Abstract
A novel bovine astrovirus (BoAstV CH13/NeuroS1) has been associated with encephalitis in cattle in Europe and the USA. We retrospectively analyzed feedlot cattle with encephalitis of unknown etiology for this virus by in-situ hybridization. Results suggest that BoAstV CH13/NeuroS1 is a major cause of encephalitis in western Canadian feedlot cattle.
Résumé
Infection par l’astrovirus bovin chez les bovins de parcs d’engraissement atteints d’une maladie neurologique dans l’Ouest canadien. Un nouvel astrovirus bovin (BoAstV CH13/NeuroS1) a été associé à l’encéphalite chez les bovins en Europe et aux États-Unis. Nous avons effectué une analyse rétrospective des bovins des parcs d’engraissement atteints d’encéphalite d’étiologie inconnue pour ce virus à l’aide d’une hybridation in situ. Les résultats suggèrent que BoAstV CH13/NeuroS1 est une cause majeure d’encéphalite chez les bovins des parcs d’engraissement dans l’Ouest canadien.
(Traduit par Isabelle Vallières)
Neurological disease due to non-suppurative encephalitis often occurs sporadically in cattle and in many instances it is difficult to determine the etiology. Such cases have been registered in a number of countries over the past decades and first reports date back to the 1930s (1). In a recent study, Sanchez et al (2) investigated neurologically severely diseased feedlot beef cattle from western Canada with the histopathological diagnosis of non-suppurative encephalitis and undetermined etiology. Animals were between 7 and 24 mo old and had been diagnosed between 1987 and 2010. In total, brains of 37 animals were characterized by histopathology and tested by immunohistochemistry or polymerase chain reaction (PCR) protocols for several viral, bacterial, and parasitic agents that are known to cause encephalitis. These analyses included bovine viral diarrhea virus, various members of the family Herpesviridae, parainfluenza virus 3, West Nile virus, rabies virus, Listeria monocytogenes, Histophilus somni, Mycoplasma bovis, Chlamydophila spp., Neospora spp., and Toxoplasma gondii. Results of this study showed that rabies virus was found in 1 animal, but in the other cases all tests were negative and the cause of the disease remained inconclusive.
In 2013, Li et al (3) and our laboratory (4) used a metagenomics approach to detect a novel bovine astrovirus (BoAstV CH13/NeuroS1) in brains of cattle with non-suppurative encephalitis of unknown etiology (4). Until then, astroviruses had primarily been associated with enteric infections in various mammalian species (5). However, recently they have increasingly been identified in brain tissues of human patients and animals with neurological disease and encephalitis (6–8). This has led to the hypothesis that astroviruses are one of the causes for sporadic bovine non-suppurative encephalitis.
The aim of the present study was to investigate brain samples of unresolved non-suppurative bovine encephalitis cases from Canada for the presence of BoAstV CH13/NeuroS1.
We analyzed formalin-fixed paraffin-embedded (FFPE) tissue from different areas of the central nervous system of 9 out of 37 feedlot cattle from western Canada that had been previously diagnosed as non-suppurative encephalitis of unknown etiology and included in the first report by Sanchez et al (2). Material was provided by the Prairie Diagnostic Services, Western College of Veterinary Medicine, University of Saskatchewan, Saskatoon. Cases were selected based on lesions observed in astrovirus-positive cattle brains reported in a previous study (3) and comprised case numbers 7 to 9, 13, 22, 23, 31, 35 and 37 (2). Two BoAstV CH13/NeuroS1 positive controls and 7 negative controls from healthy animals were obtained from the archives of the Vetsuisse Faculty, University of Bern, Switzerland. A non-radioactive in-situ hybridization (ISH) was used for the detection of BoAstV CH13/NeuroS1 RNA as described previously (4). Briefly, sections of 4 μm were deparaffinized, rehydrated, treated with 0.2 M HCl, Proteinase K (Roche), and 4% paraformaldehyde. The prehybridization mixture included 50% deionized formamide, 0.05% ficoll, 0.05% polyvinylpyrrolidone, 0.05% BSA, 4 × SSC, and 0.25% yeast RNA. This mixture was added at a temperature of 50°C for 2 h, after which a digoxygenin-UTP (DIG) labelled antisense RNA probe (probe A) was added at a concentration of 1 ng/mL to the hybridization mixture (0.5% yeast RNA, 10% dextran sulfate, 50% deionized formamide, 0.05% ficoll, 0.05% polyvinylpyrrolidone, 0.05% BSA, 4 × SSC). Probe A is complementary to a 167 nucleotide sequence at the 5′ end of open reading frame 2 (ORF2), which encodes the viral capsid proteins. Sections were incubated in the hybridization mixture for 15 h at 50°C. After washing steps and RNAse treatment the probe was detected with anti-digoxygenin-AP-Fab fragments (Roche) and NBT/BCIP substrate (Roche).
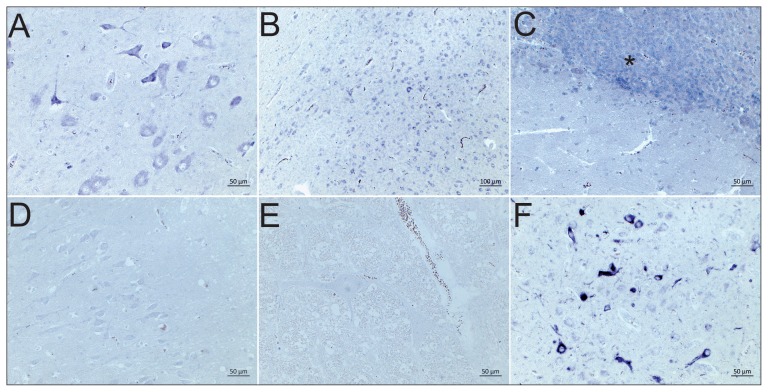
BoAstV CH13/NeuroS1 RNA was detected in several tested brain regions in 4 of the 9 cases (Table 1), appearing as a deep purple staining in the cytoplasm of neurons, similar to that observed in positive controls (Figure 1). All negative controls remained unstained. Consistent with our previous observations, mainly neurons were labelled. We did not always have comparable brain structures available in these animals, but BoAstV CH13/NeuroS1 was frequently detected in the midbrain, the thalamus, the hippocampus, and also in the cerebellum. However, the number of cells infected with BoAstV CH13/NeuroS1 did not correlate with the severity of inflammation (Table 1). We made a similar observation in a series of BoAstV CH13/NeuroS1 positive cases in Switzerland (9). This divergence may be related to clearance of the virus in areas with prominent lesions at the time of death, specific mechanisms of neuroinvasion as well as intracerebral spread or to other factors that influence disease pathogenesis, such as co-infections with other pathogens.
Table 1.
Correlation of viral RNA and histopathological lesions in brains of BoAstV CH13/NeuroS1 positive feedlot cattle with non-suppurative encephalitis
| Case IDa | Brain structure | BoAstV CH13/NeuroS1 in-situ hybridization gradeb | Histopathology | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Grade of inflammation | Meningitis | Gliosis | ||||
|
| ||||||
| Percent of blood vessels with peri-vascular cuffs (%)c | Number of cell layers in the peri-vascular cuffsd | |||||
| 31 | Medulla oblongata | 1 | 1 | +++ | N | Y |
| Midbraine,f | 3 | 1 | +++ | N | Y | |
| Cerebellume | 0 | 1 | +++ | N | Y | |
| Cerebrum | 3 | 0 | — | N | N | |
| Thalamus | 3 | 1 | + | N | Y | |
| 37 | Medulla oblongata | 0 | 1 | +++ | N | N |
| Cerebellum | 2 | 0 | — | N | Y | |
| Cerebrum | 3 | 0 | — | N | N | |
| 35 | Medulla oblongata | 0 | 1 | + | N | Y |
| Midbraine | 2 | 1 | + | N | Y | |
| Cerebellum | 0 | 0 | — | Y | Y | |
| Cerebrume | 1 | 1 | + | Y | Y | |
| Hippocampus | 0 | 1 | + | N | N | |
| Thalamuse | 2 | 2 | + | N | Y | |
| 23 | Medulla oblongata | 0 | 1 | +++ | N | Y |
| Midbraine | 2 | 1 | ++ | N | Y | |
| Cerebellum | 0 | 0 | — | N | Y | |
| Cerebrum | 1 | 0 | — | N | N | |
| Hippocampus | 3 | 0 | — | N | N | |
| Thalamus | 1 | 0 | — | N | N | |
Case identification from Sanchez et al (2).
ISH grading (0–3): 0 — astrovirus negative; 1 — mild astrovirus infection (1 to 10 cells stained positive); 2 — moderate astrovirus infection (11 to 50 cells); 3 — severe astrovirus infection (> 50 cells).
Percentage of affected blood vessels per section: 0 — none; 1 — < 25%; 2% — 25% to 50%; 3 — > 50%.
Number of cell layers in the perivascular cuffs: + — scant number of cells not forming a complete concentric ring around the vessel to 1 complete cell layer; ++ — 2 to 3 complete cell layers; +++ — more than 3 complete cell layers.
Glial nodules present
Neuronophagia observed.
Y — present; N — absent.
Figure 1.
Detection of BoAstV CH13/NeuroS1 in brain sections by RNA in-situ hybridization. A — Case 23, midbrain and B — case 31, cerebral cortex with strong dark purple positive labeling of neurons; C — Case 37, cerebellum; the granular layer shows a strong neuronal positive RNA labelling in almost all granular cells (asterisk); D — BoAstV Ch13/NeuroS1 negative case 8, brainstem; E — BoAstV CH13/NeuroS1 negative control; F — BoAstV CH13/NeuroS1 positive control.
So far, BoAstV CH13/NeuroS1 has been reported in cattle with encephalitis from the USA, Switzerland and the UK (3,4,10). Our results suggest that some of the cases investigated by Sanchez et al (2) (4 out of 9 tested) were indeed infected with BoAstV CH13/NeuroS1 and raise the possibility that this virus is a major cause of neurological disease in feedlot cattle in western Canada. This is in agreement with our findings in Switzerland, where ~34% of the cases of non-suppurative bovine encephalitis are BoAstV CH13/NeuroS1 positive in the brain (9). Taken together, these results provide further evidence that this virus is i) associated with disease, and ii) geographically widespread.
It remains to be studied whether or not BoAstV CH13/NeuroS1 replicates in the bovine enteric system or in other extraneuronal tissues. Also, we do not know at which frequency astrovirus associated encephalitis occurs, because affected animals may not be reported or further investigated, especially in intensive industrial farming. There is a need to investigate neurologically diseased cattle and related farms thoroughly in order to gain knowledge on the epidemiology of the disease and its importance for animal health and livestock production. Basic diagnostic tools for this purpose are available and are being further developed in our laboratory. CVJ
Footnotes
This study was supported by the Alberta Beef Producers, the Swiss Food Safety and Veterinary Office, and the Swiss National Science Foundation.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Frauchiger E, Hofmann W. Nervous diseases of cattle. Bern, Switzerland: Medizinischer Verlag Hans Huber; 1941. [Google Scholar]
- 2.Sanchez S, Clark EG, Wobeser GA, Janzen ED, Philibert H. A retrospective study of non-suppurative encephalitis in beef cattle from western Canada. Can Vet J. 2013;54:1127–1132. [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Diab S, McGraw S, et al. Divergent astrovirus associated with neurologic disease in cattle. Emerg Infect Dis. 2013;19:1385–1392. doi: 10.3201/eid1909.130682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouzalas IG, Wuthrich D, Walland J, et al. Neurotropic astrovirus in cattle with nonsuppurative encephalitis in Europe. J Clin Microbiol. 2014;52:3318–3324. doi: 10.1128/JCM.01195-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosch A, Pinto RM, Guix S. Human astroviruses. Clin Microbiol Rev. 2014;27:1048–1074. doi: 10.1128/CMR.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cordey S, Vu DL, Schibler M, et al. Astrovirus MLB2, a new gastro-enteric virus associated with meningitis and disseminated infection. Emerg Infect Dis. 2016;22:846–853. doi: 10.3201/eid2205.151807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quan PL, Wagner TA, Briese T, et al. Astrovirus encephalitis in boy with X-linked agammaglobulinemia. Emerg Infect Dis. 2010;16:918–925. doi: 10.3201/eid1606.091536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wunderli W, Meerbach A, Gungor T, et al. Astrovirus infection in hospitalized infants with severe combined immunodeficiency after allogeneic hematopoietic stem cell transplantation. PloS One. 2011;6:e27483. doi: 10.1371/journal.pone.0027483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selimovic-Hamza S, Boujon CL, Hilbe M, Oevermann A, Seuberlich T. Frequency and pathological phenotype of bovine astrovirus CH13/NeuroS1 infection in neurologically-diseased cattle: Towards assessment of causality. Viruses. 2017;9:12. doi: 10.3390/v9010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.APHA Disease Surveillance Report. Bovine astrovirus associated with encephalitis in cattle. Vet Rec. 2015;177:91–95. doi: 10.1136/vr.h3860. [DOI] [PubMed] [Google Scholar]