Abstract
Two dogs were presented, each with a large solitary pulmonary mass, and cytology confirmed mast cell tumor (MCT) in each dog. One dog was euthanized following diagnosis. Thoracic computed tomography scan and exploratory thoracotomy of the second dog revealed a right pulmonary mass that would require a radical lung resection. The patient was euthanized and histopathology confirmed a poorly granulated MCT with characteristics suggestive of epitheliotropism, an uncommon finding with MCT. These represent the first reported cases of presumptive primary pulmonary MCT in dogs.
Résumé
Mastocytome primaire pulmonaire présumé chez deux chiens. Deux chiens ont été présentés avec une volumineuse masse pulmonaire dont l’analyse cytopathologique confirma le diagnostic de mastocytome (MCT). L’un des chiens a été euthanasié suite au diagnostic. Un CT scan thoracique et une thoracotomie exploratrice du second chien ont révélé une masse pulmonaire droite nécessitant une résection pulmonaire radicale; le chien fut euthanasié. L‘histopathologie a confirmé un MCT peu granulé avec des caractéristiques suggestives d’épithéliotropisme, une trouvaille inhabituelle lors de MCT. Il s’agit des premiers cas rapportés de MCT pulmonaires primaires présumés chez le chien.
(Traduit par les auteurs)
Canine primary lung cancer is relatively uncommon in comparison to metastatic pulmonary disease. Canine primary pulmonary tumors include carcinomas, histiocytic sarcoma, primary pulmonary lymphoma (previously known as lymphomatoid granulomatosis), and various other sarcomas (1). Mast cell tumors (MCT) are usually not considered as a differential diagnosis for solitary pulmonary masses.
Mast cell tumors are the most common cutaneous malignancy in dogs, accounting for 16% to 21% of all canine skin tumors (2). Other primary sites include the oral cavity (3) and visceral organs such as the gastrointestinal tract, liver, or spleen (4). Common metastatic sites for canine MCT include the draining lymph nodes, skin, spleen, liver, and bone marrow. Lungs are rarely affected with metastatic disease (2,4,5), although cases have been reported in many species including dogs (4,6–8), cats (2), humans (9), a hen (10), cattle (11), and a goat (12). In dogs, only a few cases of MCT have been described to involve the lungs (4,6–8) or other thoracic organs (13,14). Minimal pulmonary involvement has also been reported in a dog with mast cell leukemia (15).
Mast cell disease involving visceral organs is referred to as visceral mast cell tumors (vMCT) (4). This form of the disease is often preceded by an aggressive primary cutaneous lesion (2,4), but has also been reported without a previous diagnosis of primary cutaneous MCT (4,6–8,13,14,16). In a retrospective study, 10 dogs with histologically confirmed vMCT had no evidence of past or present cutaneous MCT (4). A single dog with an aggressive pulmonary and splenic MCT has been described without cutaneous involvement at diagnosis (8). In contrast with the dog, primary splenic and intestinal MCTs without pre-existing primary cutaneous involvement are more common in cats (2).
No reference to canine primary pulmonary MCT could be found by the authors. In all reported cases of intrathoracic MCT, neoplastic mast cell disease was also disseminated to extra-thoracic organs (4,6–8,13–15). A case of focal mastocytosis in 2 tracheobronchial lymph nodes has been reported without mast cell infiltration in the lungs or any other tissue (17). However, in that case, the lesions were of unknown significance and could have been non-neoplastic. To the best of our knowledge, and based on the presentation with a massive solitary lung lesion and absence of previous cutaneous MCT, the patients described here represent the first 2 reported cases of canine presumptive primary pulmonary MCT.
Case descriptions
Case 1
A 6-year-old, 21.6-kg (body condition score 2 out of 9) spayed female American bulldog was presented to Centre Vétérinaire Rive-Sud (CVRS) with lethargy, weight loss, profuse vomiting, and diarrhea.
Upon presentation, the dog was hyperthermic (39.6°C), apathetic, dehydrated (estimated at 7%), tachycardic (150 beats/min), had a grade II out of VI systolic murmur, a left-sided purulent nasal discharge, and tenderness upon abdominal palpation. Medical history included bacterial bronchopneumonia with right middle lung lobe consolidation 18 mo earlier, with complete clinical resolution following antibiotic therapy. The most recent thoracic radiographs, 3 mo before presentation, only revealed a mild bronchointerstitial pattern in the ventral right hemithorax.
Abdominal and thoracic imaging were recommended but only blood analysis and supportive therapy were initially accepted. A complete blood (cell) count (CBC) (LaserCyte; IDEXX, Westbrook, Maine, USA), serum biochemistry profile (Catalyst Dx; IDEXX), and venous blood gases and electrolytes (VetStat; IDEXX) were performed. All results were within reference limits. The dog received intravenous crystalloid fluid therapy and was administered ampicillin (Ampicillin; Novopharm, Toronto, Ontario), 22 mg/kg body weight (BW), IV, q8h, and famotidine (Famotidine; Omega, Montreal, Quebec), 0.5 mg/kg BW, IV, q24h.
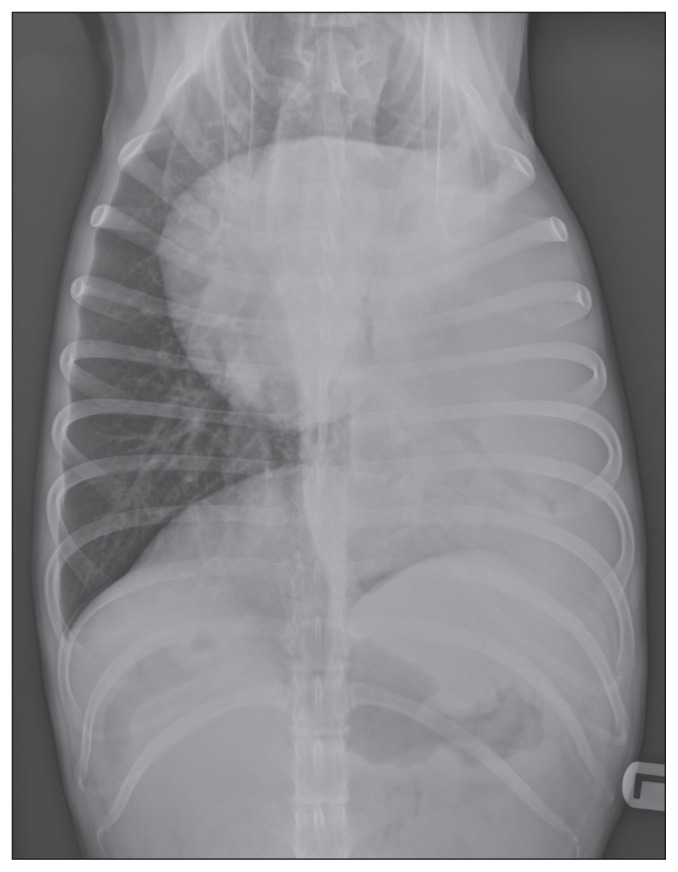
Occasional episodes of productive cough were observed during hospitalization. The day following presentation, the owner agreed to additional diagnostic imaging tests. Abdominal radiographs were unremarkable, but thoracic radiographs revealed a large mass effect of soft tissue density in the left caudal lung lobe (Figure 1). The cranial, medial, and dorsal edges of the lesion were irregular and well-defined. There was a right mediastinal shift, and an air bronchogram in the left caudal bronchus, which was displaced medially. Other thoracic structures were within normal limits.
Figure 1.
Thoracic radiograph of the dog of Case 1, ventrodorsal view. Note the mediastinal shift towards the right hemithorax and the air bronchogram in the left caudal bronchus. The lesion measured approximatively 9 cm lateromedially.
Given the patient’s medical history, the main differential diagnosis was bronchopneumonia with lung lobe consolidation, this time in the left hemithorax. Enrofloxacin (Baytril; Bayer Animal Health, Toronto, Ontario), 5 mg/kg BW, IV, q24h, was added to the therapeutic regimen, as was periodic coupage of the left hemithorax (5 min, q4h). The dog was no longer febrile after 1 day of hospitalization but occasional vomiting was observed and maropitant (Cerenia; Pfizer Animal Health, Kirkland, Quebec), 1 mg/kg BW, SQ, q24h, was administered.
After 3 d of supportive therapy the dog had improved, was adequately hydrated, no longer vomited, and began eating. The patient was released with prescriptions for oral enrofloxacin (Baytril; Bayer Animal Health), 6.6 mg/kg BW, PO, q24h, amoxicillin (Amoxil; Pfizer Animal Health), 33 mg/kg BW, PO, q12h, famotidine (Famotidine; Apotex, Toronto, Ontario), 0.44 mg/kg BW, PO, q24h, and maropitant (Cerenia; Pfizer Animal Health), 2.6 mg/kg BW, PO, q24h. The owner was instructed to perform coupage on the left hemithorax for 5 min, q6h, and to return 5 d later for recheck physical examination and thoracic radiographs.
The dog was readmitted 6 d later for inappetence, hyper-salivation, and vomiting that began following cessation of the maropitant. Concurrent complaints included diarrhea, productive cough, and lethargy. Repeat thoracic radiographs provided similar findings to the ones obtained upon initial presentation. The dog was hospitalized and administered intravenous crystalloid fluid (Plasmalyte; Baxter, Mississauga, Ontario), enrofloxacin (Baytril; Bayer Animal Health), ticarcillin-clavulanate (Timentin; GlaxoSmithKline, Montreal, Quebec), and famotidine (Omega).
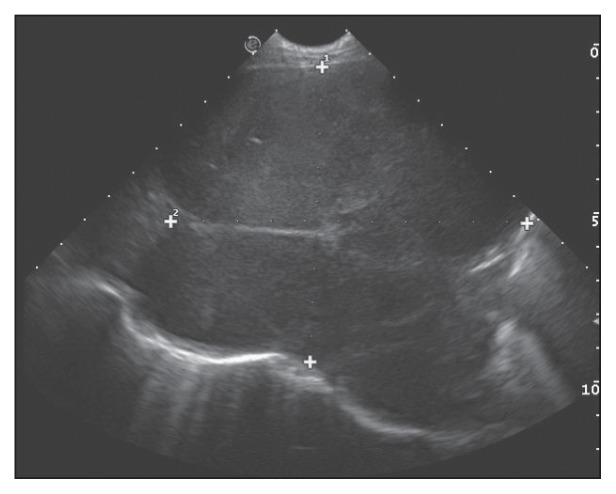
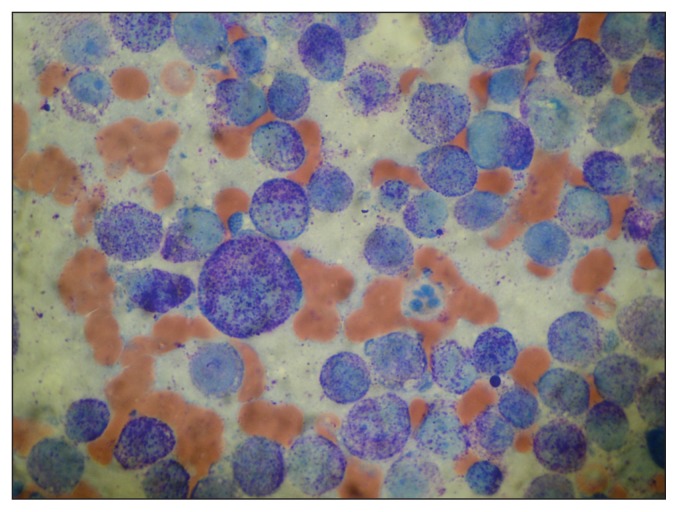
Based on the radiographic findings and poor response to antibiotic therapy, the list of differentials was expanded to include resistant bronchopneumonia, primary pulmonary tumor, and lung lobe torsion. Thoracic ultrasonography (BioSound MyLab 70 × Vision; Esaote, Genoa, Italy) confirmed a mass involving most of the left caudal lung lobe and measuring at least 87 mm × 106 mm (Figure 2). The lesion was hypoechoic and heterogeneous with smooth borders, and contained rare air bubbles. Lung lobe torsion was ruled out based on the vasculature observed with color Doppler. Mild pleural and moderate pericardial effusions were present, and portions of the left caudal lung lobe were atelectatic. Percutaneous transthoracic fine-needle (22G) aspirates were obtained and submitted for cytopathologic analysis and bacterial culture with sensitivity testing. Cytopathology demonstrated mast cells with atypia including moderate to marked anisocytosis and anisokaryosis, a variable N:C ratio, a slightly basophilic cytoplasm that occasionally contained vacuoles, and variable amounts of fine metachromatic granules (Figure 3). Nuclei were central to paracentral and contained granular chromatin and 1 to 2 round nucleoli. Binucleation and macrocytosis were observed. These findings confirmed the mass to be a mast cell tumor with numerous criteria of malignancy, as observed in cutaneous MCT of high histologic grade (17,18). There was no growth on bacterial culture.
Figure 2.
Thoracic ultrasound finding in the dog of Case 1 involving the left caudal lung lobe. Note the hypoechoic and heterogeneous nature of the mass with its smooth borders and rare air bubbles. The mass measured at least 87 mm × 106 mm.
Figure 3.
Photomicrograph of a fine-needle aspirate from the pulmonary mass in the dog of Case 1. Note the moderate anisocytosis and anisocaryosis of the mast cells and their moderate to high quantity of fine granules. Red blood cells are also visible in the background. Wright-Giemsa stain; Magnification 1000×.
In the following 24 h the dog had diarrhea with melena and episodes of vomiting with partially digested blood. Abdominal discomfort and tachypnea were noted, and the lethargy progressed. The owner was informed of the diagnosis of a pulmonary MCT, with systemic signs possibly caused by hyperhistaminemia. Clinical staging with abdominal ultrasound and thoracic computed tomography (CT) scan, and therapeutic options including surgery, anticancer therapy, and supportive treatments were discussed. However, based on the predicted guarded prognosis and observed deteriorating quality of life, the owner elected to have the dog euthanized. Postmortem examination was not authorized.
Case 2
A 14-year-old, 11-kg (body condition score 5 out of 9) spayed female mixed-poodle dog was presented to CVRS with labored breathing, coughing, diarrhea, and vomiting.
Previous history included chronic cough for 1 y attributed to tracheal collapse. The dog had been presented to the referring veterinarian 1 wk earlier for wheezing. Thoracic radiographs were then obtained and pulmonary edema was suspected. Furosemide 3.6 mg/kg BW, PO, q12h was administered for 3 d, with no improvement. Vomiting, diarrhea, and anorexia were first noted following that visit.
Upon presentation to CVRS, the dog was calm, panting, and dehydrated (estimated at 7% to 8%). Increased respiratory sounds were present on auscultation, and the abdomen was mildly tense upon palpation.
Blood biochemistry profile (Catalyst Dx; IDEXX), CBC (LaserCyte; IDEXX), and urinalysis were performed. The results indicated moderate azotemia with serum urea at 23.3 mmol/L [reference interval (RI): 2.5 to 9.6 mmol/L], and creatinine at 230 μmol/L (RI: 44 to 159 μmol/L). Alkaline phosphatase (ALP) was increased at 402 U/L (RI: 23 to 212 μ/L). The CBC results were within normal limits. The urine specific gravity was low at 1.020 (RI > 1.035), and white blood cells with phagocytized bacteria were present in the urine sediment. Urinary tract infection with chronic kidney disease were suspected. Abdominal and thoracic radiographs were obtained. Abdominal radiographs were unremarkable; however, a severe alveolar pattern was observed in the right caudal lung lobe.
The dog was hospitalized and received intravenous fluid therapy (NaCl 0.45%, Baxter; Dex 50, Rafter Product, Calgary, Alberta), ticarcillin-clavulanate (Timentin; GlaxoSmithKline) 40 mg/kg BW, IV, q8h, famotidine (Famotidine; Omega) 0.5 mg/kg BW, IV, q24h, and maropitant (Cerenia; Pfizer Animal Health), 1 mg/kg BW, SC, q24h. Vomiting subsided during hospitalization, but profuse liquid and mucoid diarrhea persisted, and metronidazole (Metronidazole; McKesson, Toronto, Ontario), 11.4 mg/kg BW, PO, q12h was therefore added to the therapeutic regimen. During hospitalization, the breathing worsened and the dog had to be placed in an oxygen rich environment (40% oxygen).
Abdominal ultrasonography showed no detectable evidence of neoplasia or metastasis in the abdomen. Bilateral chronic degenerative renal changes were observed along with pyelectasis of the left kidney. These changes may have explained the azotemia. Thoracic ultrasonography revealed a heterogenous and hypoechoic mass effect of 30 mm × 20 mm in the right caudal lung lobe. Percutaneous transthoracic fine-needle (22G) aspirates were performed for cytopathologic analysis.
Cytology revealed well-differentiated epithelial cells compatible with benign mesothelial or pulmonary cells, and a second population composed of mast cells distributed individually and in small clusters. Mast cells were large with mild anisocytosis and anisokaryosis. Intra-cytoplasmic metachromatic granules were observed in variable amounts. There was no sign of inflammation indicating that the presence of mast cells was not secondary to an inflammatory reaction. A pulmonary MCT was suspected, but the small number of mast cells precluded a definitive diagnosis.
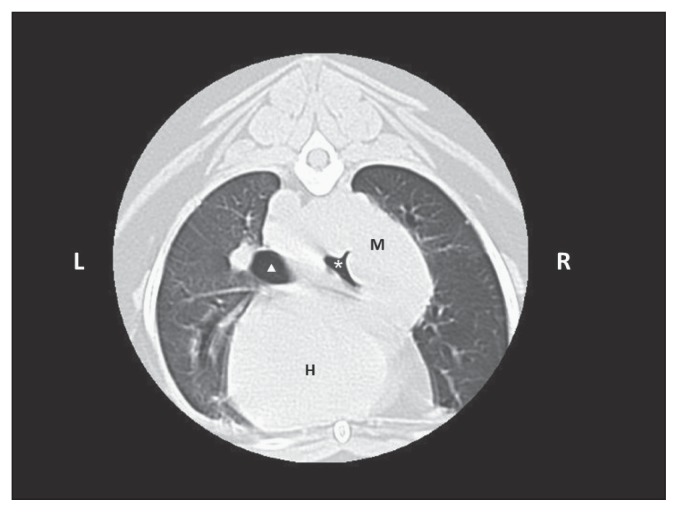
A thoracic CT scan confirmed the presence of a soft-tissue mass in the caudal right lung lobe that extended cranially towards the carina, and towards the left side surrounding half of the main left bronchus. Compression of both main bronchi was present, but was more severe on the right side (Figure 4). The resectability of the mass was questionable, and the likelihood that complete margins could be obtained was considered low. The owner was advised of the poor surgical potential and risks involved, but nevertheless opted to proceed with an exploratory thoracotomy. Preoperative prothrombin and activated partial thromboplastin times (Coag Dx; IDEXX) were both normal.
Figure 4.
Computed tomographic image of the dog of Case 2 at the level of the 6th rib, caudally to bronchi bifurcation. Note the mass (M) in the caudal right lobe, dorsal to the heart (H), associated with a severe compression of the main right bronchus (white asterisk) and a mild compression of the left main bronchus (white triangle).
Thoracotomy at the 6th right intercostal space was performed. Exploration showed an irregular and firm right caudal lung lobe. Other lung lobes appeared normal with appropriate expansion, but the mass encompassed and compressed the bronchi of the cranial and middle right lung lobes. The mass also expanded into the cranial mediastinum, up to the carina, and covered the right atrium. The right vagal nerve travelled through the mass at the mediastinal level. In order to surgically debulk the mass, the whole right lung would have to be removed (Figure 5). It was noted that the left lung also did not adequately ventilate, and a collapsed left main bronchus was suspected. During anesthesia, the dog developed marked hypotension (systolic pressure of 55 mmHg), with no response to crystalloid fluid boluses. Given the poor prognosis, the owners opted for intraoperative euthanasia.
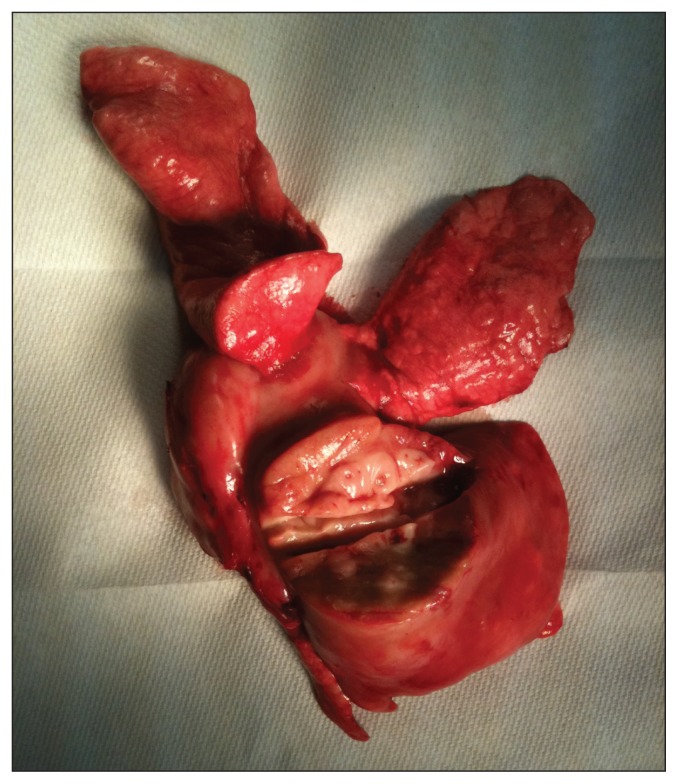
Figure 5.
Photograph of the resected right lung of the dog of Case 2 showing the mass (bottom) incised for fixation.
Direct impression slides of the mass were submitted for cytopathology. Mast cells with atypia were observed, including mild to marked anisocaryosis and anisocytosis, and a mildly to moderately increased N:C ratio. The cytoplasm was mildly basophilic with a moderate to high quantity of metachromatic granules. Binucleation was occasionally observed. Individual eosinophils and macrophages were observed in small numbers. Cohesive clusters of epithelial cells morphologically compatible with pulmonary cells were also noted; those cells showed no atypia, except for occasional small dysplastic clumps. A cytopathologic diagnosis of MCT was obtained.
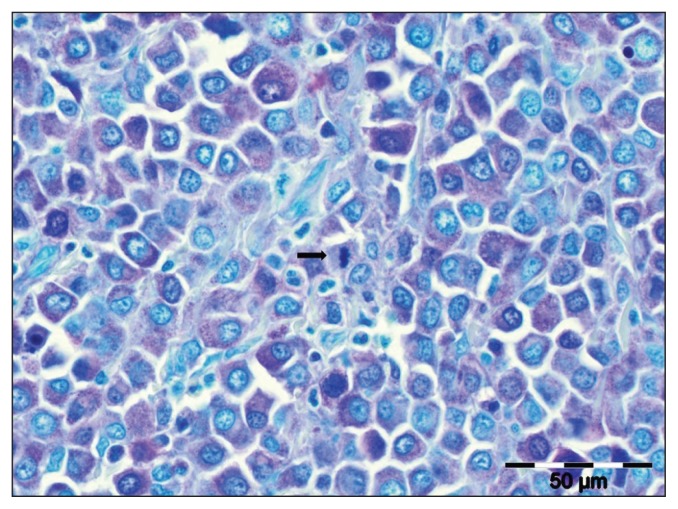
A complete postmortem examination was declined but the owners permitted histopathology of the pulmonary mass. The sample was highly cellular and composed of a population of round to polygonal cells arranged in sheets. It was nonencapsulated, infiltrative, and effaced the pulmonary architecture. Cells had variably discrete cell borders, a moderate amount of amphophilic cytoplasm, and a variably prominent nucleolus. Moderate cellular atypia was present, and the mitotic rate was highly variable with a mitotic count of 7 per 10 high-powered fields (400×). Rare neoplastic cells contained pale eosinophilic granules. Eosinophils were admixed with the neoplastic population. The neoplastic cells and eosinophils were occasionally observed within the lumen of bronchi and bronchioli. Low numbers of alveolar macrophages and neutrophils were scattered amongst the neoplastic cell population and present within airways. There were regions in which the atypical population appeared to be infiltrating the bronchiolar epithelium, suggesting epitheliotropism. Vascular invasion by neoplastic cells was not observed. There was no histopathological evidence of intrapulmonary metastases within other lung lobes. Histologic features were compatible with a pulmonary round cell tumor, and a poorly granulated MCT was suspected. Staining with Giemsa and toluidine blue (Figure 6) showed metachromatic granules and confirmed the neoplastic cells to be of mast cell phenotype. Additional sections of the submitted tissues were also evaluated and lymph nodes were identified. A large number of neoplastic cells infiltrated and effaced large portions of the tracheobronchial lymph nodes, confirming regional metastasis of the pulmonary MCT.
Figure 6.
Photomicrograph of a histopathological section of the pulmonary mass (Case 2), confirming a mast cell tumor. Note the mitotic figure in center (black arrow). Toluidine blue stain; Bar = 50 μm.
Discussion
This report describes presumptive primary pulmonary MCT in 2 dogs, neither of which had a previous history of cutaneous MCT. Cytology is a central tool in the diagnosis of MCT and has been shown in many instances to be more sensitive than histopathology (19). A cytologic diagnosis of MCT with atypia was obtained in both cases, and histopathology with toluidine blue staining further supported the diagnosis in Case 2. Importantly, both dogs were presented with massive solitary pulmonary MCT, a very unusual presentation. A similar presentation of voluminous pulmonary MCT has only been reported once previously, in a dog with a concurrent splenic mass (6). The clinical presentation of both dogs described herein differs from subtle (15,20) and multifocal (7) pulmonary lesions, which may be less unusual.
The presence of neoplastic mast cells infiltrating the epithelium, as observed in Case 2, is also a very uncommon finding. The 2 main canine round cell tumors known for epitheliotropism are cutaneous epitheliotropic lymphoma, and cutaneous histiocytoma (21). The presence of neoplastic mast cells demonstrating epitheliotropism has only previously been described in a report on 3 canine cutaneous MCT (21). Two were of grade II (22) or low grade (23), and were near mucocutaneous junctions without mutation of the c-kit gene. The third case was a grade III (22), or high grade (23), with a mutated c-kit gene. Since the first 2 cases were located near mucocutaneous junctions, it was suggested that epitheliotropic MCTs may have a tropism for mucosal surfaces. It was also suspected that the release of proteolytic enzymes such as matrix metalloproteinases (MMPs) may be involved in the epitheliotropism by causing damage to the basement membrane (21). Epitheliotropism has also been described in a cat with disseminated cutaneous MCT and systemic mastocytosis (24). Case 2 in the present report is the first in which an MCT affecting the lung with histopathological characteristics suggestive of epitheliotropism has been described.
An important limitation for Case 1 is the lack of abdominal ultrasound or advanced imaging; it therefore remains possible that abdominal mast cell disease was present and unidentified. It can only be presumed that this case may represent a primary pulmonary MCT. Even if abdominal involvement had been documented, the presentation would remain unusual because no primary cutaneous MCT was documented, a large solitary pulmonary lesion was identified, and pulmonary involvement with vMCT is a rarely described phenomenon in dogs. Abdominal ultrasonography was performed on Case 2 and no detectable lesion suggestive of abdominal dissemination or primary abdominal MCT was observed. Sensitivity of ultrasonography for detection of mast cell infiltration in liver and spleen has been reported to be low compared with cytopathologic analysis (25). Cytology of the spleen and liver could have been performed in this case even though both appeared normal on ultrasonography. Advanced medical imaging, such as whole body CT scan or magnetic resonance imaging (MRI), was not performed on either case. Additional lesions may consequently have gone undetected. Knowing that the clinical staging of Case 2 only showed intrathoracic mast cell disease, it can be presumed that it is the first reported case of canine primary pulmonary MCT.
Limitations of our case presentations also include the lack of additional analyses such as buffy coat smear, bone marrow cytology or biopsy, and postmortem examination. A buffy coat smear is seldom performed in the clinical evaluation of canine MCT because of low sensitivity and specificity for the detection of circulating malignant mast cells (26). It may have been indicated in our cases since there is currently no information concerning the buffy coat smear analysis of dogs with primary and secondary pulmonary MCT. Bone marrow examination and complete postmortem examination could have revealed additional lesions. Concurrent mast cell disease at nonpulmonary sites may therefore have gone undetected. There is also a possibility that an underlying process may have caused a mast cell hyperplastic condition that could have been misinterpreted as MCT. For example, canine pulmonary lymphoma (lymphomatoid granulomatosis) lesions often demonstrate infiltrates of leucocytes, such as lymphocytes, eosinophils, and plasma cells (1). Lymphocyte immunochemistry markers could therefore have been used to exclude a primary lymphoproliferative cause. Similarly, immunochemistry could have further supported the diagnosis of MCT by excluding other round cell neoplasia. With convincing cytology results in both cases, and the histopathologic diagnosis with special stains suggesting a mast cell phenotype in Case 2, it seems unlikely that a different diagnosis would have been obtained.
In both cases, the KIT protein staining pattern and the c-kit gene mutation status were not evaluated. In the last decade, tyrosine kinase inhibitors (TKIs), such as toceranib (Palladia, Pfizer) and masitinib (Masivet/Kinavet, AB Science), were developed to treat canine MCT by inhibiting KIT signaling. Investigating the c-kit mutation status in our 2 cases may have justified the therapeutic use of a TKI and could also have been a prognostic indicator. However, because the most common side effects of TKIs are gastrointestinal (vomiting and diarrhea), their use could have been problematic herein since both dogs were presented with serious gastrointestinal signs.
Mast cells have cytoplasmic secretory granules that contain numerous biologically active pre-formed substances including histamine, heparin, and various proteases. Hyperhistaminemia may lead to gastrointestinal ulceration via histamine binding to the H2 receptors on gastric parietal cells, stimulating hydrochloric acid production (2). It has been demonstrated that plasma histamine concentration is significantly higher in dogs with MCT compared with clinically healthy dogs (27), and progressive elevation in plasma histamine concentration may correlate with advanced disease (28). Gastrointestinal signs suggestive of mast cell degranulation and probable hyperhistaminemia with secondary ulceration of the digestive tract were prominent features of both cases in this report. Release of preformed amines from cytoplasmic granules can also lead to vasodilation (2). The marked hypotension observed during surgery in Case 2 may have been caused by mast cell degranulation and resultant hyperhistaminemia. Plasma histamine measurements are not commonly evaluated in clinical practice, but could have helped to correlate the observed clinical signs with hyperhistaminemia. Postmortem examination could also have shown changes suggestive of decreased gastric pH secondary to hyperhistaminemia, such as gastroduodenal erosions or ulcerations.
In conclusion, the 2 dogs discussed here were presented with large, solitary, pulmonary masses; a very unusual presentation for canine MCT. Another uncommon finding compared with previously reported cases of canine intrathoracic mast cell disease, is that no primary cutaneous or visceral MCT was detected elsewhere. Prognosis was poor and both dogs had systemic signs suggestive of mast cell degranulation and probable hyperhistaminemia. Characteristics suggestive of epitheliotropism were observed in 1 case; another uncommon finding with canine MCT. Although rare, MCT should be included in the differential diagnosis of solitary pulmonary masses in dogs, even in the absence of cutaneous lesions.
Acknowledgments
The authors acknowledge the VCA Canada for providing the funds necessary for publication of this article. The authors also thank Dr. Yasmine Raphaël for kindly reviewing the manuscript prior to submission. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Rebhun RB, Culp WTN. Pulmonary neoplasia. In: Withrow SJ, MacEwen EG, editors. Small Animal Clinical Oncology. 5th ed. Philadelphia, Pennsylvania: Saunders; 2013. pp. 453–462. [Google Scholar]
- 2.London CA, Thamm DH. Mast cell tumors. In: Withrow SJ, MacEwen EG, editors. Small Animal Clinical Oncology. 5th ed. Philadelphia, Pennsylvania: Saunders; 2013. pp. 335–355. [Google Scholar]
- 3.Elliott JW, Cripps P, Blackwood L, Berlato D, Murphy S, Grant IA. Canine oral mucosal mast cell tumours. Vet Comp Oncol. 2016;14:101–111. doi: 10.1111/vco.12071. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T, Kadosawa T, Nagase M, et al. Visceral mast cell tumors in dogs: 10 cases (1982–1997) J Am Vet Med Assoc. 2000;216:222–226. doi: 10.2460/javma.2000.216.222. [DOI] [PubMed] [Google Scholar]
- 5.Welle MM, Bley CR, Howard J, Rüfenacht S. Canine mast cell tumours: A review of the pathogenesis, clinical features, pathology and treatment. Vet Dermatol. 2008;19:321–339. doi: 10.1111/j.1365-3164.2008.00694.x. [DOI] [PubMed] [Google Scholar]
- 6.Davies AP, Hayden DW, Perman V. Noncutaneous systemic mastocytosis and mast cell leukemia in a dog: Case report and literature review. J Am Anim Hosp Assoc. 1981;17:361–368. [Google Scholar]
- 7.Pollack MJ, Flanders JA, Johnson RC. Disseminated malignant mastocytoma in a dog. J Am Anim Hosp Assoc. 1991;27:435–440. [Google Scholar]
- 8.Spann DR1, Sellon RK, Thrall DE, Bostian AE, Boston GT. Computed tomographic diagnosis: Use of computed tomography to distinguish a pulmonary mass from alveolar disease. Vet Radiol Ultrasound. 1998;39:532–535. doi: 10.1111/j.1740-8261.1998.tb01645.x. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt M, Dercken C, Loke O, et al. Pulmonary manifestation of systemic mast cell disease. Eur Respir J. 2000;15:623–625. doi: 10.1034/j.1399-3003.2000.15.32.x. [DOI] [PubMed] [Google Scholar]
- 10.Hafner S, Latimer K. Cutaneous mast cell tumours with pulmonary metastasis in a hen. Avian Pathol. 26:657–663. doi: 10.1080/03079459708419242. 199. [DOI] [PubMed] [Google Scholar]
- 11.Hill JE, Langheinrich KA, Kelley LC. Prevalence and location of mast cell tumors in slaughter cattle. Vet Pathol. 1991;28:449–450. doi: 10.1177/030098589102800514. [DOI] [PubMed] [Google Scholar]
- 12.Khan KN, Sagartz JE, Koenig G, Tanaka K. Systemic mastocytosis in a goat. Vet Pathol. 1995;32:719–721. doi: 10.1177/030098589503200616. [DOI] [PubMed] [Google Scholar]
- 13.Cowgill E, Neel J. Pleural fluid from a dog with marked eosinophilia. Vet Clin Pathol. 2003;32:147–149. doi: 10.1111/j.1939-165x.2003.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 14.Harris BJ, Constantino-Casas F, Archer J, Herrtage ME. Loeffler’s endocarditis and bicavity eosinophilic effusions in a dog with visceral mast cell tumour and hypereosinophilia. J Comp Pathol. 2013;149:429–433. doi: 10.1016/j.jcpa.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Hikasa Y, Morita T, Futaoka Y, et al. Connective tissue-type mast cell leukemia in a dog. J Vet Med Sci. 2000;62:187–190. doi: 10.1292/jvms.62.187. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs T, Hoppe B, Moore F. Visceral mast cell tumour in a dog with haemabdomen and mastocytaemia. J Small Anim Pract. 2007;48:180. doi: 10.1111/j.1748-5827.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- 17.Lund JE, Park JF. Focal mastocytosis in lymph nodes from a Beagle dog. Vet Pathol. 1978;15:64–67. doi: 10.1177/030098587801500108. [DOI] [PubMed] [Google Scholar]
- 18.Scarpa F, Sabattini S, Bettini G. Cytological grading of canine cutaneous mast cell tumours. Vet Comp Oncol. 2016;14:245–251. doi: 10.1111/vco.12090. [DOI] [PubMed] [Google Scholar]
- 19.Duncan JR, Prasse KW. Cytology of canine cutaneous round cell tumors. Mast cell tumor, histiocytoma, lymphosarcoma and transmissible venereal tumor. Vet Pathol. 1979;16:673–679. doi: 10.1177/030098587901600605. [DOI] [PubMed] [Google Scholar]
- 20.Hottendorf GH, Nielsen SW. Pathologic report of 29 necropsies on dogs with mastocytoma. Pathol Vet. 1968;5:102–121. doi: 10.1177/030098586800500202. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira FN, Elliott JW, Lewis BC, et al. Cutaneous mast cell tumor with epitheliotropism in 3 dogs. Vet Pathol. 2013;50:234–237. doi: 10.1177/0300985812451626. [DOI] [PubMed] [Google Scholar]
- 22.Patnaik AK, Ehler WJ, MacEwen EG. Canine cutaneous mast cell tumor: Morphologic grading and survival time in 83 dogs. Vet Pathol. 1984;21:469–474. doi: 10.1177/030098588402100503. [DOI] [PubMed] [Google Scholar]
- 23.Kiupel M, Webster JD, Bailey KL, et al. Proposal of a 2-tier histologic grading system for canine cutaneous mast cell tumors to more accurately predict biological behavior. Vet Pathol. 2011;48:147–155. doi: 10.1177/0300985810386469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamm CG, Stern AW, Smith AJ, Cooper EJ, Ullom SW, Campbell GA. Disseminated cutaneous mast cell tumors with epitheliotropism and systemic mastocytosis in a domestic cat. J Vet Diagn Invest. 2009;21:710–715. doi: 10.1177/104063870902100520. [DOI] [PubMed] [Google Scholar]
- 25.Book AP, Fidel J, Wills T, Bryan J, Sellon R, Mattoon J. Correlation of ultrasound findings, liver and spleen cytology, and prognosis in the clinical staging of high metastatic risk canine mast cell tumors. Vet Radiol Ultrasound. 2011;52:548–554. doi: 10.1111/j.1740-8261.2011.01839.x. [DOI] [PubMed] [Google Scholar]
- 26.McManus PM. Frequency and severity of mastocytemia in dogs with and without mast cell tumors: 120 cases (1995–1997) J Am Vet Med Assoc. 1999;215:355–357. [PubMed] [Google Scholar]
- 27.Fox LE, Rosenthal RC, Twedt DC, Dubielzig RR, MacEwen EG, Grauer GF. Plasma histamine and gastrin concentrations in 17 dogs with mast cell tumors. J Vet Intern Med. 1990;4:242–246. doi: 10.1111/j.1939-1676.1990.tb03116.x. [DOI] [PubMed] [Google Scholar]
- 28.Ishiguro T, Kadosawa T, Takagi S, et al. Relationship of disease progression and plasma histamine concentrations in 11 dogs with mast cell tumors. J Vet Intern Med. 2003;17:194–198. doi: 10.1111/j.1939-1676.2003.tb02433.x. [DOI] [PubMed] [Google Scholar]