Abstract
Occupational medical surveillance is highly desirable in manufacturing facilities where exposure to chemicals is significant. The insecticide fipronil is generally considered safe for humans but with increasing use, exposure to fipronil is of concern. Identification of urinary metabolites of fipronil may allow development of affordable, cheap and rapid procedures for human exposure evaluation. In this study we developed a fast and easy approach for synthesis of hydroxy-fipronil, a potential urinary metabolite of fipronil. This standard was used to develop a sensitive analytical LC-MS/MS method with a limit of quantification (LOQ) of 0.4 ng/mL. Fipronil sulfone, a known metabolite, and hydroxy-fipronil were quantified in urine samples from rats treated with a fipronil containing diet. Fipronil sulfone concentration centered around 20 ng/mL, while the concentration of hydroxy-fipronil was dose-dependent ranging in 10–10 000 ng/mL and thus being a more sensitive marker of fipronil exposure. A fipronil immunoassay with cross-reactivity to hydroxy-fipronil showed a good correlation in signal intensity with LC-MS data. It was also used to demonstrate the applicability of the method for sample screening in the evaluation of exposure levels.
Keywords: insecticide, GABA receptor bioassay, metabolite, LC/MS, antibody, immunoassay, screening, sensor
Introduction
Fipronil is a broad spectrum insecticide from the phenylpyrazole family. It is often used in four major domains: pest control in a wide variety of field crops, urban pest management, veterinary applications (especially in topical pet care products), and to control rice water weevil infestation in rice-crawfish rotation (EPA 2011; Simon-Delso et al. 2015; Watts 2012). Fipronil is a neurotoxic compound acting on invertebrate GABA-gated chloride channels in the central nervous system and thus has found a wide application mainly because the target insects have not developed resistance to the pesticide. Fipronil is also relatively persistent in the environment providing long-term protection, with half-life (t1/2) over 100 days in certain conditions (Gunasekara et al. 2007). A recent report by the U.S. Environmental Protection Agency (EPA) indicated a significant increase in the amount of fipronil used between 1998 and 2008 (Simon-Delso et al. 2015).
With increasing amounts of insecticide use, human exposure to fipronil is of greater concern, in particular in the case of people directly working with the chemical at manufacturing plants and those applying it in the fields and other corresponding sites. In addition to occupational exposure, the general population is also exposed to fipronil through household uses of fipronil. In particular, human exposure may occur through application of fipronil to animals and interaction with pets. Animals may spread fipronil residues by rubbing against carpet and furniture, suggesting subchronic or chronic exposure in the general population (Cochran et al. 2015). Although, fipronil is accepted to be relatively safe for humans because of its lower binding affinity to vertebrate GABA-gated chloride channels (Hainzl et al. 1998), it has been recently demonstrated that exposure to micromolar concentrations of fipronil induced cell death (Vidau et al. 2009; Vidau et al. 2011). Interestingly, cytotoxic effects of fipronil were shown to be stronger with lower micromolar concentrations and less pronounced with higher concentrations (Das et al. 2006), indicating that continuous exposure to low levels of fipronil as in the case of occupational exposure may have a pronounced impact on human health. A number of studies with animals showed that continuous exposure to fipronil leads to significant hepatic effects (e.g. periacinar hypertrophy) in mice and rats even at low doses. Mice showed reduced bodyweight gains at the high doses tested (4.5 mg/kg body weight (bw)/day for 13 weeks) (Hamernik 1998). Rats, in particular females, showed a significant increase in liver weight following treatment with fipronil even with low doses (3.4 mg/kg bw) (Hamernik 1998). Dogs treated with a diet containing low fipronil doses (up to 3 mg/kg bw/day) did not experience abnormal weight gain or severe neurological effects (usually 6–30 mg/kg bw for flea and tick control). However, other signs of toxicity were observed including convulsions, head nodding and muscles twitching (Hamernik 1998; Woodward 2012). Herin et al. (2011) assessed serum concentrations of thyroid-stimulating hormone (TSH), fipronil and fipronil sulfone in 159 workers in a factory manufacturing fipronil-containing veterinary drugs. They found a significant positive correlation between exposure and fipronil/fipronil sulfone concentration in the blood. In addition, fipronil sulfone concentration correlated negatively with serum TSH. Authors suggested the possibility that fipronil has a central inhibitory effect on TSH secretion in humans that potentially leads to adverse health consequences, the major one being loss of bone density and osteoporosis (Clark et al. 2010; Ozkaya et al. 2015). Thereby they emphasized that close occupational medical surveillance is highly desired to monitor the exposure of factory workers to fipronil in order to maintain health and well-being.
A number of studies were conducted to determine fipronil distribution and metabolism in mammals, as well as to identify its metabolites as potential biomarkers of exposure to fipronil (Cravedi et al. 2013; McMahen et al. 2015). Fipronil sulfone is a commonly accepted and proven major metabolite in all tissues, excrement, and blood (Cravedi et al. 2013; McMahen et al. 2015). Patients with signs of distress indicating potential poisoning with fipronil are usually subjected to blood analysis to identify the parent compound or fipronil sulfone (Mohamed et al. 2004). However, blood screening is an invasive method causing discomfort, pain and carries a risk of developing infection. Therefore, urine is a much more convenient biospecimen for screening tests. Two recent studies performed a detailed analysis of urine samples obtained from rats treated with a fipronil-containing diet (Cravedi et al. 2013; McMahen et al. 2015). Based on mass fragmentation of the compound both teams assigned a structure of hydroxy-fipronil but it has not been proven by analytical methods. Nevertheless, based on signal ratios in chromatograms McMahen et al. (2015) provided an estimate of concentration of metabolites present in the urine. From these data, hydroxy-fipronil appears to be a dominant metabolite among the other identified metabolites of fipronil.
The specific objectives of this study were to develop an approach for the synthesis of hydroxy-fipronil and use it as a standard to verify the identity of the discovered metabolite. A synthetic standard of hydroxyl-fipronil was tested on mammalian GABAA receptors to characterize the biological activity of the new compound. It was also used to assess its toxicity to insects. Finally, hydroxy-fipronil was used to develop a high performance liquid chromatography method (HPLC) coupled with tandem mass spectrometry detection (MS/MS) to provide a quantitative estimate of the biomarker in the urine of treated animals.
Materials and methods
Information concerning chemicals, instruments, buffers and reagents synthesis is detailed in the Supporting Information (SI) or in the sections below.
Urine samples
Urine samples were generated as part of a study reported by Freeborn et al. (2015). Briefly, rats were treated by oral gavage with a fipronil-containing corn oil using an 18 Ga feeding needle with a blunt tip. Animals were treated daily at 5 or 10 mg/kg either for two weeks (repeated) or on a single day only. Urine was collected in a syringe either from voids on a clean table or via bladder puncture and transferred to a micro-centrifuge tube, immediately frozen on dry ice, and stored at −80°C.
Enzymatic hydrolysis of urine
Urine aliquots of 250 μL were mixed with 150 μL of methanol and hydrolyzed using a 100 μL solution containing 33 μL (85000/7500 U/mL) of β-glucuronidase/sulfatase and 1.1 mL of 1 M ammonium acetate buffer at pH 5.5. The reaction was left overnight at 37°C with gentle mixing.
Hydroxy-fipronil identification
Analysis was carried out with a method reported by McMahen et al. (2015). Briefly, an Agilent 1100 HPLC interfaced with an Agilent 6210 (TOF) mass spectrometer fitted with an electrospray ionization (ESI) source was used. The HPLC was performed with a Zorbax Eclipse Plus C18 column (2.1 × 50 mm, 3.5 μm, Agilent Technologies) fitted with a Phenomenex guard column (Torrance, CA). The method consisted of the following: 0.2 mL/min flow rate; at 30 °C; mobile phases: A: ammonium formate buffer (0.4 mM) and DI water:methanol (95:5 v/v), and B: ammonium formate (0.4 mM) and methanol:DI water (95:5 v/v); gradient: 0–5 min a linear gradient from 50:50 A:B to 100% B; 5–15 min, 100% B; 15–18 min re-equilibration to 50% A and 50% B.
Method 1 (the Hammock group). For fipronil sulfone and hydroxy-fipronil
Rat urine samples (40 μL) were mixed with 10 μL of 1 μM 12-(3-cyclohexyl-ureido)-dodecanoic acid (CUDA) methanol solution. We used CUDA as an external standard since it showed a retention time close to fipronil sulfone, as well as spike-recovery studies with blank urine matrix showed CUDA to be an appropriate standard to account for ion suppression. The samples were cleared with a centrifugal filter device under 20,000 g for 5 min. The resulting solutions were transferred to vials with 100 μL volume inserts, and stored at −20 °C prior to analysis. Separation of the target compounds was performed on an UPLC system (Waters Corp., Milford, MA). Samples were stored in an autosampler at 4 °C, and 10 μl were injected by a partial loop with needle overfill. The UPLC column Kinetex C18 (1.7 μm, 2.1 × 100 mm, 1.7 μm particle size, Kinetex, Phenomenex) was kept at 40 °C. Mobile phases were composed of water with 0.1% acetic acid (phase A) and acetonitrile with 0.1% acetic acid (phase B). The following gradient was applied: 0–3 min 20% B, 3.1–6 min, a linear gradient from 20 to 80% B, 6.1–8 min, a linear gradient from 80 to 100% B, 8.1–9.1 min, 100% B, 9.1–10 min re-equilibration to 20%. The flow rate remained constant at 0.4 mL/min. The run event was designed to pre-purify samples online, 0–3 min to waste; 3–8 min to MS; 8–10 min to waste. The UPLC system was interfaced with the Quattro Premier MS equipped with an ESI source operated in positive ionization mode. MS operating parameters and compound specific information are provided in the SI, Table S1 and S2. The data were acquired and processed using Masslynx 4.1 software with instrument and Masslynx 4.1 with TargetLynx.
LC/MS/MS analysis. Method 2 (the EPA group). For fipronil sulfone only
Method 2 is an alternative method for fipronil sulfone quantification and for independent evaluation of the accuracy of method 1. Analysis was carried out with a method reported by McMahen et al. (2015). Briefly, rat urine (100μL) was precipitated with 900 μL of cold acetonitrile and centrifuged for 8 min at 12,500 ×g. An aliquot of the supernatant was extracted and mixed 50:50 with 10 mM ammonium acetate buffer before LC/MS analysis. Quantification analysis (LC/triple-quad) was carried out using an Agilent 1100 HPLC interfaced with a Sciex 3000 triple quadrupole mass spectrometer (Applied Biosystems/MDS Sciex) fitted with an ESI operated in the negative ionization mode. Fipronil sulfone specific transitions used for quantification were 451.1/415, 451.1/281.9, 451.1/243.9. The HPLC system consisted of a Phenomenex Luna C18 column (50 × 3 mm, 5 μm; Torrance, CA, USA) with a Security-guard guard column (Phenomenex). The method consisted of the following: 0.4 mL/min flow rate which increased to 0.75 mL/min at time = 2 min; temperature: 30 °C; mobile phases — A: ammonium acetate buffer (0.2 mM) and DI water:methanol (95:5, v/v), and B: ammonium acetate buffer (0.2 mM) and acetonitrile:DI water (95:5, v/v); gradient: 0–2 min 50% A and 50% B; 2.1–4 min, a linear gradient from 50:50 A:B to 10:90 A:B; 4–6 min 10% A and 90% B; 6.1–10 min re-equilibration to 50% A and 50% B.
Insect Bioassay
Rearing of Spodoptera frugiperda (Fall armyworm)
The eggs of S. frugiperda were obtained from Benzon Research (Carlisle, PA) and maintained at 26 ± 2 °C until hatching. The larvae were reared on a premixed artificial diet (Bioserv#F9781B, Frenchtown, NJ) and maintained at 26 ± 2 °C, with a photoperiod of 14:10 h (L:D).
Bioassays procedure
Bioassays were conducted on second instar (~3.5 days old) larvae of S. frugiperda using a diet surface contamination method. Five concentrations ranging from 1–100 μg/mL from each agent were prepared in DI water and used with 3 replicates for each concentration. The diet was prepared according to the manufacturer instructions by adding 139.7 g dry mix (BioServ, NJ; Product #F9781B) and 4.8 g potassium hydroxide solution into 24 g boiled agar in 831 mL dH2O to form 1 L of diet. An aliquot of 15 mL was poured in 120 mL cup (Frontier Agricultural Services, Newark, DE) (with base and top diameters equal to 6 and 6.5 cm, respectively). One mL of each concentration or dH2O (control) was separately spread on the diet surface, and then cups moved several times to ensure covering the whole surface of the diet with solution. Immediately after covering the whole diet surface, the remainder of the solution was completely removed using a pipette. Cups were left for 15 min at RT to dry. Ten 2nd instar larvae of S. frugiperda were transferred into each cup with a total number of 30 larvae/concentration or control. A total of 180 larvae were used for each bioassay and control, and repeated in triplicate. Data were recorded at 12 h intervals for 3 days. The larvae were considered dead when they did not respond to a gentle touch with a brush to provoke movement. Median lethal concentrations (LC50) and median lethal times (LT50) were calculated using the Probit analysis 3.1 program and ViStat 2.1 program respectively.
GABA receptor bioassay
Preparation of cells expressing the α1β3γ2L GABAA receptors
The human GABAA receptors α1, β3 and γ2L cloned into pcDNA3.1 expression vectors were a gift from Dr. Robert L. Macdonald, Vanderbilt University, TN. Fibroblast L929 cells were cultured in Dulbecco’s modified Eagle’s medium (Lonza) supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 mg/mL streptomycin (Invitrogen) and maintained in humidified 95% air and 5% CO2 air at 37°C. Cells were transfected using FuGENE 6 (Roche) transfection reagent with an equal amount of each of the subunits in combination with pEGFP-C1. The transfection ratio of total cDNA to transfection reagent was 3:1. 36 h post-transfection, cells were plated on glass coverslips and transfected cells were identified using an epifluorescence microscope for electrophysiological whole-cell voltage-clamp studies.
Electrophysiological recordings
Whole-cell voltage-clamp recordings were performed at RT with an EPC-10 HEKA amplifier. Cells were bathed in an external Ringer solution consisting of 160 mM NaCl, 4.5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, pH 7.4, 311 mOsm. Recording electrodes were pulled and fire-polished to resistances of 1.4–1.8 MΩ and filled with an internal solution consisting of 154 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES and 10 mM EGTA with pH 7.3 and 308 mOsm. Cells were voltage clamped at −80 mV and control currents were recorded under the application of 25 μM GABA, using a gravity-fed fast perfusion system, for 5–6 s followed by a 30–40 s wash with external solution. Inhibition of currents were determined by the reduction in current level elicited by the same amount of GABA after the pre-application of fipronil and its derivatives for at least 3 min. Test solutions of fipronil and its derivatives were freshly prepared immediately before each application onto cells. Data analysis was performed using Excel (Microsoft) and Origin 7.0 (OriginLab Corp.) software. Data fitting to the Hill equation to obtain IC50 values was performed with Origin 7.0. Data are presented as the mean ± S.D.
Indirect competitive Fipronil ELISA
For ELISA all buffers and water solutions were prepared with DI water; phosphate-buffered saline (PBS, 10 mM, pH 7.5); wash buffer PBST (PBS containing 0.05% Tween 20); coating buffer (14 mM Na2CO3, 35 mM NaHCO3, pH 9.8); blocking buffer (1% BSA in PBST); substrate buffer (0.1 M sodium citrate/acetate buffer, pH 5.5). Substrate solution contained 0.2 mL of 0.6% TMB (in dimethyl sulfoxide, DMSO w/v), 0.05 mL of 1% H2O2 in 12.5 mL of substrate buffer. Stop solution was 2 MH2SO4.The immunoassay was performed as previously described (Vasylieva et al. 2015a). Briefly, plates were coated with 1 μg/mL antigen BDH-297-7-CON diluted in coating buffer (100 μL/well). After incubation for 1 h at room temperature (RT), the solution was replaced with blocking buffer (200 μL/well) and plates were incubated over night at 4 °C or for 1–4 h at RT. Plates were washed with 300 μl/well of PBST 3 times prior to sample loading. Standards in assay buffer were prepared in glass vials and loaded onto the coated plate in triplicate (50 μL/well). Urine samples were directly loaded onto the plate at 5 μL in 45 μL of the assay buffer. An equal volume (50 μL/well) of anti-fipronil serum #2268 diluted in PBS was added at 1:4000 dilution, giving final 1:8000 dilution in the plate. The plate was incubated for 1 h at RT and then washed 5 times with wash buffer. Goat anti-rabbit IgG-HRP secondary antibody was added at 100 μL/well in a 1:10 000 dilution as instructed by manufacturer. The plate was incubated for 1 h at RT and washed 5 times. Substrate solution was added (100 μL/well). The color development was stopped by addition of 2 M H2SO4 (50 μL/well) and absorbance was read at 450 nm. SigmaPlot 11.0 software was used for curve fitting and data analysis.
Results
1. Synthesis
5-Amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-hydroxy-1H-pyrazole-3-carbonitrile (hydroxy-fipronil, (5)) and methyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-1H-pyrazole-3-carbimidate (imidate derivative of fipronil desulfinyl, (6)) were synthesized as described in Scheme 1. The synthesis of hydroxy-fipronil (5) began with preparation of the aceto-intermediate (3) according to the previously described procedure (Banks 2000). Briefly, iodination of the fipronil detrifluoromethylsulfinyl with N-iodosuccinimide followed by the Sonogashira coupling of the resulting iodo derivative with trimethylsilyl acetylene gave the acetylenic derivative (2) which was further subjected to potassium carbonate catalyzed cleavage of the trimethylsilyl group and acid catalyzed hydration of the acetylene moiety to give the desired intermediate (3). Baeyer-Villiger oxidation of (3) with mCPBA in the presence of para-toluensulfonic acid provided the acetoxy intermediate (4) in 33 % yield (68 % based on the recovered starting material). Hydrolysis of the acetoxy function completed the synthesis of the hydroxy-fipronil 5. The imidate (6) was synthesized in 54% yield (quantitative based on the recovered starting material) by stirring the solution of fipronil detrifluoromethylsulfinyl (1) and sodium hydroxide in methanol under an atmosphere of ambient air or oxygen. NMR and HRMS spectra obtained for the synthesized compound were in a good agreement with expected structure. The detailed analysis is provided in the SI.
Scheme 1.
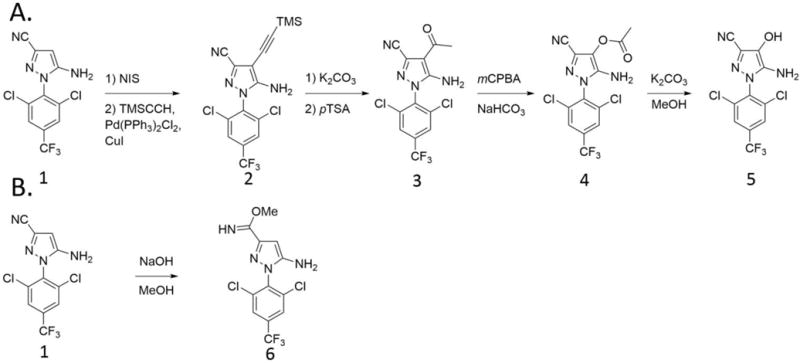
Synthetic approach toward hydroxy-fipronil (5) and the imidate derivative of fipronil desulfinyl (6).
2. Identification and quantification analysis
High resolution TOF MS in negative ionization mode was used to evaluate compound (5) (scheme 1). Hydroxy-fipronil was observed as a formate adduct with a major ion at m/z 380.9786 while lower intensity ions at m/z 382.9751 and 384.9716 appeared due to the presence of chlorine isotopes (Fig.S1). The mass-to-charge values obtained experimentally correspond perfectly to calculated values compared at three decimal places. This isotopic pattern is very characteristic of compounds containing two chlorine atoms. There were also additional chlorinated peaks in the spectrum, most probably related to hydroxy-fipronil (reaction side products or intermediate materials) suggesting that the standard contained some impurities. However, the compound was purified by flash column chromatography and as judged by TLC and NMR spectroscopy it was pure at >95%. Therefore, chlorinated peaks observed in the spectra may indicate presence of impurities at no more than 5 %. The spectrum looked similar when hydroxy-fipronil was spiked in the urine matrix (Fig. S2). Prior to quantitative analytical method development, we ran a standard solution of hydroxy-fipronil and a sample of hydrolyzed but non-spiked urine sample from a treated rat on LC/TOF to compare retention times of the standard and the metabolite. We showed clearly a match in retention times of the peaks with the same exact molecular mass of 380.9786 corresponding to the formic adduct of hydroxy-fipronil thus further supporting the identity of the metabolite (Fig. S3).
The LC/MS/MS chromatograms of a standard mixture containing hydroxy-fipronil, fipronil sulfone and CUDA as an internal standard are provided in the SI (Fig. S4). The limit of quantification (LOQ) was determined as 10 times the signal-to-noise ratio. In methanol the LOQ was 0.4 ng/mL and 1.5 ng/mL for hydroxy-fipronil and fipronil sulfone, respectively. The LOQ for hydroxy-fipronil in the urine matrix was determined to be 1.0 ng/mL. The sensitivity achieved is comparable to other methods reported in the literature (Bichon et al. 2008; Cazorla-Reyes et al. 2011 ; Lacroix et al. 2010). All replicates for all experiments had a relative standard deviation (RSD) of <18%. Recoveries were determined from spiked urine samples with CUDA used as an external standard. Recovery values for hydroxy-fipronil spiked at 1.0 ng/mL were 110 ± 6 %.
Prior to quantitative analysis all urine samples were hydrolyzed to cleave possible glucuronide and sulfate conjugates of hydroxy-fipronil which occure according to literature reports (Cravedi et al. 2013; McMahen et al. 2015). For this purpose, we used an enzymatic solution of β-glucuronidase/sulfatase since it provides mild conditions for hydrolysis, decreasing the possibility of destroying the hydroxy-fipronil by harsh acidic hydrolysis conditions. The hydrolyzed urine samples were loaded on the chromatography column directly without a preliminary extraction step. To remove any urinary components that could suppress ionization, after loading and prior to elution, the column was washed excessively with aqueous phase followed by elution and MS/MS detection. Table 1 shows a good relationship between the concentration of hydroxy-fipronil detected in the samples and the amount of fipronil in the diet received by the corresponding rats. Thus, when rats received a single meal with a fipronil dose of 5 or 10 mg/kg, the hydroxy-fipronil concentration in the urine did not differ much and was about 20 ng/mL. However, when animals received continual treatment over 14 days, the concentration of hydroxy-fipronil was 2025 and 5340 ng/mL for animals treated with 5 mg/kg (2 rats tested) and 10200 ng/mL for the animal treated with 10 mg/kg. The developed method for hydroxy-fipronil quantification was also applied for the quantification of fipronil sulfone. Fipronil sulfone was detected only in samples obtained from animals treated with fipronil repeatedly (samples 42, 81 and 82). All samples were tested blindly and results agreed between two laboratories that tested the samples independently with different sample preparation protocols and instrumental set-up (Table 1).
Table 1.
Quantification of fipronil sulfone and hydroxy-fipronil in urine samples.
| Sample ID | Dose info | Conc sulfone, ng/mL LOQ* 1 ng/mL | Conc sulfone, ng/mL LOQ** 10 ng/mL | Conc sulfone, ng/ml LOQ$ 1.5 ng/mL | Conc FipOH, ng/mL LOQ$ 0.4 ng/mL |
|---|---|---|---|---|---|
| 17 | 0 mg/kg | 2 | n/a | N/D | N/D |
| 35 | 0 mg/kg | 2 | < 10 | N/D | N/D |
| 90 | 0 mg/kg | 8 | < 10 | N/D | N/D |
| 3 | 5 mg/kg single | 8 | n/a | N/D | 23 |
| 60 | 5 mg/kg single | 13 | < 10 | N/D | 24 |
| 42 | 5 mg/kg repeated | 12 | 26 | 17 | 5340 |
| 81 | 5 mg/kg repeated | 18 | 24 | 14 | 2025 |
| 52 | 10 mg/kg single | 7 | < 10 | N/D | 14 |
| 82 | 10 mg/kg repeated | 29 | 23 | 19 | 10200 |
Determined with different LC-MS/MS methods by the EPA group in:
the study of McMahen et al. (2015);
this study
determined in this work with a LC-MS/MS method different from the EPA group. n/a, not tested, N/D-non-detectable
3. Insect toxicity
Table S3 indicates that fipronil is toxic to S. frugiperda larvae with an LC50 of 4.1 μg/mL. The level of mortality increased with increasing concentrations and reached 100 % after 48 h of treatment using 50 μg/mL, and after 12 h of exposure using 100 μg/mL. On the other hand, the fipronil analogues, both hydroxy and imidate, did not cause any mortality on 2nd instar larvae of S. frugiperda even at doses as high as 100 μg/mL which was sufficient to kill 100 % of the treated S. frugiperda larvae with fipronil in less than 12 h.
4. Human GABA receptor bioassay
To test the activity of fipronil and its derivatives in inhibiting GABA-induced Cl− currents, their effect on currents produced by cells transiently expressing the α1 β3γ2L Gabaa receptor were measured using whole-cell voltage-clamp. Fipronil showed the highest potency in reducing currents and had an inhibitory effective concentration (EC50) of 1.15 ± 0.25 nM (n = 6, Fig. 1), in agreement with its previously published nanomolar activity (Hainzl and Casida 1996). Fipronil desulfinyl imidate blocked GABA-induced currents with a similar EC50 of 2.78 ± 1.42 nM (n = 23). In contrast, the metabolite hydroxy-fipronil was not as potent and required up to a 1000 fold higher concentrations to elicit similar current inhibition (EC50 = ~1 μM).
Figure 1.
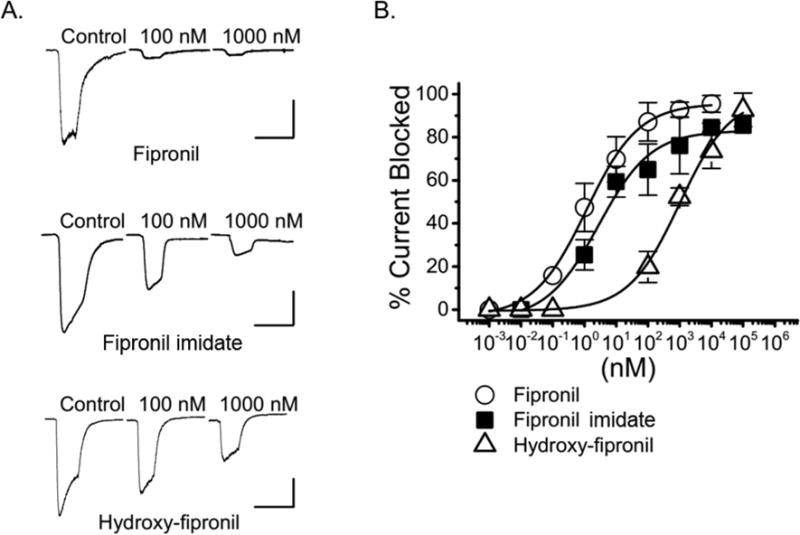
Inhibition of GABAA current by fipronil and its derivatives. A) Whole-cell patch-clamp recordings showing the inhibition of GABA-induced Cl− currents by fipronil (top), fipronil desulfinyl imidate (middle) and hydroxy-fipronil (bottom) at concentrations of 100 nM and 1000 nM. Scale bars indicate 1 nA and 5 s, respectively. B) Concentration-response curves showing the percentage of α1β3γ2L GABAA currents blocked by increasing concentrations of fipronil, fipronil desulfinyl imidate and or hydroxy-fipronil. The inhibitory EC50 values are 1.2 ± 0.2 nM (n = 6) for fipronil, 2.8 ± 1.4 nM (n = 23) for fipronil desulfinyl imidate, and 1000 ± 341 nM (n = 7) for hydroxy-fipronil.
5. Immunoassay screening for independent evaluation
We previously developed 2 immunoassays using different sera for the detection of fipronil (Vasylieva et al. 2015b). Cross-reactivity (CR) to hydroxy-fipronil was tested with these polyclonal antibodies 2265 and 2268 (pAbs). In both assays sensitivity to hydroxy-fipronil was significantly lower with CR being only 3.2 (n=5) and 0.7 % (n=4) for sera 2265 and 2268 respectively (Fig. S5). The assay 2268 is more robust to changing sample conditions like pH or ionic strength (Vasylieva et al. 2015a). We therefore chose this assay to assess the applicability of the immunoassay for screening of urine samples for the presence of biomarkers of exposure to fipronil. Although minimal CR is observed, based on our previously determined concentrations for hydroxy-fipronil, it should be high enough to produce a pronounced result in the immunoassay. Since the literature data indicates that urine may contain a number of fipronil derivatives and the sera cross-reactivity is not known for all derivatives, the signal from the immunoassay was given a (+) when the concentration of analyte was lower than 5 ng/mL but still significantly detectable; (++) for the concentration range between 10 and 1000 ng/mL and (+++) for >1000 ng/mL. Table 2 shows a relatively good correlation between data obtained with LC/MS/MS and immunoassay screening of hydrolyzed urine samples.
Table 2.
Screening urine samples for Hydroxy-fipronil using immunoassay
| Animal | Dose | Sulfone, μg/L | Fip-OH, μg/L | ELISA screening |
|---|---|---|---|---|
| 17 | 0 mg/kg | N/D | N/D | − |
| 35 | 0 mg/kg | N/D | N/D | + |
| 90 | 0 mg/kg | N/D | N/D | + |
|
| ||||
| 3 | 5 mg/kg single | N/D | 23 | + |
| 60 | 5 mg/kg single | N/D | 24 | + |
|
| ||||
| 42 | 5 mg/kg repeated | 17 | 5340 | +++ |
| 81 | 5 mg/kg repeated | 14 | 2025 | +++ |
|
| ||||
| 52 | 10 mg/kg single | N/D | 14 | ++ |
|
| ||||
| 82 | 10 mg/kg repeated | 19 | 10200 | +++ |
non-detectable, + < 5 ng/mL, ++ > 10 ng/mL, +++ > 1000 ng/mL
N/D – non-detectable
Discussion
Serum and urine are two common body fluids used for general health analysis but also for monitoring of human exposure to chemicals. Even though both specimens bring complementary information, serum samples are more often used to monitor global health parameters. Nevertheless, urine is much more suitable for large monitoring campaigns since the sample collection is fast, cheap, does not require special training, could be accomplished by the patient and provides large amounts if needed. It is also noninvasive and thus does not have any side effects such as pain or inflammation. However, its use is limited only to the target analytes that are cleared from the body into the urine in the form of the initial parent compound or its corresponding metabolites. To date, fipronil has mainly been detected in the serum of patients in its parent form or as fipronil sulfone (Mohamed et al. 2004). Two recent studies performed a detailed analysis of urine samples obtained from rats treated with fipronil-containing diet (Cravedi et al. 2013; McMahen et al. 2015). Both research teams identified a novel potential metabolite in urine, hydroxy-fipronil (UM6, Scheme 2), in its conjugated form (UM10, Scheme 2). Despite the actual match in exact mass and fragments analysis, the identity of the compound has not been proven by comparison with a synthetic standard.
Scheme 2.
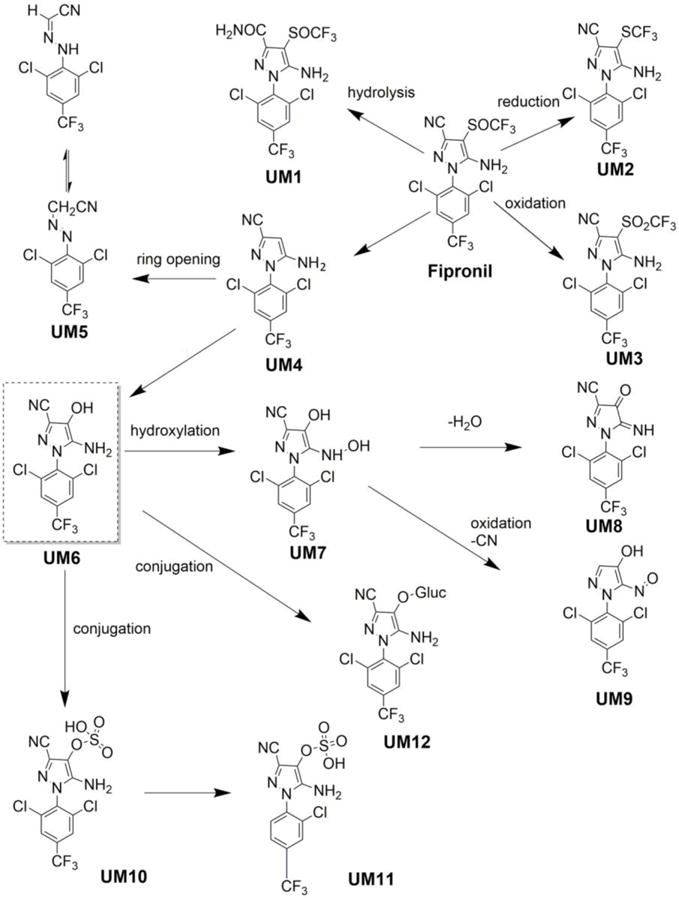
Urinary fipronil metabolites identified by high resolution MS and fragment analysis. Summary from 3 independent studies (Cravedi et al. 2013; McMahen et al. 2015; Powles et al. 1992).
A novel and easy approach to the synthesis of hydroxy-fipronil was developed here to extend previous studies and provide data supported with a synthetic standard for detection of hydroxy-fipronil. Since fipronil detrifluoromethylsulfinyl (1), a late-stage intermediate for the synthesis of fipronil, is commercially available and differs from the structure of hydroxy-fipronil by only the absence of the oxygen atom at the 4th position of the pyrazole moiety that could be potentially introduced by an oxidation reaction, it was considered as an attractive starting material for the synthesis. The pyrazole moiety of fipronil detrifluoromethylsulfinyl has a high degree of similarity with enols of β-ketocarbonyl compounds (Fig.2). Keeping in mind that enols and enolates are very reactive toward molecular oxygen and undergo addition reactions with it at ambient temperature (Cubbon and Hewlett 1968; Schottner et al. 2010; Wang et al. 2011) yielding α-hydroperoxy carbonyl compounds, a natural question arose: is a similar reaction possible for the appropriately substituted heterocycles and in particular for the pyrazole moiety of fipronil detrifluoromethylsulfinyl? Unfortunately, treatment of (1) with sodium hydroxide in methanol in the presence of ambient air only resulted in formation of the imidate (6) but not the desired peroxide. The precise structural assignment for compound (6) was proven by spectroscopic analyses. Since the peroxidation-reduction approach failed to give the desired hydroxy-fipronil product, a new successful approach was designed that involved Baeyer-Villiger oxidation of known compound (3) as the key step (Scheme 1A).
Figure 2.
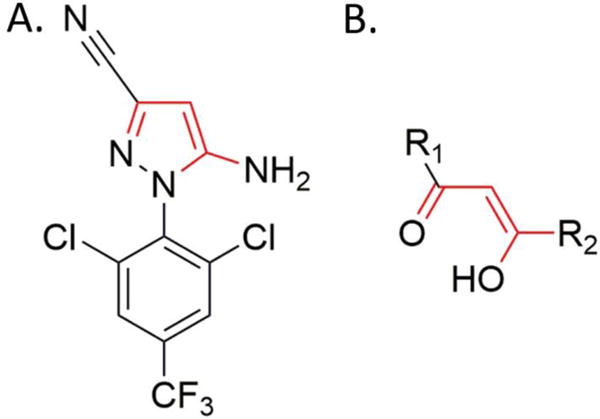
Similarity between aminopyrazol moiety (A) and enols of β-ketocarbonyl compounds (B).
The newly developed synthetic standard of hydroxy-fipronil and commercial fipronil sulfone were used to develop an analytical method for their separation and quantification with LC/MS/MS. Prior to the quantification of hydroxy-fipronil the developed method was used to quantify fipronil sulfone in urine samples. These results were compared to the data obtained using a different analytical set up (McMahen et al. 2015), tested independently by the EPA group in a blind fashion (Table 1). The concentrations of fipronil sulfone determined in urine samples using the LC/MS/MS method developed in this work (17, 14 and 19 ng/mL for rat samples 42, 81 and 82 respectively) were very close to the values provided by the EPA group (26, 24 and 23 ng/mL) that were obtained with two separate methods having different LOQs: 26, 24 and 23 ng/mL at LOQ 10 ng/mL; and 12, 19 and 29 ng/mL at LOQ 1 ng/mL (the EPA data provided for this study). These urine samples were previously tested for free fipronil and no detectable amount was reported (McMahen et al. 2015), which also agrees with literature reports (Cravedi et al. 2013; Powles et al. 1992).
Using the analytical method developed here to quantify hydroxy-fipronil in the urine of rats treated with fipronil containing diet, we obtained a very good positive trend between the concentration of metabolite detected and fipronil dose in the diet (Table 1). Control animals did not have detectable hydroxy-fipronil (LOQ 0.4 ng/mL). Those rats that received a single treatment dose of 5 or 10 mg/kg of fipronil had similar hydroxy-fipronil concentrations in the urine at about 20 ng/mL. However, when the animals were treated with the same doses for a period of two weeks, about 80 to 700 times difference in metabolite concentration was observed with the highest values detected in animals treated with dose of 10 mg/kg. Interestingly, Powles et al.(Powles et al. 1992) in metabolism studies with 14C-fipronil and a similar dosing regimen showed that after a single dose at 4 mg/kg, recovery of radioactivity in the urine of treated rats was 5.6%, while after repeated dosing over 14 days, the recovery of radioactivity in the urine was 14–16%. They also showed that a single high dose (150 mg/kg) resulted in an even higher recovery of 22–30%. Thus, our results correlate with data on fipronil metabolism reported in the literature (Powles et al. 1992). Theoretically, a single exposure to a low amount of fipronil may lead to a distribution of the compound over the body and mostly to the adipose tissue. However, when repeated exposure occurs and the compound is no longer accumulating, a higher amount is excreted, leading to higher detected amounts in the urine.
In the same study with 14C-fipronil Powles et al. (1992) reported high levels of very polar radiolabeled material detected in unextracted undiluted urine samples using HPLC. Deconjugation with a β-glucuronidase/sulfatase did not change the elution profile. Fourteen compounds were resolved of which seven were identified, while 4 remained unknown. These authors concluded that fipronil sulfone was a minor constituent in deconjugated and original urine. Our results strongly suggest that at least one of those unidentified metabolites might be hydroxy-fipronil because in addition to other evidence shown above, it also possesses polar properties after enzymatic deconjugation as described by Powles et al. (1992). As an interesting addition, we also observed that concentration of hydroxy-fipronil detected in the samples before and after enzymatic hydrolysis did not change significantly (table S4).
Bioactivity studies
Since the data reported here demonstrate strong evidence that hydroxy-fipronil is a urinary metabolite in humans, further characterization of its bioactivity properties was conducted. Our goal was to identify if the metabolite had any remaining activity and if it presented any potential concern after being released from the body. Its toxicity was studied in an insect bioassay, as well as its activity on a major human Gabaa receptor to which fipronil has a high affinity. In addition, in the bioassay studies we included the imidate derivative of fipronil. This compound has never been characterized before and since it has a chemical structure similar to fipronil, we tested it to extend our knowledge about this compound class. In our hands, fipronil showed an LC50 (4.1 μg/mL) (Table S3) on 2nd larval instar of S. frugiperda very close to what has been previously detected in other lepidopterans (Li et al. 2006) even though the method of treatment was different (diet surface contamination method vs incorporation in the food). In the current study hydroxy-fipronil and fipronil desulfinyl imidate did not show any mortality against 2nd instar larvae of S. frugiperda even when used in high concentrations (i.e. 24-fold higher than fipronil LC50). Larvae treated with both fipronil derivatives did not show any differences compared with control and survivors completed the life cycle normally at least until the pupae stage. We did not monitor possible abnormalities with adult emergence. Our results reflect the apparent safety of these compounds in the environment.
The activity of fipronil and derivatives on Gabaa α1β3γ2L receptor currents was then evaluated. As expected, hydroxy-fipronil did not have a significant inhibitory effect on GABAA receptor currents and thus this metabolite might be considered safe in terms of neurotoxicity to humans. Surprisingly, the imidate derivative that did not show any insecticidal activity turned out to be approximately as potent to fipronil on human α1β3γ2L receptors. This discrepancy is even more surprising in light of previous research suggesting that the fipronil binding site lies within the second trans-membrane region of the β3 subunit, a region that shares high amino-acid sequence homology to the analogous region of the insect GABAR, the target of the fipronil (Ratra and Casida 2001). One possible explanation for this observation could be the metabolic instability of fipronil desulfinyl imidate.
Urine samples screening by immunoassay
A sensitive fipronil immunoassay was employed to assess the presence of fipronil-like compounds in the urine of treated animals. It was used to evaluate the presence of fipronil metabolites in the samples as an independent method that has a different way of sensing the target analyte as an alternative to LC/MS. Two false positive read outs for samples 35 and 90 were detected. It could have been any other metabolite that the pAb may recognize, but since these samples were from negative control animals, it was more likely due to contamination of the sample or presence of a small matrix effect. Aside from this, there is a strong trend in signal intensity with (++) read out for samples containing hydroxy-fipronil and no fipronil sulfone and (+++) for samples with a significant amount of fipronil sulfone and hydroxy- fipronil detected with LC/MS/MS. These data further support the hypothesis that hydroxy-fipronil is a urinary metabolite of fipronil. In addition, this demonstrates that an immunoassay for analysis of urine samples could be a useful and convenient method for exposure assessment and evaluation. Immunoassays are proven to be the tools of choice for high throughput large screening studies because they provide an inexpensive and fast alternative to instrumental methods. Immunoassays can also be converted into a field portable lateral flow sensor format. Together with non-invasive urine sample collection the availability of field portable sensors would simplify the screening campaigns at manufacturing plants and other related sites of exposure. This would also make occupational medical surveillance affordable and allow timely treatment of affected persons in order to maintain health and well-being.
In conclusion, we have developed an easy and fast method for synthesis of hydroxy-fipronil, a urinary metabolite of the fipronil. The newly developed synthetic standard of hydroxy-fipronil was used to develop an analytical LC/MS/MS method for its quantification in the urine of treated animals. Immunoassay with cross-reactivity to fipronil metabolites and to hydroxy-fipronil was used as alternative method to confirm the presence of the metabolite in the urine and to evaluate its level. Our results obtained in a blind fashion, correlated well with the data obtained in the second laboratory that used a different analytical set-up. New compounds synthesized in this work were evaluated in bioassays for neurotoxicity, and results suggested that they did not show GABAergic activity.
Supplementary Material
Highlights.
Fast and easy approach was developed for the first time synthesis of hydroxy-fipronil, a urinary metabolite of fipronil.
Hydroxy-fipronil standard was used to confirm the metabolite identity and to develop a sensitive analytical LC-MS/MS method with a limit of quantification (LOQ) of 0.4 ng/mL.
This is the first study to show that hydroxy-fipronil is a more sensitive dose-dependent biomarker of exposure to fipronil than currently accepted fipronil-sulfone.
Fipronil immunoassay with cross-reactivity to hydroxy-fipronil was used for sample screening in the evaluation of exposure levels.
A standard of hydroxyl-fipronil was tested on mammalian GABAA receptors to characterize its biological activity. It was also used to assess its toxicity to insects.
Acknowledgments
This work was supported by National Institute of Environmental Health Sciences, Superfund Research Program, P42 ES04699 and the National Institute for Occupational Safety and Health Western Regional Center for Agricultural Health Science U50 OH07550. The research was also supported by the CounterACT Program, National Institutes of Health Office of the Director, and the National Institute of Neurological Disorders and Stroke, Grant Number U54 NS079202.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- FAO fipronil. Accessible at: http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Evaluation01/08_Fipronil.pdf.
- National Pesticide Information Center, Technical fact sheet: Fipronil. Oregon State University; http://npic.orst.edu/factsheets/fiptech.pdf. [Google Scholar]
- Banks BJ. Parasiticidal compounds. USA: Pfizer Inc.; 2000. [Google Scholar]
- Bichon E, Richard CA, Le Bizec B. Development and validation of a method for fipronil residue determination in ovine plasma using 96-well plate solid-phase extraction and gas chromatography-tandem mass spectrometry. Journal of chromatography A. 2008;1201:91–99. doi: 10.1016/j.chroma.2008.06.024. [DOI] [PubMed] [Google Scholar]
- Cazorla-Reyes R, Fernandez-Moreno JL, Romero-Gonzalez R, Frenich AG, Vidal JL. Single solid phase extraction method for the simultaneous analysis of polar and non-polar pesticides in urine samples by gas chromatography and ultra high pressure liquid chromatography coupled to tandem mass spectrometry. Talanta. 2011;85:183–196. doi: 10.1016/j.talanta.2011.03.048. [DOI] [PubMed] [Google Scholar]
- Clark L, Mohammed A, Pillai A, Klotsas A, Masters P, Lawson N. Hyperthyroidism, an overlooked cause of severe hypercalcaemia. Grand Rounds,Specialities: Endocrinology. 2010;10:110–112. [Google Scholar]
- Cochran RC, Yu L, Krieger RI, Ross JH. Postapplication Fipronil Exposure Following Use on Pets. J Toxicol Env Heal A. 2015;78:1217–1226. doi: 10.1080/15287394.2015.1076363. [DOI] [PubMed] [Google Scholar]
- Cravedi JP, Delous G, Zalko D, Viguie C, Debrauwer L. Disposition of fipronil in rats. Chemosphere. 2013;93:2276–2283. doi: 10.1016/j.chemosphere.2013.07.083. [DOI] [PubMed] [Google Scholar]
- Cubbon RCP, Hewlett C, Organic Peroxides Containing Functional Groups .1 Preparation and Properties of Some Alpha-Oxo-Hydroperoxides. J Chem Soc C. 1968:2978–&. [Google Scholar]
- Das PC, Cao Y, Cherrington N, Hodgson E, Rose RL. Fipronil induces CYP isoforms and cytotoxicity in human hepatocytes. Chem Biol Interact. 2006;164:200–214. doi: 10.1016/j.cbi.2006.09.013. [DOI] [PubMed] [Google Scholar]
- EPA. Fipronil Summary Document Registration Review: Initial Docket June 2011. 2011 Docket Number: EPA-HQ-OPP-2011-0448. Case No.7423. [Google Scholar]
- Freeborn DL, McDaniel KL, Moser VC, Herr DW. Use of electroencephalography (EEG) to assess CNS changes produced by pesticides with different modes of action: Effects of permethrin, deltamethrin, fipronil, imidacloprid, carbaryl, and triadimefon. Toxicology and applied pharmacology. 2015;282:184–194. doi: 10.1016/j.taap.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Gunasekara AS, Truong T, Goh KS, Spurlock F, Tjeerdema RS. Environmental fate and toxicology of fipronil. J Pest Sci. 2007;32:189–199. [Google Scholar]
- Hainzl D, Casida JE. Fipronil insecticide: Novel photochemical desulfinylation with retention of neurotoxicity. PNAS. 1996;93:12764–12767. doi: 10.1073/pnas.93.23.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainzl D, Cole LM, Casida JE. Mechanisms for selective toxicity of fipronil insecticide and its sulfone metabolite and desulfinyl photoproduct. Chem Res Toxicol. 1998;11:1529–1535. doi: 10.1021/tx980157t. [DOI] [PubMed] [Google Scholar]
- Hamernik KL. Pesticide Residues in Food-1997, Evaluations 2005 Part II- Toxicological Joint FAO/WHO Meeting on Pesticide Residues. World Organization; Geneva: 1998. [Google Scholar]
- Herin F, Boutet-Robinet E, Levant A, Dulaurent S, Manika M, Galatry-Bouju F, Caron P, Soulat JM. Thyroid Function Tests in Persons with Occupational Exposure to Fipronil. Thyroid. 2011;21:701–706. doi: 10.1089/thy.2010.0449. [DOI] [PubMed] [Google Scholar]
- Lacroix MZ, Puel S, Toutain PL, Viguie C. Quantification of fipronil and its metabolite fipronil sulfone in rat plasma over a wide range of concentrations by LC/UV/MS. J Chromatogr B. 2010;878:1934–1938. doi: 10.1016/j.jchromb.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Li AG, Yang YH, Wu SW, Li C, Wu YD. Investigation of resistance mechanisms to fipronil in diamondback moth (Lepidoptera : Plutellidae) J Econ Entomol. 2006;99:914–919. doi: 10.1603/0022-0493-99.3.914. [DOI] [PubMed] [Google Scholar]
- McMahen RL, Strynar MJ, Dagnino S, Herr DW, Moser VC, Garantziotis S, Andersen EM, Freeborn DL, McMillan L, Lindstrom AB. Identification of fipronil metabolites by time-of-flight mass spectrometry for application in a human exposure study. Environ Int. 2015;78:16–23. doi: 10.1016/j.envint.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed F, Senarathna L, Percy A, Abeyewardene M, Eaglesham G, Cheng R, Azher S, Hittarage A, Dissanayake W, Sheriff MH, Davies W, Buckley NA, Eddleston M. Acute human self-poisoning with the N-phenylpyrazole insecticide fipronil - a GABA(A)-gated chloride channel blocker. J Toxicol Clin Toxicol. 2004;42:955–963. doi: 10.1081/clt-200041784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaya HM, Keskin FE, Haliloglu OA, Senel TE, Kadioglu P. Life-Threatening Hypercalcemia due to Graves’ Disease and Concomitant Adrenal Failure: A Case Report and Review of the Literature. Case Rep Endocrinol. 2015 doi: 10.1155/2015/684648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles P, Biol C, Biol MI. 14C Fipronil (14C-M&B 46 030): Absorption, Distribution, Metabolism, and Excretion in the Rat. EPA. 1992 [Google Scholar]
- Ratra GS, Casida JE. GABA receptor subunit composition relative to insecticide potency and selectivity. Toxicology letters. 2001;122:215–222. doi: 10.1016/s0378-4274(01)00366-6. [DOI] [PubMed] [Google Scholar]
- Schottner E, Wiechoczek M, Jones PG, Lindel T. Enantiospecific Synthesis of the Cubitane Skeleton. Org Lett. 2010;12:784–787. doi: 10.1021/ol902854t. [DOI] [PubMed] [Google Scholar]
- Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, Furlan L, Gibbons DW, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke CH, Liess M, Long E, McField M, Mineau P, Mitchell EAD, Morrissey CA, Noome DA, Pisa L, Settele J, Stark JD, Tapparo A, Van Dyck H, Van Praagh J, Van der Sluijs JP, Whitehorn PR, Wiemers M. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut R. 2015;22:5–34. doi: 10.1007/s11356-014-3470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasylieva N, Ahn KC, Barnych B, Gee SJ, Hammock BD. Development of an Immunoassay for the Detection of the Phenylpyrazole Insecticide Fipronil. Environmental science & technology. 2015a;49:10038–10047. doi: 10.1021/acs.est.5b01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasylieva N, Ahn KC, Barnych B, Gee SJ, Hammock BD. Development of an Immunoassay for the Detection of the Phenylpyrazole Insecticide Fipronil. Environmental science & technology. 2015b doi: 10.1021/acs.est.5b01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidau C, Brunet JL, Badiou A, Belzunces LP. Phenylpyrazole insecticides induce cytotoxicity by altering mechanisms involved in cellular energy supply in the human epithelial cell model Caco-2. Toxicol In Vitro. 2009;23:589–597. doi: 10.1016/j.tiv.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Vidau C, Gonzalez-Polo RA, Niso-Santano M, Gomez-Sanchez R, Bravo-San Pedro JM, Pizarro-Estrella E, Blasco R, Brunet JL, Belzunces LP, Fuentes JM. Fipronil is a powerful uncoupler of oxidative phosphorylation that triggers apoptosis in human neuronal cell line SHSY5Y. Neurotoxicology. 2011;32:935–943. doi: 10.1016/j.neuro.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Wang GW, Lu QQ, Xia JJ. Three Types of Products Obtained Unexpectedly from the Reaction of Dimedone with Chalcones. Eur J Org Chem. 2011:4429–4438. [Google Scholar]
- Watts M. Pesticide Action Network Asia and the Pacific, Highly Hazardous Pesticides: Fipronil. A PAN AP Factsheet Series. 2012 http://archive.panap.net/en/p/post/pesticides-info-database/1209.
- Woodward KN. In: Chapter 12.2.6 Fipronil Mammalian Toxicology of Insecticides. Marrs TC, editor. The Royal Society of Chemistry; 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


