Abstract
Bovine astrovirus (BoAstV) was identified by reverse transcriptase polymerase chain reaction on brain tissue of 2 feedlot cattle that died of non-suppurative encephalitis. Sequencing demonstrated a high degree of identity with neurotropic US and Swiss BoAstV strains. To our knowledge, this is the first confirmed report of BoAstV-associated encephalitis in cattle residing in eastern Canada.
Résumé
Identification de l’astrovirus bovin dans des cas d’encéphalite bovine non suppurative dans l’Est du Canada. L’astrovirus bovin (BoAstV) a été identifié par amplification en chaîne par la polymérase avec la transcriptase réverse sur des tissus du cerveau de 2 bovins de parcs d’engraissement qui étaient morts d’encéphalite non suppurative. Le séquençage a démontré un haut niveau d’homologie avec les souches neurotropiques américaine et suisse de BoAstV. À notre connaissance, il s’agit du premier rapport confirmé d’une encéphalite associée à BoAstV chez le bétail résidant dans l’Est du Canada.
(Traduit par Isabelle Vallières)
In the spring of 2016, the Animal Health Laboratory, University of Guelph received fresh and formalin-fixed samples of brain from 2 cattle located in separate small feedlots, each comprised of 25 to 50 animals. Animal #1 (Can1_16-035113) was a 2-year-old Limousin bull that was born and resided in Saskatchewan for the first 14 months of its life before being sold to an operation in southern Ontario. It lived in southern Ontario for a subsequent 14-month period where it co-mingled with a few other translocated western Canadian cattle. Animal #2 (Can2_16-047183) was a 1-year-old Hereford steer that was born and raised in a closed herd located in southern Ontario. Both animals were found dead after exhibiting neurologic signs for several days. In 1 animal, tonic-clonic seizures were elicited by stimulation, and repetitive licking and chewing movements were also described. Rabies remains a differential diagnosis in cattle dying of neurologic disease in Ontario, and therefore, hemisected brains were submitted to the Canadian Food Inspection Agency (Ottawa Laboratory, Fallowfield, Ontario) for rabies fluorescent antibody testing. Both tested negative.
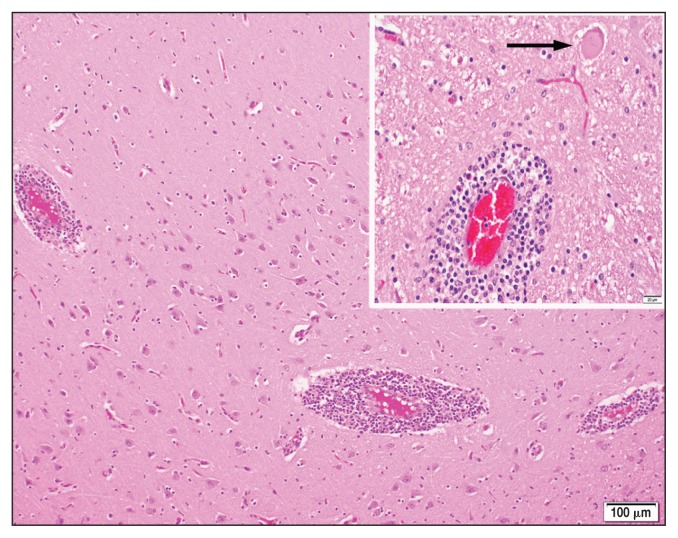
Histologic examination of routine hematoxylin and eosin-stained sections of brain revealed generalized, broad perivascular cellular cuffs extending from the cerebral cortex into the brain-stem. Leukocytic cuffs expanding Virchow-Robin spaces were comprised of 3 to 8 layers of lymphocytes with a few admixed histiocytes, rare neutrophils, and plasma cells (Figure 1). Foci of mild microgliosis and neuronal necrosis were also observed within some sections. Necrotic neurons were distributed sporadically throughout the neuropil, and were characterized by either rounded, hypereosinophilic perikarya (Figure 1, inset), or pale-staining, swollen indistinct structures. Regional non-suppurative meningitis within several cerebral sulci was typified by coalescing non-suppurative perivascular cuffs surrounding meningeal blood vessels; however, overt vasculitis was not observed. Based upon the histologic lesions, the negative rabies test result, and regional diagnostic frequency, listerial meningoencephalitis and infections with bovine herpesvirus-1 (BoHV-1) and malignant catarrhal fever (MCF) virus were considered for ancillary test selection. Immunohistochemical staining for Listeria monocytogenes in paraffin-embedded sections of brain was negative in both cases, as were polymerase chain reaction (PCR) tests for BoHV-1 and MCF virus in fresh sections of brain (MCF PCR performed by Prairie Diagnostic Services, Saskatoon, Saskatchewan).
Figure 1.
Non-suppurative perivascular cuffing in cerebral cortex.
Inset — arrow indicates necrotic neuron. Hematoxylin and eosin stain.
Recently, 2 novel groups of astrovirus have been detected in cases of bovine non-suppurative encephalitis. These include: i) BoAstV CH13 identified by Bouzalas et al (1) in Switzerland which is closely related to NeuroS1 found by Li et al (2) in the US; and ii) BoAstV CH15 identified by Seuberlich et al (3) in Switzerland which is similar to the virus described by Schlottau et al (4) in Germany. Therefore, samples of fresh brain from these 2 Canadian cattle were tested by a reverse transcriptase (RT)-PCR targeting the coding region of the mammalian astrovirus RNA-dependent RNA polymerase. Brain samples were swab-stabbed and swabs were agitated in 1 mL of sterile phosphate-buffered saline. Nucleic acids were extracted from 50-μL aliquots using the MagMAX-96 Viral RNA Isolation Kit in a MagMAX Express-96 Magnetic Particle Processor (Thermo Fisher Scientific, Mississauga, Ontario). The RT-PCR was performed with Qiagen One-Step RT-PCR kit (Qiagen, Mississauga, Ontario) by following the manufacturer’s instructions and using the primers described by Mittelholzer et al (5): MiAstV_MA2 5′-GGCTTTACCCACATICCAAA-3′ and MiAstV_MA4 5′-TGGACCCGCTATGATGGCACIAT-3′. The RT-PCR was carried out with a LightCycler 96 (Roche, Laval, Quebec) employing the following performance criteria: RT, 50°C 30 min; polymerase activation, 95°C 15 min; 40 cycles of amplification, 52°C 45 s, 72°C 1 min; final extension, 72°C 10 min. The PCR products were visualized with an E-Gel Imager (Thermo Fisher Scientific) then sequenced in a molecular biology facility (Laboratory Services Division, University of Guelph, Guelph, Ontario). The nucleotide sequences were analyzed with DNAstar software (DNASTAR, Madison, Wisconsin, USA).
Test results were positive in both cases, and the 428 base pair PCR products were sequenced and sequences were compared to other mammalian astrovirus sequences in GenBank. The sequences from both Canadian cases (Can1_16-035113 and Can2_16-047183) showed the highest percentages of identity, 94.4% and 94.6%, to a bovine astrovirus (BoAstV) detected in the brain of cattle with neurologic disease in the US (2) (Table 1). High percentages of identity, 91.1% to 92.5% were also noted to BoAstV CH13 detected in brain samples from cattle with encephalitis in Switzerland (1) (Table 1). A diagnosis of presumptive BoAstV-associated encephalitis was therefore assigned to both Canadian cases. A subsequent 3rd confirmed case was a 7-year-old Holstein cow that had been imported from California into eastern Ontario in 2012.
Table 1.
Pairwise sequence distances of partial, 428 bp fragments of RNA-dependent RNA polymerase gene of representative bovine astroviruses (BoAstV) from Canada (Can), United States (US) and Switzerland (CH). (GenBank accession numbers for bovine astrovirus nucleotide sequences reported in this manuscript are KY614055 and KY614056.)
| Divergence | Percent identity | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | ||||
| 1 | 97.2 | 94.4 | 92.5 | 91.6 | 91.4 | 1 | Can1_16-035113_KY614055 | ||
| 2 | 2.9 | 94.6 | 92.5 | 91.4 | 91.1 | 2 | Can2_16-047183_KY614056 | ||
| 3 | 5.9 | 5.6 | 91.6 | 92.1 | 92.3 | 3 | US_NeuroS1_KF233994 | ||
| 4 | 8.0 | 8.0 | 9.2 | 93.5 | 92.8 | 4 | CH13_NeuroS1_26730_KX266902 | ||
| 5 | 9.1 | 9.4 | 8.6 | 7.0 | 98.8 | 5 | CH13_NeuroS1_23871_KX266901 | ||
| 6 | 9.4 | 9.7 | 8.3 | 7.8 | 1.2 | 6 | CH13_NeuroS1_NC_024498 | ||
| 1 | 2 | 3 | 4 | 5 | 6 | ||||
Idiopathic non-suppurative meningoencephalitis is a not uncommon default diagnosis in cattle dying of neurologic disease, because it is often the case that a causative etiology cannot be identified despite testing for a wide range of viral, bacterial, and protozoal agents. A recent retrospective study of non-suppurative encephalitides in 37 beef cattle from western Canada employed immunohistochemistry (IHC) to test paraffin-embedded sections of inflamed brain for the presence of bovine viral diarrhea virus, BoHV-1 and 5, rabies, Listeria monocytogenes, Neospora sp. Toxoplasma gondii, and Sarcocystis spp. (6). One case tested positive for rabies; all other cases were negative for the presence of listed antigens. A smaller subset of cases was also tested by IHC, with similarly negative results, for West Nile virus, Histophilus somni, Mycoplasma bovis, and parainfluenza virus type 3. Polymerase chain reaction tests for Chlamydophila sp. and for MCF (ovine herpesvirus 2 and caprine herpesvirus 2) employing paraffin-embedded tissue were also negative. Since members of the current contingent of circulating infectious agents capable of inducing non-suppurative encephalitis could not be identified in 36 out of 37 cases, the authors speculated that an immune-mediated mechanism may be involved. While this is certainly an important avenue for further investigation, other studies also highlight the crucial role of metagenomic analysis for discovery of novel virus infections (4,7). Molecular tools such as next-generation sequencing (NGS) are proving to be increasingly important during this current era in which laboratories are shuttering their virus cell culture capabilities. However, it is well-recognized that the use of these exquisitely sensitive techniques must be counterbalanced with validation studies that prove causality, rather than mere detection.
Mammalian astroviruses (family Astroviridae, genus Mammastrovirus) have been identified in the feces of a wide range of animal species, including cattle (8). In most animals, they cause minimal or no apparent clinical disease. However, they are considered to be a significant cause of gastroenteritis in children, and are implicated in sporadic cases of neurologic disease in immunocompromised humans (9). In domesticated animals, the use of NGS has identified astrovirus infection as the probable cause of shaking mink syndrome (10). A link between porcine astrovirus and congenital tremor type AII in piglets is conjectured, but not conclusively demonstrated (11). It is suspected that neurotropic astroviruses have been circulating in global cattle populations for several decades. Swiss researchers performing in situ hybridization (ISH) on paraffin-embedded brain from historical cases of bovine idiopathic encephalitis from the 1960s and 1970s found astrovirus RNA in a large proportion of samples (12). However, viral association with histologic lesions was inconsistent, and the authors could not conclusively rule out a bystander role for this virus. The same authors subsequently employed immunohistochemistry in addition to ISH to demonstrate a somewhat stronger association between BoAstV CH13/NeuroS1 proteins or RNA and histologic lesions in 34% of brain sections from a group of cattle with undiagnosed non-suppurative encephalitis (13). However, causality remains unproven. Infection studies would assist in confirming the neurovirulent potential of BoAstV, but cannot be performed at present because the virus has yet to be isolated in cell culture. Whether the current rapid expansion of the range of BoAstV-associated encephalitis is related to increased scrutiny by sophisticated tools such as NGS, or to the emergence of divergent neurovirulent strains remains to be elucidated. Clinical cases appear sporadically within herds, hence the appellation “European sporadic bovine encephalomyelitis” by Fankhauser who first described a case series in Switzerland in the 1960s (14). Similar to the reported cases of human astrovirus-associated encephalitis, cattle immunocompromised by concurrent viral infections or management stressors may be more susceptible to developing neurologic disease when infected with BoAstV.
Acknowledgments
The authors thank Drs. Rose Rumney, Rex Crawford, and Janet Shapiro for submitting these cases and providing clinical information. The technical assistance of histotechnology and virology staff at the Animal Health Laboratory is gratefully acknowledged. Dr. Murray Hazlett is thanked for providing photographic expertise. The administrative support of Dr. Grant Maxie is appreciated. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Bouzalas IG, Wüthrich D, Selimovic-Hamza S, Drögemüller C, Bruggmann R, Seuberlich T. Full-genome based molecular characterization of encephalitis-associated bovine astrovirus. Infect Genet Evol. 2016;44:162–168. doi: 10.1016/j.meegid.2016.06.052. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Diab S, McGraw S, et al. Divergent astrovirus associated with neurologic disease in cattle. Emerg Infect Dis. 2013;19:1385–1392. doi: 10.3201/eid1909.130682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seuberlich T, Wüthrich D, Selimovic-Hamza S, et al. Identification of a second encephalitis-associated astrovirus in cattle. Emerg Microbes Infect. 2016;5:e5. doi: 10.1038/emi.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlottau K, Schulze C, Bilk S, et al. Detection of a novel bovine astrovirus in a cow with encephalitis. Transbound Emerg Dis. 2016;63:253–259. doi: 10.1111/tbed.12493. [DOI] [PubMed] [Google Scholar]
- 5.Mittelholzer C, Hedlund K-O, Englund L, Dietz H-H, Svensson L. Molecular characterization of a novel astrovirus associated with disease in mink. J Gen Virol. 2003;4:3087–3094. doi: 10.1099/vir.0.19267-0. [DOI] [PubMed] [Google Scholar]
- 6.Sánchez S, Clark EG, Wobeser GA, Janzen ED, Philibert H. A retrospective study of non-suppurative encephalitis in beef cattle from western Canada. Can Vet J. 2013;54:1127–1132. [PMC free article] [PubMed] [Google Scholar]
- 7.Bouzalas IG, Wüthrich D, Walland J, et al. Neurotropic astrovirus in cattle with nonsuppurative encephalitis in Europe. J Clin Microbiol. 2014;52:3318–3324. doi: 10.1128/JCM.01195-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woode GH, Bridger JC. Isolation of small viruses resembling astroviruses and caliciviruses from acute enteritis of calves. J Med Microbiol. 1978;11:441–452. doi: 10.1099/00222615-11-4-441. [DOI] [PubMed] [Google Scholar]
- 9.Brown JR, Morfopoulou S, Hubb J, et al. Astrovirus VA1/HMO-C: An increasingly recognized neurotropic pathogen in immunocompromised patients. Clin Infect Dis. 2015;60:881–888. doi: 10.1093/cid/ciu940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blomström A-L, Widén F, Hammer A-S, Belák S, Berg M. Detection of a novel astrovirus in brain tissue of mink suffering from shaking mink syndrome by use of viral metagenomics. J Clin Microbiol. 2010;48:4392–4396. doi: 10.1128/JCM.01040-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blomström A-L, Ley C, Jacobson M. Astrovirus as a possible cause of congenital tremor type AII in piglets? Acta Vet Scand. 2014;56:82–87. doi: 10.1186/s13028-014-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selimovic-Hamza S, Bouzalas IG, Vandevelde M, Oevermann A, Seuberlich T. Detection of astrovirus in historical cases of European sporadic bovine encephalitis, Switzerland 1958–1976. Front Vet Sci. 2016;3:1–8. doi: 10.3389/fvets.2016.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selimovic-Hamza S, Boujon CL, Hilbe M, Oevermann A, Seuberlich T. Frequency and pathological phenotype of bovine astrovirus CH13/NeuroS1 infection in neurologically-diseased cattle: Towards assessment of causality. Viruses. 2017;9:12. doi: 10.3390/v9010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fankhauser R. Sporadic meningo-encephalomyelitis in cattle. Schweiz Arch Tierheilkd. 1961;103:225–235. [Google Scholar]