effect of betamethasone ointment on imiquimod (IMQ)-induced skin inflammation in mice. In BALB/c mice, topical IMQ at the dose of 250 μg each on both sides of the ear induced marked psoriasis-like skin inflammation within 5 days. The same dose of IMQ produced only slight to moderate skin inflammation even on Day 7 in CB-17 scid mice. IMQ-induced skin inflammation was associated with increased levels of mRNA transcripts expression of signature cytokines of T helper (Th)1/Th17 cells, i.e., interferon-γ, interleukin (IL)-17 and IL-22 on Day 5. In addition, levels of mRNA expression of the markers of keratinocytes, i.e., IL-1β, S100A8, and S100A9, were dramatically elevated in IMQ-treated mice. The IMQ-induced changes in cytokine expression were significantly suppressed by topical treatment with betamethasone ointment. IMQ failed to produce significant changes in the mRNA levels of tumor necrosis factor-α as a marker of macrophages and NK1.2 as a marker of natural killer cells and natural killer T cells. In contrast, mRNA level of a Th2 cytokine IL-13 was significantly decreased by IMQ treatment and further suppressed by betamethasone. These findings provide the first pharmacological evidence that the topical application of betamethasone prevents IMQ-induced psoriasis-like skin inflammation in mice by inhibiting gene expressions of various cytokines related to Th1 cells, Th17 cells and keratinocytes.
Keywords: Imiquimod, Psoriasis, Skin Inflammation, Betamethasone, Cytokines
INTRODUCTION
Psoriasis is a chronic inflammatory skin disease characterized by skin thickening and sharply demarcated erythematous plaques covered by silvery white scales due to hyperproliferation and excessive accumulation of epidermal keratinocytes [1,2]. In patients with psoriasis, not only physical, but also psychological and social dimensions of quality of life is significantly impaired since psoriatic plaques and inflammation on uncovered skin are easily recognized by others in daily life [3,4,5]. Particularly, female patients suffer from feelings of shame, self-esteem, and rejection [5,6,7]. Although the precise mechanism is not fully elucidated, it is commonly accepted that dysregulated auto-reactive immune system is involved in the pathogenesis of this chronic skin disease [8,9]. Growing clinical and preclinical evidence strongly suggests that T lymphocytes, particularly T helper (Th) 1 cells and Th17 cells, play a pivotal role in progress and maintenance of psoriasis by secreting interferon (IFN)-γ, interleukin (IL)-17 and IL-22, while implicating other types of cells surrounding T cells in the psoriatic skin such as dendritic cells (DCs) and keratinocytes [10,11,12,13,14]. In this context, strong immunosuppressors, including cyclosporine and methotrexate, are used for treatment of severe, widespread psoriasis, although clinical use of these drugs is often significantly limited by serious side effects, e.g., hepatic and renal toxicity [15,16].
More recently, several biologics, including anti-tumor necrosis factor (TNF)-α, anti-IL12/23 and anti-IL-17 antibodies, have been proven to achieve significant remission in patients with moderate to severe psoriasis. However, there remains several concerns, including non-responder rate, increased risks of infections, cancer development, and financial burden due to high drug prices [17]. Over the last 2 years, apremilast, a new orally active inhibitor of phosphodiesterase 4, has been approved and launched for psoriasis treatment in the United States and Europe [18,19]. In addition, a new Kv1.3 channel blocker, dalazatide (ShK-186), has been shown to be effective in psoriasis [20]. These new drugs have made a considerable contribution to establishment of a wider range of therapeutic options for moderate to severe psoriasis patients who need systemic drug treatment.
On the other hand, mild to moderate psoriasis, that is majority of the patients, can be controlled by topical steroids [1,2]. Although topical steroids are widely prescribed for those patients, their mechanisms of action remain to be fully elucidated, at least partly, because experimental approaches were hampered by lack of reliable animal models. Recently, it was reported that topical application of imiquimod (IMQ), a toll-like receptor (TLR)-7 and TLR-8 agonist, to skin induced psoriasis-like skin inflammation in mice, and that DCs/Th1/Th17 axis plays an important role in inducing the skin inflammation [22]. The IMQ-induced skin inflammation in mice is demonstrated to manifest diffuse epidermal hyperplasia and inflammatory cell infiltration that human psoriasis patients have in common [22,23]. In addition, recent clinical studies have revealed that topical IMQ application induced and even exacerbated psoriasis in human, and that drugs used for the treatment of psoriasis could also suppress IMQ-induced psoriasis [24,25,26,27]. Based on these experimental and clinical findings, IMQ-induced skin inflammation in mice is thought to be a valid animal model of psoriasis.
In this study, in order to confirm and further extend the pharmacological rationales of topical steroids in psoriasis therapy, we investigated the effect of betamethasone ointment on IMQ-induced skin inflammation in mice. Additional experiments were carried out to measure mRNA transcripts expression levels of various cytokines and substances relevant to DCs/Th1/Th17 axis to assess the underlying mechanism of action of topical betamethasone in IMQ-induced psoriasis model.
MATERIALS AND METHODS
Materials
Imiquimod (IMQ, 5% cream, Beselna®) was purchased from Mochida Pharmaceutical (Tokyo, Japan). Betamethasone butyrate propionate (0.05% ointment, Antebate®) was purchased from Torii Pharmaceutical (Tokyo, Japan). Real-time PCR probes and related agents were purchased from Applied Biosystems (Massachusetts, USA). All other reagents were purchased from Wako Pure Chemical (Osaka, Japan) unless otherwise stated.
Animals
Female BALB/c mice and male CB-17 scid mice aged 7–12 weeks old were obtained from SLC (Hamamatsu, Japan) and from Charles River Laboratories Japan Inc. (Yokohama, Japan), respectively. The mice were housed in-house under specific pathogen-free conditions at a room temperature of 23 ± 3 °C and air humidity of 55 ± 15% in a 12-hour light/dark cycle environment, and they were provided with food and water ad libitum. All animal experiments in this study were reviewed and approved by the Institutional Animal Care and Use Committee of Japan Tobacco Inc. and were conducted in strict accordance with related ethical regulations.
Induction of Skin Inflammation
IMQ 5% cream was applied on inner and/or outer sides of the left ear skin once daily. The dose of IMQ was either 250 μg on outer side, 500 μg on outer side, or 250 μg on both inner and outer sides of ear skin. Betamethasone ointment or relevant ointment base was applied twice daily on to the left ear skin, at a volume of 5 μL to both the inner and/or outer sides. Thickness of the left ear was measured as a quantitative index of skin inflammation utilizing a thickness gauge (IDA-112M, Mitutoyo, Kawasaki, Japan) once daily before the application of IMQ. Age-matched normal control animals received neither IMQ nor vehicle cream. The investigator who measured skin thickness was blinded to the treatment groups. For the experiments to assess mRNA transcript expression, mice were euthanized on Day 5 by carbon dioxide gas, and the left ear was harvested after examination of gross appearance for occurrence of inflammatory changes including erythema and scaling. Part of the harvested tissue was sliced, fixed with buffered 10% formalin solution, and processed for preparation of histological paraffin sections. The sections were stained with hematoxyline and eosin, and subjected to light microscopic examination. The rest of the harvested ear tissue was stored at −80 °C for mRNA analysis by real time PCR assays.
Real Time PCR Assays
Total RNA samples in the ear tissues were obtained with RNeasy® Lipid Tissue Mini Kit (QIAGEN, Venlo, the Netherlands), following the manufacturer’s instruction. In short, harvested ear tissues stored at −80 °C were homogenized with TissueLyser II (QIAGEN, Venlo, the Netherlands) using the buffer provided in the kit, and RNA samples were extracted and purified by applying the homogenates to the kit column followed by eluting RNA samples binding to the column by RNA-free water. The expression levels of mRNA transcripts coding cytokines of interest in the present study were measured by the TaqMan Gene Expression Assays with the RNA-to-Ct™ 1-Step Kit, using relevant probes recommended by the manufacturer. The probes employed in the present study were: IFN-γ, Mm01168134_m1; IL-13, Mm00434204_m1; IL-17, Mm00439618_m1; IL-22, Mm01226722_g1; IL-23, Mm00518984_m1; TNF-α, Mm00443258_m1; IL-1β, Mm00434228_m1; S100A8, Mm00496696_g1; S100A9, Mm00656925_m1; and NK1.2, Mm00824341_m1. The GAPDH (Mm99999915 _g1) was used as an endogenous control gene and the expression levels of cytokines were corrected for the GAPDH levels to normalize for input.
Statistical Analysis
Values of ear thickness were shown as the increases from the pre-treatment values measured at Day 1 and were expressed as mean ± standard deviation (S.D.). Statistical significance was analyzed by F-test followed by Aspin-Welch’s t-test and Bartlett’s test followed by Dunnett’s test or Steel test in ear thicknesses, and by Bartlett’s test followed by Tukey’s test or Steel-Dwass test in mRNA transcript levels. A p value of less than 0.05 was considered statistically significant.
RESULTS
Chronological Changes of IMQ-induced Skin Inflammation
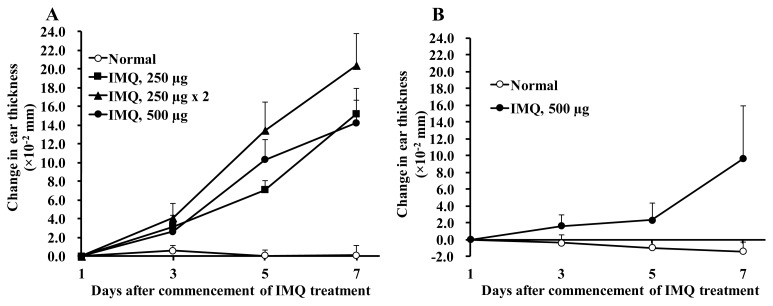
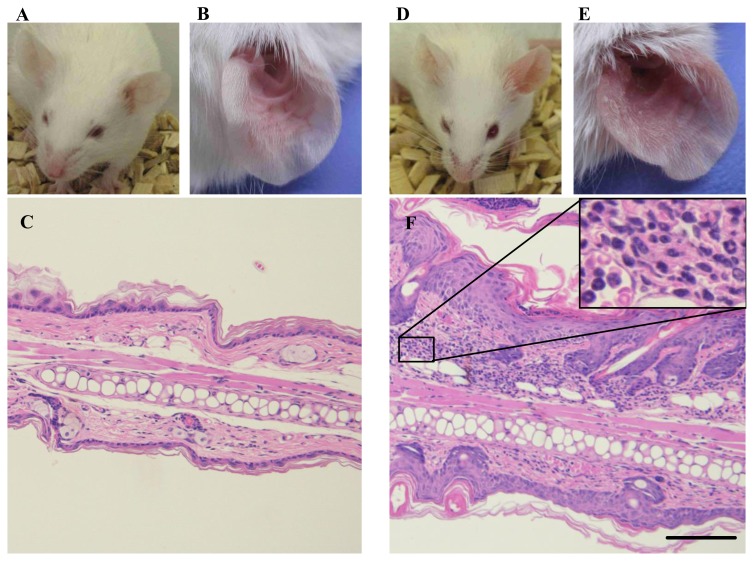
In BALB/c mice, IMQ applied to the ear skin induced ear swelling measured as a quantitative index of skin inflammation on Days 3, 5 and 7 (Figure 1A). Most profound ear swelling was achieved at the dose of 250 μg each on both sides of the ear through the whole observation period. As shown in Figure 2, redness, erythema, thickening and scaling were observed in the left ear skin of BALB/c mice treated with IMQ at 250 μg on both the outer and inner sides of the left ear once daily for 5 days. Light microscopic examination demonstrated that the IMQ-treated ear had marked parakeratosis, hyperkeratosis, acanthosis and infiltration of inflammatory cells, such as lymphocytes and neutrophils, in the thickened dermis. Based on these results, the protocol of applying 250 μg of IMQ each to both sides of the left ear once daily for 5 days was employed in the following experiments using BALB/c mice. It was confirmed that, the same dose of IMQ produced only slight to moderate skin inflammation even on Day 7 in CB-17 scid mice that inherently lack T cells and B cells (Figure 1B).
Figure 1.
IMQ-induced skin inflammation in BALB/c mice (A) and in CB-17 scid mice (B). (A) IMQ was applied once daily with a dose of either 250 μg only to outer side of the ear skin, 250 μg to both outer side and inner side of the ear skin, or 500 μg only to outer side of the ear skin. (B) 500 μg of IMQ was applied once daily only to outer side of the ear skin. Normal control animals received neither IMQ nor vehicle cream. Data are expressed as mean change in ear thickness (× 10−2 mm) + S.D. n = 5–7.
Figure 2.
Typical phenotypic images, close-ups, and histological images of left ear on Day 5. (A, B, C) Normal control animals received neither IMQ nor vehicle cream. (D, E, F) IMQ application on left ear skin. F, inset, a close-up image of inflammatory cells in dermis. Ear thickening, erythema, and scaling are observed on gross appearance. The histological image shows parakeratosis, hyperkeratosis, acanthosis, infiltration of lymphocytes and neutrophils in thickened dermis. Scale bar = 100 μm
Effect of Betamethasone on IMQ-induced Skin Inflammation
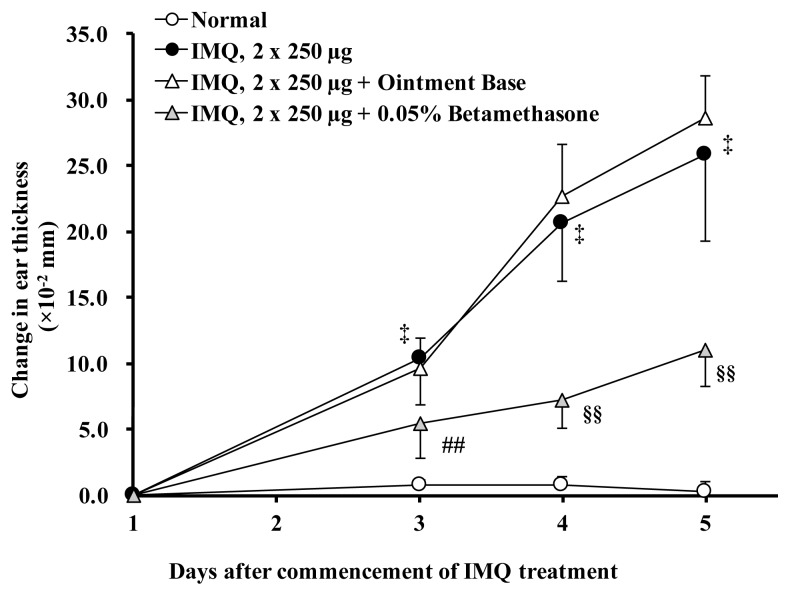
Figure 3 illustrates the effect of betamethasone ointment on the skin inflammation induced by topical IMQ once daily for 5 days in BALB/c mice. Treatment of the ear with betamethasone ointment twice daily significantly (p < 0.01) inhibited the development of IMQ-induced skin inflammation on Days 3, 4 and 5. Morphological changes of the IMQ-affected skin, such as redness, erythema, thickening and scaling, were also markedly diminished by treatment with topical betamethasone. Sham operation with relevant ointment alone produced little or negligible effect on the IMQ-induced skin thickening and morphological changes throughout the 5-day observation period.
Figure 3.
Effects of betamethasone on IMQ-induced skin inflammation. Normal control animals received neither IMQ nor vehicle cream. Each data represents mean change in ear thickness (× 10−2 mm) ± S.D. n = 10 except for the IMQ group (n = 8). ‡: p < 0.01 vs. Normal group (Aspin-Welch’s t-test), ##: p < 0.01 vs. IMQ group (Dunnett’s test), §§: p < 0.01 vs. IMQ group (Steel test).
Effects of Betamethasone on Th cells-related Cytokine Expression in IMQ-induced Skin Inflammation
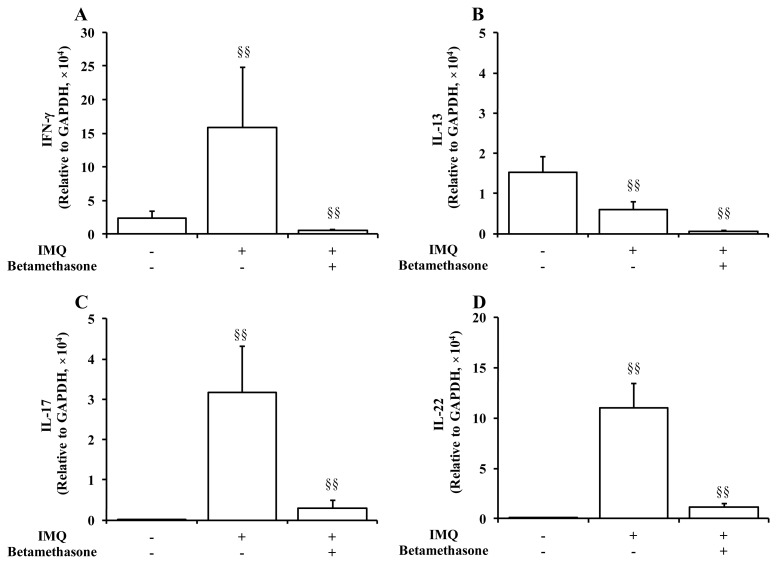
Figure 4 summarizes the effects of betamethasone on mRNA transcripts expression levels of Th cells-related cytokines in the IMQ-affected ear tissues of BALB/c mice. Repeated application of IMQ resulted in remarkable and significant (p < 0.01) increases in the mRNA levels of signature cytokines of Th1/Th17, i.e., IFN-γ, IL-17 and IL-22 on Day 5. In contrast, mRNA level of a Th2 cytokine IL-13 was significantly (p < 0.01) decreased by IMQ treatment. Topical treatment of IMQ-applied ear skin with betamethasone twice daily for 5 days dramatically inhibited the increases in the expression levels of IFN-γ, IL-17, IL-22 and IL-13, compared to those of the disease control group.
Figure 4.
Effects of application of 2 x 250 μg of IMQ and 5 μL of 0.05% betamethasone on mRNA transcripts expression levels of Th cells-related inflammatory cytokines in ear tissue on Day 5. Normal control animals received neither IMQ nor vehicle cream. Data represent mean expression levels in the normal group, the IMQ group, and the IMQ + betamethasone treatment group, relative to a control house-keeping gene (GAPDH) + S.D. (n = 8–10). §§: p < 0.01. IMQ vs. Normal group or IMQ + betamethasone vs. IMQ group (Steel-Dwass test).
Effects of Betamethasone on Psoriasis-related Cytokine Expression in IMQ-induced Skin Inflammation
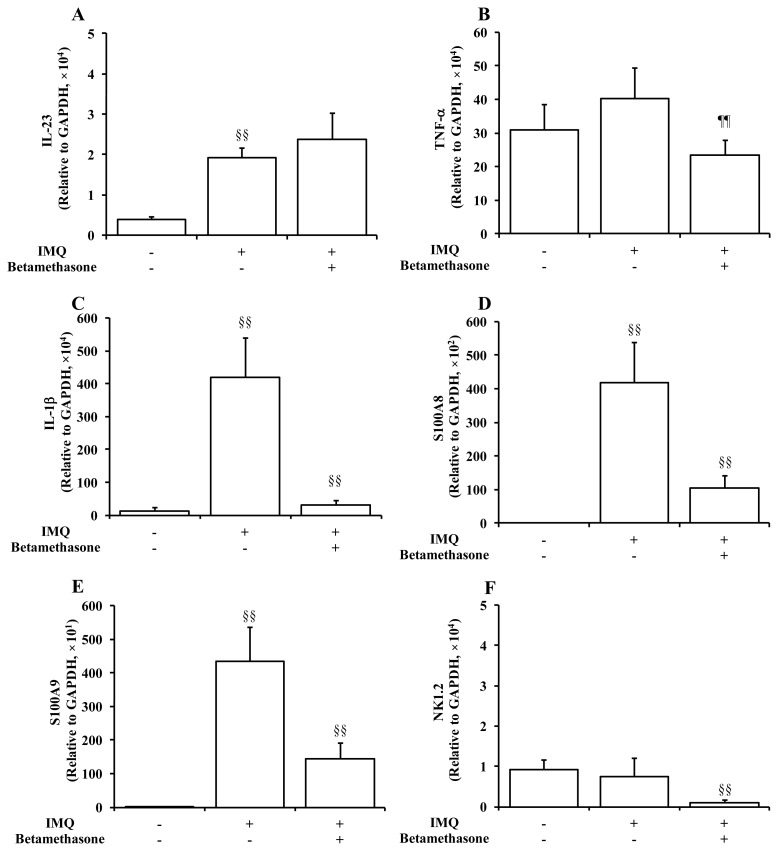
Following to the measurement of Th cells-related inflammatory cytokines, the mRNA transcripts levels of other cytokines and substances related to the pathophysiology of psoriasis were also measured in the ear tissues harvested on Day 5 (Figure 5). IMQ elicited a marked and significant (p < 0.01) increase in the expression level of IL-23. In addition, the mRNA levels of markers of keratinocytes, i.e., IL-1β, S100A8, and S100A9, were dramatically elevated in IMQ-treated mice, while there were no significant changes in the mRNA levels of TNF-α as a marker of macrophages and NK1.2 as a marker of natural killer cells and natural killer T cells. Topical application of betamethasone significantly (p < 0.01) decreased the mRNA levels of IL-1β, S100A8, S100A9, and NK1.2, compared to those of the group treated with IMQ alone. On the other hand, betamethasone was with only little or negligible effects on the expression levels of IL-23 and TNF-α in the IMQ-treated ear, although the statistical significance was achieved for the result of TNF-α expression.
Figure 5.
Effects of application of 2 x 250 μg of IMQ and 5 μL of 0.05% betamethasone on mRNA transcripts expression levels of inflammatory cytokines and substances in ear tissue on Day 5. Normal control animals received neither IMQ nor vehicle cream. Data represent mean expression levels in the normal group, the IMQ group, and the IMQ + betamethasone treatment group, relative to a control house-keeping gene (GAPDH) + S.D. (n = 8–10). §§: p < 0.01. IMQ vs. Normal group or IMQ + betamethasone vs. IMQ group (Steel-Dwass test), ¶¶: p < 0.01. IMQ + betamethasone vs. IMQ group (Tukey’s test).
DISCUSSION
In the present study, we confirmed that repeated application of IMQ to the ear skin induced severe skin inflammation characterized by skin thickening, parakeratosis, hyperkeratosis, acanthosis and inflammatory cell infiltration in BALB/c mice within 5 days. The morphological features of IMQ-induced skin inflammation were similar to those of human psoriasis, as has been reported in a previous study [22]. Furthermore, IMQ produced only mild to moderate skin thickening of slower onset in male CB-17 scid mice even after repeated application once daily for 7 days, whereas it elicited fully established, severe psoriasis-like skin inflammation in female BALB/s mice within 5 days. Since we found no gender-related difference in development of IMQ-induced skin thickening in either CB-17 scid or BALB/c mice (unpublished data), these results suggest that immunological mechanisms are involved, at least partly, in the development of IMQ-induced skin inflammation in mice. We also found that topical application of betamethasone significantly suppressed IMQ-induced skin thickening and other associated morphological changes. This finding provides the first experimental evidence that betamethasone ointment, like the most potent steroid clobetasol [28], could suppress the experimental psoriasis model.
Activation of skin DCs via TLR-7 and TLR-8 triggers the development and chronicity of skin inflammation in psoriasis [29]. An endogenous ligand to TLR-7 and TLR-8 is the complex of an antimicrobial peptide LL37 and self-derived single-stranded RNA released by dying cells [30,31,32]. Recently, it was demonstrated that, in the psoriatic skin, the LL37-self RNA complex was elevated in the keratinocytes, and that activated DCs release various cytokines, including IL-12 and IL-23, triggering the directed differentiation of naïve Th cells into Th1 and Th17 cells, respectively [29,33]. IMQ is a potent agonist to TLR-7 and TLR-8 [22], and known to activate DCs on topical application onto the skin in humans and mice [22,34]. These clinical and experimental findings strongly suggest that Th1/Th17 axis, in concert with other types of cells such as DCs and keratinocytes, play pivotal roles in the pathogenesis of psoriasis. In line with this view, the present experiments showed that IMQ application on mouse ear elicited a marked increase in mRNA transcripts expression of IL-23 measured as a marker of DCs, and that expression levels of signature cytokines of Th1/Th17 cells, such as IFN-γ, IL-17, IL-22, were also profoundly increased in the IMQ-affected psoriatic skin. On the other hand, expression level of a Th2 cytokine IL-13 was significantly decreased in response to repeated IMQ application. These results confirm that IMQ activated DCs through TLR-7 and TLR-8 to produce an environment dominated by Th1 and Th17 in the skin, as seen in other autoimmune disease models [35]. The constitutive expression of IL-13 observed in this study is considered to be attributable to the proneness to Th2 of BALB/c strain used in the present study [36,37].
Our data also show that topical IMQ markedly increased the expression level of IL-1β a cytokine released by activated keratinocytes [29], and that it also profoundly increased the expression levels of S100A8 and S100A9, known as antimicrobial peptides produced by hyperproliferative keratinocytes and upregulated in psoriatic conditions [38,39]. These results indicate that keratinocytes were highly activated in the IMQ-induced skin inflammation in mice. Since signature cytokines of Th1/Th17 cells, i.e., IFN-γ, IL-17, and IL-22, are reported to be capable of activating keratinocytes [29], the IMQ-induced expression of IL-1β S100A8 and S100A9 is likely to occur secondarily in response to activation of Th1/Th17 cells, although the possibility cannot be excluded that IMQ directly activated skin keratinocytes. These analyses of mRNA expression carried out in this study may all indicate that, like in human psoriasis, Th1 and Th17 cells may play pivotal roles, in concert with activated DCs and keratinocytes, in induction and exacerbation of the IMQ-induced psoriasis model. Although a previous study reported possible involvement of natural killer cells and natural killer T cells in psoriatic condition [40], the possibility could be excluded that they are implicated in the pathogenesis of IMQ-induced skin inflammation since the mRNA transcript expression level of their marker, NK1.2, was not changed by IMQ.
TNF-α is one of the proven cytokines that play pivotal roles in the pathophysiology of psoriasis, and various biologics targeting TNF-α or the receptor of TNF-α are available for the treatment of psoriasis in the clinic, these biologics have been achieving great success as the first-line drugs for psoriasis patients who need systemic treatments [41]. In psoriatic conditions, TNF-α is produced by macrophages, natural killer T cells, keratinocytes as well as DCs, and it activates DCs to produce IL-12 and IL-23, resulting in the differentiation of naïve T cells [29]. In the present study, however, the mRNA transcript expression level of TNF-α remained the same between the normal group and the IMQ-treated group, indicating that TNF-α is not implicated in the pathogenesis of IMQ-induced skin inflammation at least under the experimental condition employed this time. Further studies are necessary to elucidate the possible roles of this highly inflammatory cytokine under different condition, e.g., more chronic state.
We also found that topical application of betamethasone ointment significantly suppressed IMQ-induced increases in mRNA transcript expression levels of IFN-γ, IL-17, IL-22, IL-1β, S100A8 and S100A9 in the mouse skin tissues. These results indicate that betamethasone exerted its anti-inflammatory effects against IMQ -induced psoriasis model by suppressing Th1 cells, Th17 cells and keratinocytes. The current finding that betamethasone exhibited marginal effect on IL-23 expression suggests that the steroid did not affect directly on DCs and left them activated by IMQ. Although the constitutive expression of TNF-α and NK1.2 was significantly decreased by treatment with betamethasone, the significance of the phenomena in the protective effect of the steroid remains unknown because expression levels of these cytokines were not changed in IMQ-affected skin tissues.
In summary, the present study provides the first pharmacological evidence that topical application of betamethasone effectively prevents IMQ-induced psoriasis-like skin inflammation in mice by inhibiting gene expressions of various cytokines related to Th1 cells, Th17 cells and keratinocytes that are known to play critical roles in the pathogenesis of human psoriasis. These findings confirm and further validate the therapeutic rationales of topical steroids for treatment of mild to moderate psoriasis. Further studies on the later stage of IMQ-induced skin inflammation are warranted to investigate any direct effects of betamethasone on keratinocytes and macrophages, as well as the possible involvement of general anti-inflammatory effects due to phospholipase A2 and/or cyclooxygenase 2 inhibition.
FUNDING
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
REFERENCES
- 1.Schön MP, Boehncke W. Psoriasis. N Engl J Med. 2005;352:1899–1912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- 2.Lebwohl M. Psoriasis. Lancet. 2003;361:1197–1204. doi: 10.1016/S0140-6736(03)12954-6. [DOI] [PubMed] [Google Scholar]
- 3.Rapp SR, Feldman SR, Exum ML, Fleischer AB, Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41:401–407. doi: 10.1016/s0190-9622(99)70112-x. [DOI] [PubMed] [Google Scholar]
- 4.Augustin M, Kruger K, Radtke MA, Schwippl I, Reich K. Disease severity, quality of life and health care in plaque-type psoriasis: a multicenter cross-sectional study in Germany. Dermatology. 2008;216:366–372. doi: 10.1159/000119415. [DOI] [PubMed] [Google Scholar]
- 5.Böhm D, Stock Gissendanner S, Bangemann K, Snitjer I, Werfel T, Weyergraf A, Schulz W, Jager B, Schmid-Ott G. Perceived relationships between severity of psoriasis symptoms, gender, stigmatization and quality of life. J Eur Acad Dermatol Venereol. 2013;27:220–226. doi: 10.1111/j.1468-3083.2012.04451.x. [DOI] [PubMed] [Google Scholar]
- 6.Schmid-Ott G, Kunsebeck HW, Jager B, Sittig U, Hofste N, Ott R, Malewski P, Lamprecht F. Significance of the stigmatization experience of psoriasis patients: a 1-year follow-up of the illness and its psychosocial consequences in men and women. Acta Derm Venereol. 2005;85:27–32. doi: 10.1080/000155550410021583. [DOI] [PubMed] [Google Scholar]
- 7.Misery L, Thomas L, Jullien D, Cambazard F, Humbert P, Dubertret L, Dehen L, Macy G, Boussetta S, Taieb C. Comparative study of stress and quality of life in outpatients consulting for different dermatoses in 5 academic departments of dermatology. Eur J Dermatol. 2008;18:412–415. doi: 10.1684/ejd.2008.0466. [DOI] [PubMed] [Google Scholar]
- 8.Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of psoriasis. Clin Rev Allergy Immunol. 2007;33:45–56. doi: 10.1007/s12016-007-0039-2. [DOI] [PubMed] [Google Scholar]
- 9.Sabat R, Philipp S, Hoflich C, Kreutzer S, Wallace E, Asadullah K, Volk HD, Sterry W, Wolk K. Immunopathogenesis of psoriasis. Exp Dermatol. 2007;16:779–798. doi: 10.1111/j.1600-0625.2007.00629.x. [DOI] [PubMed] [Google Scholar]
- 10.Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, Bowman EP, Krueger JG. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 11.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, Puig L, Nakagawa H, Spelman L, Sigurgeirsson B, Rivas E, Tsai TF, Wasel N, Tyring S, Salko T, Hampele I, Notter M, Karpov A, Helou S, Papavassilis C ERASURE Study Group, and FIXTURE Study Group. Secukinumab in plaque psoriasis – results of two phase 3 trials. N Engl J Med. 2014;371:326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 12.Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, Braun D, Banerjee S. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 13.Krueger JG, Ferris LK, Menter A, Wagner F, White A, Visvanathan S, Lalovic B, Aslanyan S, Wang EE, Hall D, Solinger A, Padula S, Scholl P. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: Safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116–124. doi: 10.1016/j.jaci.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Papp K, Thaçi D, Reich K, Riedl E, Langley RG, Krueger JG, Gottlieb AB, Nakagawa H, Bowman EP, Mehta A, Li Q, Zhou Y, Shames R. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930–939. doi: 10.1111/bjd.13932. [DOI] [PubMed] [Google Scholar]
- 15.Heydendael VM, Spuls PI, Opmeer BC, de Borgie CA, Reitsma JB, Goldschmidt WF, Bossuyt PM, Bos JD, de Rie MA. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349:658–665. doi: 10.1056/NEJMoa021359. [DOI] [PubMed] [Google Scholar]
- 16.Cather J, Menter A. Novel therapies for psoriasis. Am J Clin Dermatol. 2002;3:159–173. doi: 10.2165/00128071-200203030-00003. [DOI] [PubMed] [Google Scholar]
- 17.Patel RV, Clark LN, Lebwohl M, Weinberg JM. Treatments for psoriasis and the risk of malignancy. J Am Acad Dermatol. 2009;60:1001–1017. doi: 10.1016/j.jaad.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Rizvi S, Chaudhari K, Syed BA. The psoriasis drugs market. Nat Rev Drug Discov. 2015;14:745–746. doi: 10.1038/nrd4763. [DOI] [PubMed] [Google Scholar]
- 19.Gooderham M, Papp K. Apremilast in the treatment of psoriasis and psoriatic arthritis. Skin Therapy Lett. 2015;20:1–6. [PubMed] [Google Scholar]
- 20.Munoz-Elias EJ, Peckham D, Norton K, Duculan J, Cueto I, Li X, Qin J, Lustig K, Tarcha E, Odegard J, Krueger JG, Iadonato SP. Dalazatide (ShK-186), a first-in-class blocker of Kv1.3 potassium channel on effector memory T cells: safety, tolerability and proof of concept of immunomodulation in patients with active plaque psoriasis [abstract] Arthritis Rheumatol. 2015;2015(suppl 10):67. [Google Scholar]
- 21.Matheu MP, Beeton C, Garcia A, Chi V, Rangaraju S, Safrina O, Monaghan K, Uemura MI, Li D, Pal S, de la Maza LM, Monuki E, Flügel A, Pennington MW, Parker I, Chandy KG, Cahalan MD. Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block. Immunity. 2008;29:602–614. doi: 10.1016/j.immuni.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 23.Palamara F, Meindl S, Holcmann M, Luhrs P, Stingl G, Sibilia M. Identification and characterization of pDC-like cells in normal mouse skin and melanomas treated with imiquimod. J Immunol. 2004;173:3051–3061. doi: 10.4049/jimmunol.173.5.3051. [DOI] [PubMed] [Google Scholar]
- 24.Wu JK, Siller G, Strutton G. Psoriasis induced by topical imiquimod. Australas J Dermatol. 2004;45:47–50. doi: 10.1111/j.1440-0960.2004.00030.x. [DOI] [PubMed] [Google Scholar]
- 25.Gilliet M, Conrad C, Geiges M, Cozzio A, Thürlimann W, Burg G, Nestle FO, Dummer R. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch Dermatol. 2004;140:1490–1495. doi: 10.1001/archderm.140.12.1490. [DOI] [PubMed] [Google Scholar]
- 26.Patel U, Mark NM, Machler BC, Levine VJ. Imiquimod 5% cream induced psoriasis: a case report, summary of the literature and mechanism. Br J Dermatol. 2011;164:670–672. doi: 10.1111/j.1365-2133.2010.10124.x. [DOI] [PubMed] [Google Scholar]
- 27.Rajan N, Langtry JA. Generalized exacerbation of psoriasis associated with imiquimod cream treatment of superficial basal cell carcinomas. Clin Exp Dermatol. 2006;31:140–141. doi: 10.1111/j.1365-2230.2005.01938.x. [DOI] [PubMed] [Google Scholar]
- 28.Sun J, Dou W, Zhao Y, Hu J. A comparison of the effects of topical treatment of calcipotriol, camptothecin, clobetasol and tazarotene on an imiquimod-induced psoriasis-like mouse model. Immunopharmacol Immunotoxicol. 2014;36:17–24. doi: 10.3109/08923973.2013.862542. [DOI] [PubMed] [Google Scholar]
- 29.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 30.Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, Facchinetti V, Homey B, Barrat FJ, Zal T, Gilliet M. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med. 2009;206:1983–1994. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang Y, Homey B, Cao W, Wang Y, Su B, Nestle FO, Zal T, Mellman I, Schroder J, Liu Y, Gilliet M. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 32.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 33.Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suarez-Farinas M, Fuentes-Duculan J, Novitskaya I, Khatcherian A, Bluth MJ, Lowes MA, Krueger JG. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204:3183–3194. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vinter H, Iversen L, Steiniche T, Kragballe K, Johansen C. Aldara®-induced skin inflammation: studies of patients with psoriasis. Br J Dermatol. 2015;172:345–353. doi: 10.1111/bjd.13236. [DOI] [PubMed] [Google Scholar]
- 35.Kugyelka R, Kohl Z, Olasz K, Mikecz K, Rauch TA, Glant TT, Boldizsar F. Enigma of IL-17 and Th17 Cells in Rheumatoid Arthritis and in Autoimmune Animal Models of Arthritis. Mediators Inflamm. 2016;2016 doi: 10.1155/2016/6145810. Article ID 6145810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. [Google Scholar]
- 37.Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock. 2004;22:460–466. doi: 10.1097/01.shk.0000142249.08135.e9. [DOI] [PubMed] [Google Scholar]
- 38.Madsen P, Rasmussen HH, Leffers H, Honore B, Celis JE. Molecular cloning and expression of a novel keratinocyte protein (psoriasis-associated fatty acid-binding protein [PA-FABP]) that is highly up-regulated in psoriatic skin and that shares similarity to fatty acid-binding proteins. J Invest Dermatol. 1992;99:299–305. doi: 10.1111/1523-1747.ep12616641. [DOI] [PubMed] [Google Scholar]
- 39.Broome AM, Ryan D, Eckert RL. S100 protein subcellular localization during epidermal differentiation and psoriasis. J Histochem Cytochem. 2003;51:675–685. doi: 10.1177/002215540305100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tobin AM, Lynch L, Kirby B, O’Farrelly C. Natural killer cells in psoriasis. J Innate Immun. 2011;3:403–410. doi: 10.1159/000328011. [DOI] [PubMed] [Google Scholar]
- 41.Campa M, Ryan C, Menter A. An overview of developing TNF-α targeted therapy for the treatment of psoriasis. Expert Opin Investig Drugs. 2015;24:1343–1354. doi: 10.1517/13543784.2015.1076793. [DOI] [PubMed] [Google Scholar]