Supplemental Digital Content is available in the text.
Keywords: invasive ventilation, mortality, noninvasive ventilation, pediatrics, pediatric intensive care unit
Abstract
Objectives:
To compare outcomes of children receiving noninvasive ventilation with those receiving invasive ventilation as first-line mode of mechanical ventilation following unplanned intensive care admission.
Design:
Propensity score-matched cohort study analyzing data prospectively collected by the Pediatric Intensive Care Audit Network over 8 years (2007–2014).
Setting:
Thirty-one PICUs in the United Kingdom and Ireland; twenty-one of whom submitted Pediatric Critical Care Minimum Dataset data for the entire study period.
Patients:
Children consecutively admitted to study PICUs. Planned admissions following surgery, unplanned admissions from other hospitals, those on chronic ventilation, and those who did not receive mechanical ventilation on the day of PICU admission were excluded.
Interventions:
Use of noninvasive ventilation, rather than invasive ventilation, as the first-line mode of mechanical ventilation.
Measurements and Main Results:
PICU mortality, length of ventilation, length of PICU stay, and ventilator-free days at day 28. During the study period, there were 151,128 PICU admissions. A total of 15,144 admissions (10%) were eligible for analysis once predefined exclusion criteria were applied: 4,804 (31.7%) received “noninvasive ventilation first,” whereas 10,221 (67.5%) received “invasive ventilation first”; 119 (0.8%) admissions could not be classified. Admitting PICU site explained 6.5% of the variation in first-line mechanical ventilation group (95% CI, 2.0–19.0%). In propensity score-matched analyses, receiving noninvasive ventilation first was associated with a significant reduction in mortality by 3.1% (95% CI, 1.7–4.6%), length of ventilation by 1.6 days (95% CI, 1.0–2.3), and length of PICU stay by 2.1 days (95% CI, 1.3–3.0), as well as an increase in ventilator-free days at day 28 by 3.7 days (95% CI, 3.1–4.3).
Conclusions:
Use of noninvasive ventilation as first-line mode of mechanical ventilation in critically ill children admitted to PICU in an unplanned fashion may be associated with significant clinical benefits. Further high-quality evidence regarding optimal patient selection and timing of initiation of noninvasive ventilation could lead to less variability in clinical care between institutions and improved patient outcomes.
Invasive ventilation (IV), delivered through an endotracheal tube, has long been the mainstay of ventilator management in PICU worldwide (1). Randomized controlled trials (RCTs) in adults with chronic obstructive pulmonary disease and cardiogenic pulmonary edema, and in premature newborns with respiratory distress, have demonstrated that noninvasive ventilation (NIV) modalities such as continuous or bi-level positive airway pressure can reduce the need for endotracheal intubation and improve patient outcomes (2–6). However, there is a paucity of RCT evidence in critically ill children: just one clinical trial including only 50 patients, and three other small RCTs restricted to specific conditions such as bronchiolitis, asthma, and dengue shock syndrome exist (7–10). Despite this scarcity of evidence, national audit data from PICUs in the United Kingdom and Ireland have shown increasing use of NIV over the past 10 years (11), mirroring an international trend of greater adoption of NIV (12, 13).
The main benefits of NIV are that it may avoid several of the inherent risks associated with intubation such as upper airway trauma, postextubation vocal cord dysfunction, requirement for heavy sedation, and ventilator-associated complications such as pneumonia, barotrauma, or volutrauma to the lung (14, 15). On the other hand, a delay in intubation in a deteriorating patient on NIV itself appears to be an independent risk factor for mortality (16, 17). As such, the decision of whether to use NIV or IV as the first-line mode of mechanical ventilation (MV) is currently left to the discretion of the treating clinician. Although the choice of NIV or IV may be clear-cut in some patient groups, there is little evidence as to which mode of ventilation produces the most favorable outcomes in the majority of the remaining cases.
In the absence of RCT evidence confirming whether IV or NIV is the best initial mode of ventilation in the acute setting, we aimed to perform propensity score matching (PSM) analysis using a large observational dataset to compare patient outcomes for these two groups. PSM is a powerful, statistical, matching approach that allows the creation of quasi-randomized trial conditions to facilitate direct comparison of treatment groups (18, 19) and has produced results that are generally consistent with RCT findings across a diverse range of critical care topics (20–25).
METHODS
We performed a PSM cohort study utilizing prospectively collected data from the Pediatric Intensive Care Audit Network (PICANet) clinical audit database. A core admission dataset has been collected by PICANet on every admission to U.K. and Ireland PICUs since January 2004, containing clinical and demographic data (26, 27). An additional dataset, the Pediatric Critical Care Minimum Dataset (PCCMDS), has been collected for most PICUs since January 2007, on daily interventions for each patient. Data quality is ensured by regular training of staff, and with local and central validation checks (28). PICANet has approval to collect personally identifiable data under special circumstances from the Health Research Authority Confidentiality Advisory Group (ref: PIAG 4–07(c)/2002) and approval from the Trent Medical Research Ethics Committee (ref: 05/MRE04/17).
Data
Our intention was to study only those patients who theoretically could have received either NIV or IV as first-line therapy at PICU admission. Therefore, we extracted data only for children (< 16 yr old) admitted to PICU during the 8-year period, January 2007 to December 2014, who received either NIV and/or IV on the calendar day of PICU admission, and applied a series of exclusion criteria to restrict the sample (Fig. 1). Postoperative admissions, elective admissions, and emergency admissions from another hospital were excluded due to the greater likelihood of IV during surgery, for elective procedures, or during transport, which may have led to bias in the exposure status. Additionally, patients with a tracheostomy on admission and those receiving chronic ventilation prior to PICU admission were excluded. All remaining individuals were then classified into one of three groups, based on which type of ventilation they received first: “NIV-first,” “IV-first,” or “unable to classify.” In cases where both types of MV were recorded on the calendar day of PICU admission, we checked MV status on the next calendar day; if only IV was recorded on the next calendar day, patients were classified as NIV-first, and if only NIV was recorded on the next calendar day, patients were classified as IV-first.
Figure 1.
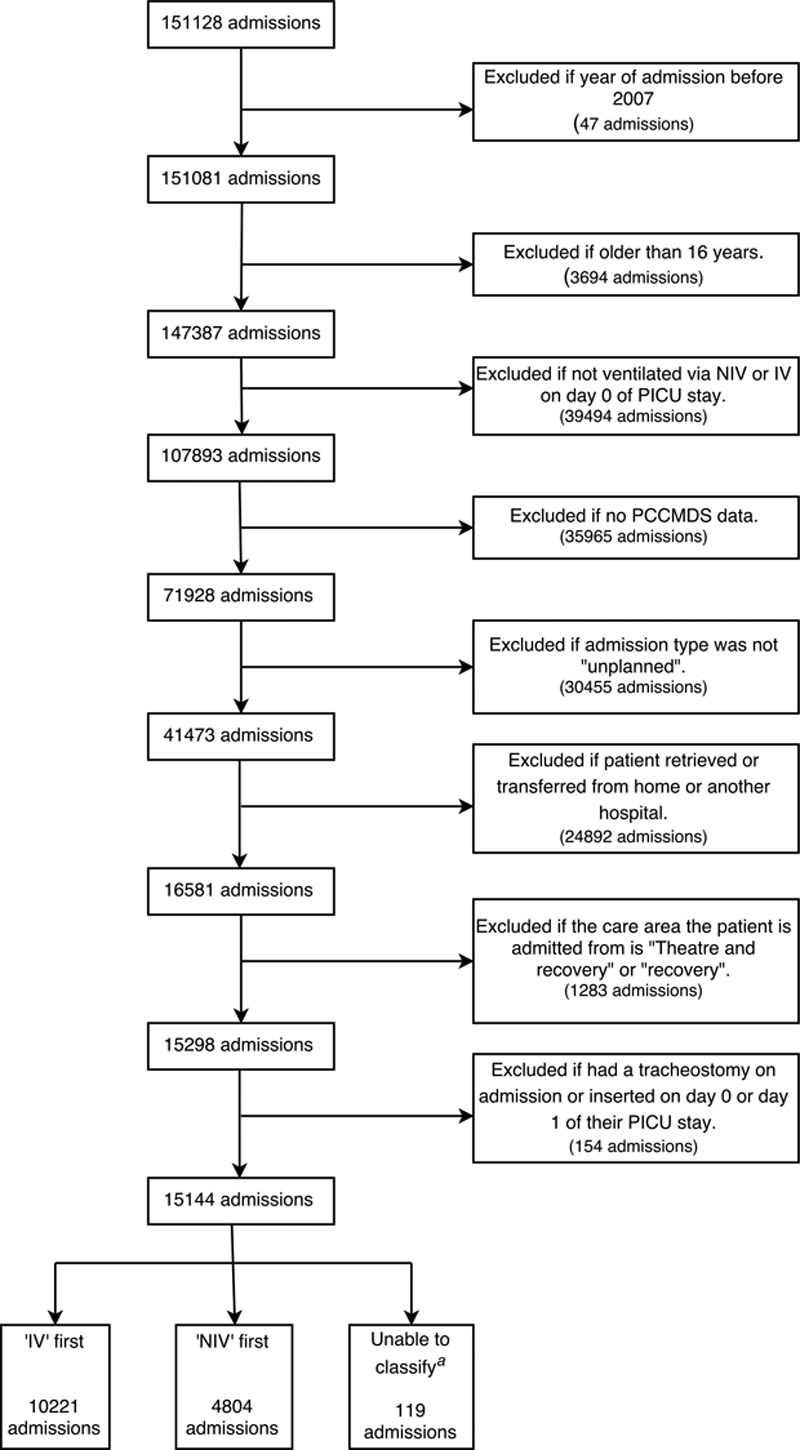
Flowchart detailing sample selection criteria. aIt was not possible to classify patients who received both noninvasive ventilation (NIV) and invasive ventilation (IV) on the day of admission, as well as the subsequent day, according to Paediatric Critical Care Minimum Dataset (PCCMDS) daily data.
Outcomes
The outcome variables were PICU mortality, length of ventilation (LOV), PICU length of stay (LOS), and ventilator-free days at 28 days (VFD-28). Participants’ VFD-28 was calculated as 28-X, where X was the number of days spent receiving MV. Patients requiring MV for greater than or equal to 28 days and those who died within 28 days of PICU admission were assigned a VFD-28 of zero (29). We performed a subgroup analysis to study outcomes in NIV-first children who failed NIV. “NIV failure” was defined as receiving IV the same or subsequent day of last receiving NIV.
Statistical Analysis
Analysis 1: Whole Cohort. Patient Characteristics Associated With Use of NIV as First-Line Treatment.
Descriptive statistics were calculated to explore differences between MV groups. Multilevel logistic regression analysis was carried out to investigate patient characteristics associated with the choice of first-line MV modality, as well as to quantify the effect of PICU site on choice of MV modality. Since Pediatric Index of Mortality (PIM)-2, the Severity of Illness Score used in U.K. PICUs, is calculated using a number of physiologic variables, including if the child is receiving MV within the first hour of PICU admission (30), the individual elements of the score (excluding the variable related to MV) were used rather than the calculated PIM-2 score. The multilevel model was created using backward elimination of covariates, and the model with the lowest Akaike’s Information Criteria value chosen as the final model (31).
Analysis 1: Whole Cohort. Association Between MV Modality and Patient Outcome.
All outcome variables were initially investigated for differences between MV modalities across the whole cohort.
Analysis 1: Whole Cohort. Propensity Score (PS) Estimates.
Several PS estimates were then created, and covariate bias was compared between estimates, to find the estimates that removed systematic differences in the covariate bias between NIV-first and IV-first patients (32).
Analysis 2a: PSM Sample.
The primary analysis was PSM, which used nearest-neighbor matching of the logit of the PS using caliper widths equal to 0.2 of the pooled sd of the logit of the PS, not allowing replacement. The “PS” is defined as the probability of treatment group assignment conditional on observed baseline covariates (18, 19). Therefore, this meant only one IV case could be matched with each NIV case, who theoretically had an equal chance of receiving NIV or IV based on key characteristics (19, 33). The PSM analysis compared patients receiving NIV-first with IV-first (baseline group) across all outcomes.
Analysis 2b: Regression Adjustment (RA) Sample.
Additionally, we performed RA using the PS and assigned treatment group (18, 34). This method allows a regression model to be specified. Logistic regression was used to investigate mortality, whereas Poisson regression was used to investigate LOV, LOS, and VFDs. RA was carried out on all patients with a calculated PS to allow comparison with other study findings and to retain a larger sample size. Participants “excluded” from PSM without a match in the opposing treatment group were therefore included in the RA sample.
Power Analysis.
Sample size calculations indicated that a total sample of 4,650 participants (3,100 IV and 1,550 NIV) would be sufficient to detect a significant increase in mortality by 2%, an increase in mean LOV and LOS by 1 day, and a decrease in mean VFD-28 by 1 day in IV-first compared to NIV-first patients with 90% power. This sample size assumes a ratio of two IV admissions to one NIV admission and uses previous mean outcome statistics for NIV and IV patients (35).
RESULTS
PICANet data were available on 151,128 consecutive admissions to 31 PICUs between January 2007 and December 2014. Twenty-one of these PICUs submitted data across the whole 8-year period. The remaining 10 varied from submitting 1 year of data (two PICUs) to 7 years of data (three PICUs). A total of 15,144 admissions (10%) met the study inclusion criteria (Fig. 1).
Patients
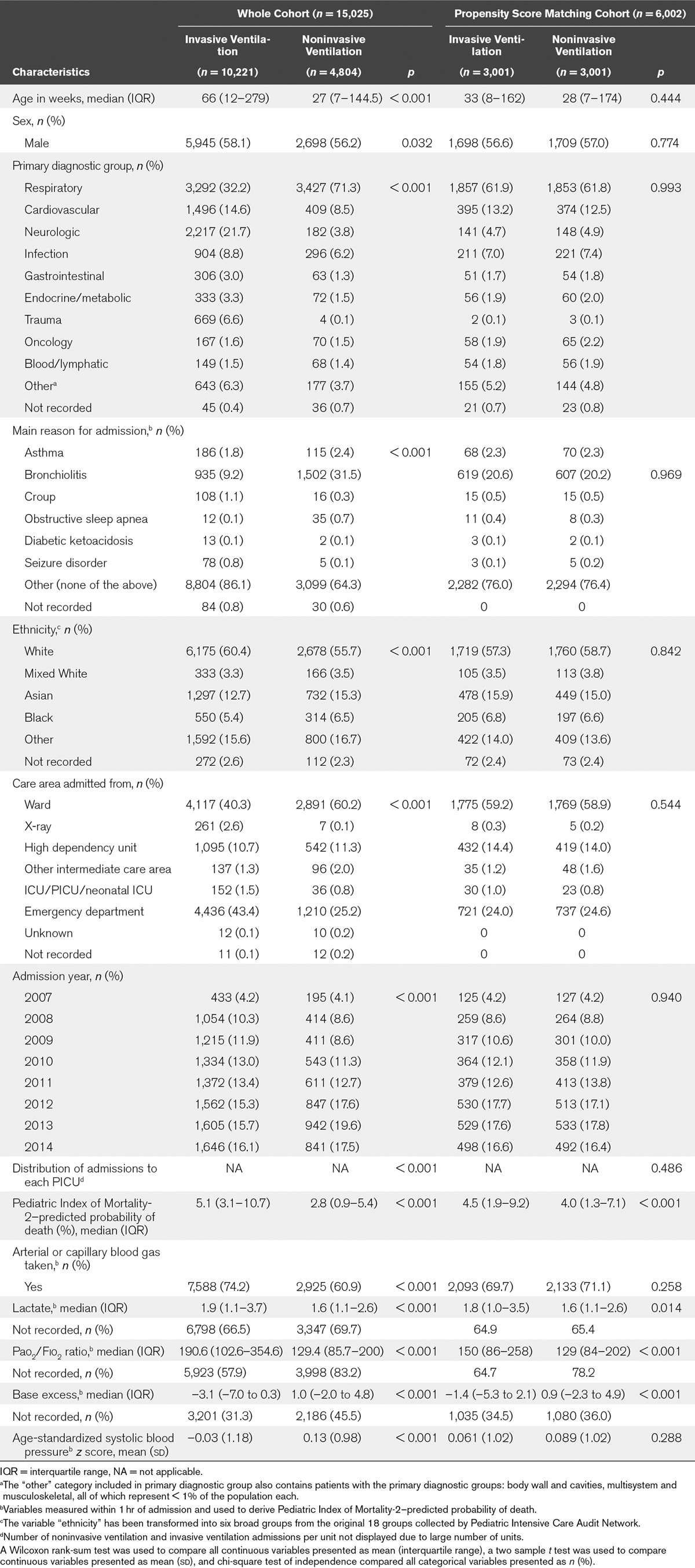
It was possible to classify 99.2% of admissions in the restricted sample into one of the two groups: NIV-first or IV-first. NIV was used as the first-line therapy in 4,804 patients (32%), whereas 10,221 patients (68%) received IV-first. Those admissions that could not be classified (119/15,144; 0.8%) were not analyzed further. Cohort demographics are described in Table 1.
TABLE 1.
Patient Demographic and Clinical Characteristics of the Whole Cohort (n = 15,025) and Propensity Score-Matched Cohort (n = 6,002)
Analysis 1: Whole Cohort (n = 15,025)
Patient Characteristics Associated With Choice of First-Line MV Modality.
Patient characteristics were significantly different between the MV groups. In particular, IV-first patients had a significantly higher admission severity of illness (median PIM-2 score, 5.1% vs 2.8%), serum lactate (median, 1.9 vs 1.6), and were more likely to have an arterial Pao2/Fio2 value recorded (42.1% vs 16.8%) compared to NIV-first patients (Table 1). In those patients who did have a Pao2/Fio2 ratio recorded, NIV-first patients had a lower Pao2/Fio2 value than IV-first patients.
Multilevel logistic regression revealed that PICU site explained 6.5% of the variation in first-line MV group (95% CI, 2.0–19.0%). The care area admitted from, primary diagnostic group, systolic blood pressure (SBP), base excess (BE), BE polarity (positive or negative), and Pao2/Fio2 ratio measured within 1 hour of PICU admission were all associated with choice of MV modality (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCM/C531). Receiver operating characteristic (ROC) analysis calculated the area under the ROC curve for this model in predicting use of IV-first to be 0.85 (95% CI, 0.83–0.88), for the 1,657 patients with values for all of the covariates included in the model.
Comparing Outcomes for NIV-First and IV-First Admissions.
Crude mortality rate was significantly lower in patients receiving NIV-first (Table 2) compared to patients who received IV-first (4.4% vs 9.6%; chi-square test p < 0.001). LOV was also significantly different between NIV-first and IV-first patients (median [interquartile range (IQR)], 4 [2–7] vs 4 [2–7]; p < 0.001), as well as LOS (median [IQR], 5 [2–9] vs 5 [3–8]; p < 0.001). Patients receiving NIV-first also had significantly greater VFD-28 than those patients receiving IV-first (median [IQR], 12 [0–22] vs 8 [0–24]; p = 0.016). The NIV failure rate of those receiving NIV-first was 25.7% (1,237 admissions). The crude outcomes for those participants who failed NIV compared to those who succeeded on NIV were mortality of 10.2% versus 2.1% (p < 0.001), median LOV of 8 (IQR, 5–14) versus 3 (IQR, 2–5) (p < 0.001), median LOS of 9 (IQR, 6–16) versus 4 (IQR, 3–6) (p < 0.001), and median VFD-28 of 0 (IQR, 0–0) versus 16 (IQR, 0–22) (p < 0.001). The median VFD-28 of NIV failure admissions remained when patients who had died within 28 days of admission were excluded.
TABLE 2.
Crude Outcomes for Patients Included in the Whole Cohort (n = 15,025) and Propensity Score-Matched Cohort (n = 6,002)
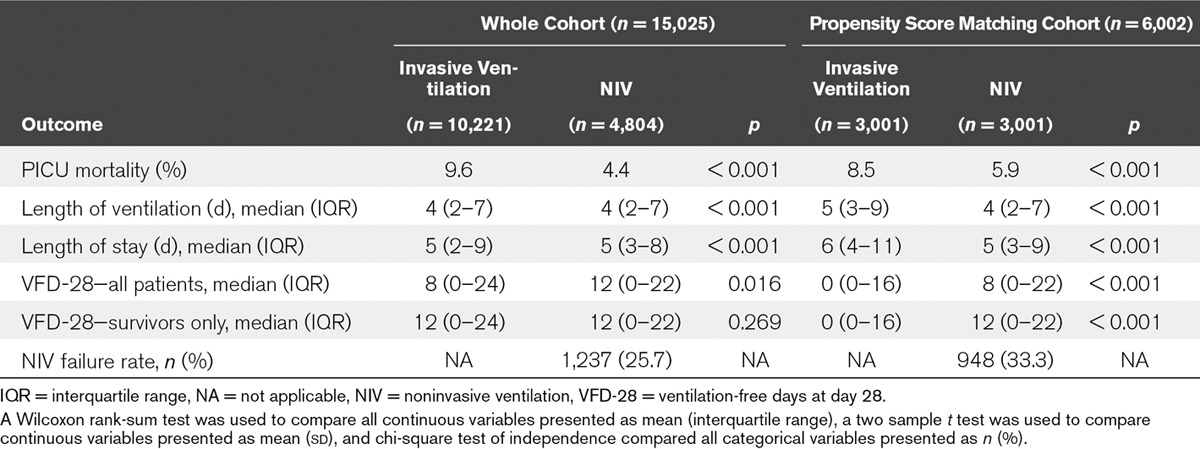
LOV, LOS, and VFD-28 were significantly different in those patients who failed NIV compared to those patients receiving IV-first (p < 0.001). However, there was no difference in mortality (p = 0.503).
PS Estimates.
PS estimates were created for 13,189 patients, who had recorded values for all of the matching variables (3,900 NIV and 9,289 IV). The final PSs adjusted for admitting PICU, primary diagnostic group, presence of a low-risk PIM-2 diagnosis, ethnicity, whether a blood gas (arterial or capillary) was measured within 1 hour of admission, the care area from which the child was admitted, age-standardized SBP z score, admission year, age, and sex. Of these 13,189 patients, 899 NIV-first patients did not have an IV-first match, and 6,288 IV-first patients did not have an NIV-first match, within the specified caliper distance. The NIV-first and IV-first patients thus excluded from the PSM analysis had significantly different PICU mortality rates (2.1% vs 9.8%; p < 0.001). Figure 2 illustrates the distribution of PSs by MV modality.
Figure 2.
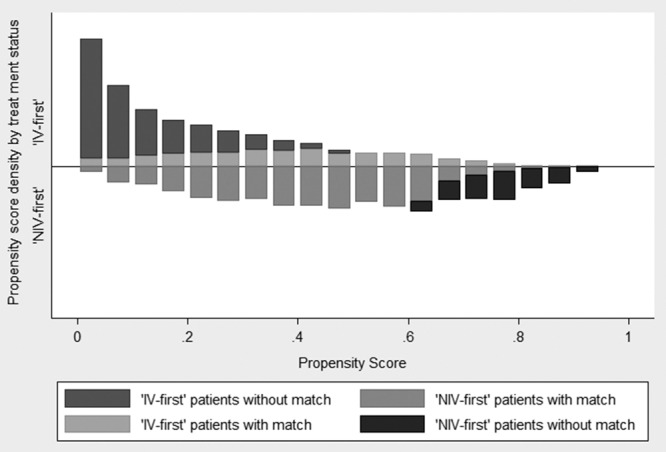
Distribution of the calculated propensity scores for noninvasive ventilation (NIV)-first and invasive ventilation (IV)-first patients, with or without a match (n = 13,189).
Analysis 2a: PSM Sample (n = 6,002)
The PSM analysis used 6,002 children (3,001 NIV and 3,001 IV). Their characteristics and crude outcomes are compared in Tables 1 and 2, and demonstrate that PSM matching produced comparable groups in terms of baseline variables. PSM analysis summarized in Table 3 found that receiving NIV-first was associated with a significant decrease in mortality by 3.1% (95% CI, 1.7–4.6%) to 5.4% compared to 8.5% in IV-first patients. LOV decreased by 1.6 days (95% CI, 1.0–2.3) from 8.4 to 6.8 days, and there was an associated decrease in LOS by 2.1 days (95% CI, 1.3–3.0) from 10.7 to 8.6 days in NIV-first patients. Use of NIV-first was associated with an increase in VFD-28 by 3.7 days (95% CI, 3.1–4.3) to 10.8 days compared to 7.1 days in IV-first patients.
TABLE 3.
Results of Analyses Comparing Patients Receiving Noninvasive Ventilation First With the Control Group (Invasive Ventilation First), Across Four Patient Outcomes, Using Both Propensity Score Matching (n = 6,002) and Regression Adjustment (n = 13,189)
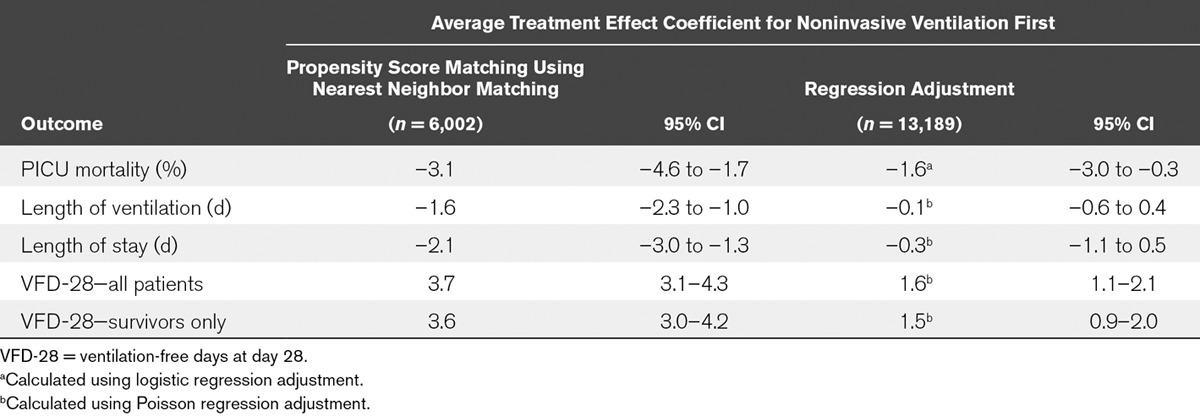
Analysis 2b: RA Sample (n = 13,189)
Similar results were obtained from the RA using the full sample of 13,189 patients in whom PSs were calculated. The use of NIV-first was associated with a significant decrease in mortality by 1.6% (95% CI, 0.3–3.0), a decrease in LOV by 0.1 days (95% CI, –0.6 to 0.4) and LOS by 0.3 days (95% CI, –1.1 to 0.5), and a significant increase in VFD-28 by 1.6 days (95% CI, 1.1–2.1) (Table 3).
In both PSM and RA, a significant increase in VFD-28 of NIV-first remained when patients who had died within 28 days of admission were excluded. In PSM, a significant increase in VFD-28 by 3.6 days was reported (95% CI, 3.0–4.2), whereas RA found a significant increase of 1.5 days (95% CI, 0.9–2.0) using the sample of 13,189 patients.
DISCUSSION
In this large study of critically ill children admitted to U.K. PICUs, we have identified several important clinical factors associated with the use of NIV as the first-line mode of MV. As well as significant inter-unit variability in the use of NIV, PSM analysis suggests that receiving NIV-first is associated with a significant reduction in mortality, LOV, LOS, and a significant increase in VFD-28. We recognize that these findings are only applicable to those patients for whom NIV is a clinically appropriate first-line option.
To our knowledge, this is the first study to compare the benefits of NIV versus IV as first-line treatment in a large cohort of critically ill children. While not an RCT, the use of PSM to analyze a national high-quality, observational dataset covering an 8-year period allows our findings to be generalized to other similar developed healthcare systems. Additionally, PSM analysis comparing NIV and IV in adult ICU patients reported findings consistent with RCTs conducted on the same topic (20–25). In our study, when the unmatched cohorts were compared, IV-first patients were considerably sicker at admission and had worse outcomes. PSM allowed the two groups to be well matched across a range of key covariates, with better outcomes seen in patients treated with NIV-first.
Characteristics that influenced the choice of first-line MV type in our study are consistent with previous studies, which identified differences between NIV and IV patients in terms of illness severity, the ratio of oxygen saturation to Fio2 (Spo2/Fio2), age, sex, whether a blood gas was taken (36–39), and variability between hospitals in the use of NIV in critically ill children (39). In our study, admitting PICU explained 6.5% of the variation in NIV or IV use, although the upper CI reached 19.0%. Our findings show that patients admitted to PICU in a more severe clinical status are more likely to receive IV so as not to delay intubation. Similarly, the presence of acute respiratory distress syndrome, indicated by a Pao2/Fio2 ratio of less than 200, has been previously shown to be a strong risk factor for NIV failure (40, 41).
Several previous small observational studies support our findings of patient benefit with the use of NIV. In a study comparing two distinct 5-year epochs in one PICU, Essouri et al (42) showed that LOV and PICU LOS decreased significantly when NIV was introduced as the main mode of MV in children with acute bronchiolitis. Similarly, a comparison of two units (one delivering IV only and the other predominantly NIV) showed that the LOV for infants with bronchiolitis was significantly shorter with NIV use (36). A retrospective analysis of bronchiolitis admissions at a single center in Australia over a 10-year period showed that PICU LOS was nearly halved with the use of NIV (43). Similar data have been reported for acute asthma (39). However, not all studies adjusted for confounding factors, and the lack of multicenter involvement prevents widespread generalizability of their findings. Findings from the few RCTs available on this topic in critically ill children do support the premise of improved outcomes for selected patients treated with NIV in preference to IV (7–10).
Our sample included data on almost all PICU admissions in the United Kingdom and Ireland, and therefore had sufficient power to detect clinically significant differences across all four outcomes. Furthermore, by excluding patients in whom PICU clinicians could not choose the first-line MV mode (planned admissions, unplanned admissions from other hospitals, patients on chronic ventilation, and those with a tracheostomy), we focused on those patients in whom our findings can be applied in the future. Several limitations of this study must also be noted: PSM can only match on measured variables and therefore cannot eliminate all potential confounding bias. PSM also reduced the sample to a smaller subset of the overall cohort, restricting the generalizability of our findings. Selection bias may be present as only 21 of 31 PICUs submitted PCCMDS data for the entire 8-year study period, and although PICANet stipulated that high-flow therapy should be classified as “supplemental oxygen,” some units may have miscoded it as NIV. Potential confounding bias may have arisen from variables with multiple unrecorded values. This was particularly evident with the Pao2/Fio2 ratio (missing in 83% of NIV-first patients). The unexpectedly lower value in the NIV-first group may be explained by the fact that only sicker patients were likely to have arterial catheters in this group. It was not appropriate to use multiple imputation to predict unrecorded values, as these values were not missing at random.
In addition, the study did not address other research questions such as complications of NIV use, nor does it clarify the reasons behind significant inter-unit variability or allow our findings to be easily generalized to postoperative patients or transported admissions.
CONCLUSIONS
The use of acute NIV (rather than IV) as first-line MV therapy may be associated with a significant decrease in mortality, LOV, and LOS, as well as an increase in the number of VFD-28. Admitting PICU has a strong association with which type of MV is used first. Variation in the use of NIV and IV between PICUs may therefore directly influence clinical outcomes. Prospective clinical trials conducted through international collaborative networks and research-driven clinical guidelines are urgently needed to guide future NIV practice in critically ill children.
Supplementary Material
Footnotes
*See also p. 1103.
All statistical analysis took place at the University of Leeds, Leeds, United Kingdom.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Barry P, Morris K, Ali T. Oxford Speciality Handbook in Paediatric Intensive Care. 2010Oxford: Oxford University Press. [Google Scholar]
- 2.Nava S, Carbone G, DiBattista N, et al. Noninvasive ventilation in cardiogenic pulmonary edema: A multicenter randomized trial. Am J Respir Crit Care Med 2003; 168:1432–1437. [DOI] [PubMed] [Google Scholar]
- 3.Lightowler JV, Wedzicha JA, Elliott MW, et al. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ 2003; 326:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plant PK, Owen JL, Elliott MW. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: A multicentre randomised controlled trial. Lancet 2000; 355:1931–1935. [DOI] [PubMed] [Google Scholar]
- 5.Antonelli M, Conti G, Rocco M, et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med 1998; 339:429–435. [DOI] [PubMed] [Google Scholar]
- 6.Ho JJ, Subramaniam P, Davis PG. Continuous distending pressure for respiratory distress in preterm infants. Cochrane Database Syst Rev 2015; 7:CD002271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milési C, Matecki S, Jaber S, et al. 6 cmH2O continuous positive airway pressure versus conventional oxygen therapy in severe viral bronchiolitis: A randomized trial. Pediatr Pulmonol 2013; 48:45–51. [DOI] [PubMed] [Google Scholar]
- 8.Yañez LJ, Yunge M, Emilfork M, et al. A prospective, randomized, controlled trial of noninvasive ventilation in pediatric acute respiratory failure. Pediatr Crit Care Med 2008; 9:484–489. [DOI] [PubMed] [Google Scholar]
- 9.Cam BV, Tuan DT, Fonsmark L, et al. Randomized comparison of oxygen mask treatment vs. nasal continuous positive airway pressure in dengue shock syndrome with acute respiratory failure. J Trop Pediatr 2002; 48:335–339. [DOI] [PubMed] [Google Scholar]
- 10.Basnet S, Mander G, Andoh J, et al. Safety, efficacy, and tolerability of early initiation of noninvasive positive pressure ventilation in pediatric patients admitted with status asthmaticus: A pilot study. Pediatr Crit Care Med 2012; 13:393–398. [DOI] [PubMed] [Google Scholar]
- 11.Paediatric Intensive Care Audit Network: A Decade of Data. Universities of Leeds and Leicester. 2014. Available at: http://www.picanet.org.uk/Audit/Annual-Reporting/PICANet_A_Decade_of_Data_2014_Annual_Report_Summary.pdf. Accessed March 1, 2015
- 12.Ducharme-Crevier L, Essouri S, Emeriaud G. Noninvasive ventilation in pediatric intensive care: From a promising to an established therapy, but for whom, when, why, and how? Pediatr Crit Care Med 2015; 16:481–482. [DOI] [PubMed] [Google Scholar]
- 13.Wolfler A, Calderini E, Iannella E, et al. ; Network of Pediatric Intensive Care Unit Study Group: Evolution of noninvasive mechanical ventilation use: A cohort study among Italian PICUs. Pediatr Crit Care Med 2015; 16:418–427. [DOI] [PubMed] [Google Scholar]
- 14.Dohna-Schwake C, Stehling F, Tschiedel E, et al. Non-invasive ventilation on a pediatric intensive care unit: Feasibility, efficacy, and predictors of success. Pediatr Pulmonol 2011; 46:1114–1120. [DOI] [PubMed] [Google Scholar]
- 15.Deis JN, Abramo TJ, Crawley L. Noninvasive respiratory support. Pediatr Emerg Care 2008; 24:331–338; quiz 339. [DOI] [PubMed] [Google Scholar]
- 16.Demoule A, Girou E, Richard JC, et al. Increased use of noninvasive ventilation in French intensive care units. Intensive Care Med 2006; 32:1747–1755. [DOI] [PubMed] [Google Scholar]
- 17.Carrillo A, Gonzalez-Diaz G, Ferrer M, et al. Non-invasive ventilation in community-acquired pneumonia and severe acute respiratory failure. Intensive Care Med 2012; 38:458–466. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70:41–55. [Google Scholar]
- 20.Kitsios GD, Dahabreh IJ, Callahan S, et al. Can we trust observational studies using propensity scores in the critical care literature? A systematic comparison with randomized clinical trials. Crit Care Med 2015; 43:1870–1879. [DOI] [PubMed] [Google Scholar]
- 21.Girou E, Brun-Buisson C, Taillé S, et al. Secular trends in nosocomial infections and mortality associated with noninvasive ventilation in patients with exacerbation of COPD and pulmonary edema. JAMA 2003; 290:2985–2991. [DOI] [PubMed] [Google Scholar]
- 22.Antonelli M, Conti G, Pelosi P, et al. New treatment of acute hypoxemic respiratory failure: Noninvasive pressure support ventilation delivered by helmet–a pilot controlled trial. Crit Care Med 2002; 30:602–608. [DOI] [PubMed] [Google Scholar]
- 23.Honrubia T, García López FJ, Franco N, et al. Noninvasive vs conventional mechanical ventilation in acute respiratory failure: A multicenter, randomized controlled trial. Chest 2005; 128:3916–3924. [DOI] [PubMed] [Google Scholar]
- 24.Matic I, Sakic-Zdravcevic K, Jurjevic M. Comparison of invasive and noninvasive mechanical ventilation for patients with chronic obstructive pulmonary disease: Randomized prospective study. Period Biol 2007; 109:137–145. [Google Scholar]
- 25.Jurjević M, Matić I, Sakić-Zdravcević K, et al. Mechanical ventilation in chronic obstructive pulmonary disease patients, noninvasive vs. invasive method (randomized prospective study). Coll Antropol 2009; 33:791–797. [PubMed] [Google Scholar]
- 26.Paediatric Intensive Care Audit Network Annual Report 2010 - 2012. Universities of Leeds and Leicester. 2013. Available at: http://www.picanet.org.uk/Audit/Annual-Reporting/Annual-Report-Archive/PICANet_Annual_Report_2013_Summary.pdf. Accessed March 1, 2015.
- 27.Slater A, Shann F, Pearson G; Paediatric Index of Mortality (PIM) Study Group: PIM2: A revised version of the Paediatric Index of Mortality. Intensive Care Med 2003; 29:278–285. [DOI] [PubMed] [Google Scholar]
- 28.Ramnarayan P, Thiru K, Parslow RC, et al. Effect of specialist retrieval teams on outcomes in children admitted to paediatric intensive care units in England and Wales: A retrospective cohort study. Lancet 2010; 376:698–704. [DOI] [PubMed] [Google Scholar]
- 29.Schoenfeld DA, Bernard GR; ARDS Network: Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med 2002; 30:1772–1777. [DOI] [PubMed] [Google Scholar]
- 30.Shann F, Pearson G, Slater A, et al. Paediatric Index of Mortality (PIM): A mortality prediction model for children in intensive care. Intensive Care Med 1997; 23:201–207. [DOI] [PubMed] [Google Scholar]
- 31.Vrieze SI. Model selection and psychological theory: A discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol Methods 2012; 17:228–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starks H, Garrido MM; Observational and Quasi-Experimental Research Methods. National Palliative Care Research Center (NSCRC) [Presentation] 2014. October 20, 2014. Available at: http://www.npcrc.org/files/NPCRC.Observational-PropensityScoreMethodsWkshop.10-20-14.pdf. Accessed March 12, 2015
- 33.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011; 10:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.StataCorp: Stata Treatment-Effects Reference Manual. 2013College Station, TX, StataCorp LP. [Google Scholar]
- 35.Macrae D, Grieve R, Allen E, et al. ; CHiP Investigators: A randomized trial of hyperglycemic control in pediatric intensive care. N Engl J Med 2014; 370:107–118. [DOI] [PubMed] [Google Scholar]
- 36.Borckink I, Essouri S, Laurent M, et al. Infants with severe respiratory syncytial virus needed less ventilator time with nasal continuous airways pressure then invasive mechanical ventilation. Acta Paediatr 2014; 103:81–85. [DOI] [PubMed] [Google Scholar]
- 37.Pancera CF, Hayashi M, Fregnani JH, et al. Noninvasive ventilation in immunocompromised pediatric patients: Eight years of experience in a pediatric oncology intensive care unit. J Pediatr Hematol Oncol 2008; 30:533–538. [DOI] [PubMed] [Google Scholar]
- 38.Lazner MR, Basu AP, Klonin H. Non-invasive ventilation for severe bronchiolitis: Analysis and evidence. Pediatr Pulmonol 2012; 47:909–916. [DOI] [PubMed] [Google Scholar]
- 39.Bratton SL, Newth CJ, Zuppa AF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network: Critical care for pediatric asthma: Wide care variability and challenges for study. Pediatr Crit Care Med 2012; 13:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayordomo-Colunga J, Pons M, López Y, et al. Predicting non-invasive ventilation failure in children from the SpO2/FiO2 (SF) ratio. Intensive Care Med 2013; 39:1095–1103. [DOI] [PubMed] [Google Scholar]
- 41.Muñoz-Bonet JI, Flor-Macián EM, Brines J, et al. Predictive factors for the outcome of noninvasive ventilation in pediatric acute respiratory failure. Pediatr Crit Care Med 2010; 11:675–680. [DOI] [PubMed] [Google Scholar]
- 42.Essouri S, Laurent M, Chevret L, et al. Improved clinical and economic outcomes in severe bronchiolitis with pre-emptive nCPAP ventilatory strategy. Intensive Care Med 2014; 40:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganu SS, Gautam A, Wilkins B, et al. Increase in use of non-invasive ventilation for infants with severe bronchiolitis is associated with decline in intubation rates over a decade. Intensive Care Med 2012; 38:1177–1183. [DOI] [PubMed] [Google Scholar]


