Abstract
This is a prospective, observational investigation without a placebo arm to evaluate the resolution rate of pneumonia when using 14 days or less of antibiotic therapy compared to longer therapy in dogs. There was no significant difference in radiographic resolution or relapse rates between the 2 treatment groups.
Résumé
Résultats chez les chiens atteints d’une présumée pneumonie bactérienne sans complication traitée à l’aide d’un traitement aux antibiotiques de courte ou de longue durée. Il s’agit d’une enquête prospective observationnelle sans groupe placebo pour évaluer le taux de résolution de la pneumonie lors du recours à 14 jours ou moins de traitement antibiotique comparativement à des thérapies plus longues chez les chiens. Il n’y avait aucune différence significative dans la résolution radiographique ou les taux de rechute entre les 2 groupes de traitement.
(Traduit par Isabelle Vallières)
The American Thoracic Society (ATS) guidelines for community acquired pneumonia in human adults recommend 7 to 10 d of antibiotic therapy for most cases based on evidence from a robust meta-analysis (1,2). Antibiotic therapy for 14 d or longer is only advised for patients who are immunocompromised or those with unusual infections (1). The Canadian guidelines for initial management of community-acquired pneumonia as well as the ATS guidelines also do not advise using the appearance of thoracic radiographs to guide the duration of antibiotic therapy but instead recommend using clinical signs and consensus-based algorithms developed to determine treatment duration (1,3). Specifically, the guidelines recommend “treatment until 72 hours after the patient becomes afebrile and until clinically stable” (1,2). In humans, only 60% of young, otherwise healthy people with Streptococcus pneumoniae pneumonia achieve radiographic resolution of pneumonia at 4 wk (1).
In contrast, veterinary recommendations for the treatment of bacterial pneumonia advise a much longer duration of antimicrobials that range from 3 to 6 wk, often with recommendations to continue therapy for 1 to 2 wk beyond radiographic resolution of the pneumonia (4–6). Unfortunately, these recommendations do not appear to have evidence-based support. There are no published investigations examining the effect of antibiotic treatment duration on outcome or relapse rates of pneumonia in dogs. The investigators suspect that the unproven, but pervasive, current clinical recommendations along with a lack of evidence indicating that shorter courses of antibiotics may be safe has hampered funding, approval, and possibly recruitment for prospective, randomized, investigator-blinded, non-inferiority investigations.
The objective of this prospective, observational study was to determine if shorter course antibiotic therapy could be used to treat dogs with uncomplicated, presumptive bacterial pneumonia with similar results to traditional, longer course therapy. It was hypothesized that dogs with uncomplicated pneumonia treated with short course antibiotics would have similar rates of radiographic resolution and relapse rates as dogs treated with a longer course of antibiotics. Short course was defined as ≤ 14 d of antibiotic therapy. Long course was defined as > 14 d of antibiotic therapy. Total length of antibiotic treatment included the days treated in hospital. Antibiotic therapy was determined at the managing clinician’s discretion.
Dogs with uncomplicated pneumonia were prospectively enrolled over a 1-year period. Pneumonia was defined as clinical signs referable to pneumonia including, but not limited to, fever, cough, increased respiratory effort, harsh lung sounds on auscultation, recent history of exposure to infectious respiratory disease, recent history of vomiting and/or recent history of anesthesia, in addition to radiographic evidence of pneumonia (aspiration or infectious based on radiographic pattern) as determined by 1 of 3 board-certified radiologists. Pneumonia was further defined as uncomplicated in otherwise healthy dogs with no pre-existing underlying lung pathology. Dogs were excluded if they had any of the following: diagnosis or treatment of presumptive pneumonia or infectious respiratory disease within 3 mo, antibiotic use within 3 mo for any reason, brachycephalic dogs with hypoplastic tracheas, and dogs with concurrent factors that might complicate treatment of pneumonia (e.g., laryngeal paralysis, megaesophagus, immunosuppression, neoplasia or those undergoing chemotherapy). Dogs younger than 6 mo were also excluded from the investigation.
Initial and all follow-up radiographs were evaluated by 1 of 3 board-certified radiologists. Follow-up radiographs were taken at 2 and 4 wk +/− 4 d regardless of antibiotic therapy. Radiographic resolution of pneumonia was defined as normal thoracic radiographs as described by 1 of 3 board-certified radiologists. Relapse was defined as re-presentation to a veterinary hospital within 3 mo of initial diagnosis for respiratory signs such as cough, increased respiratory rate or effort and/or nasal discharge, radiographic resolution of pneumonia followed by a new course of treatment for pneumonia, or owner-reported respiratory signs consistent with pneumonia. Dogs were included in the final analysis if they had at least one follow-up data point. Follow-up communication with owners at 3 mo or more was used to determine if patients experienced a clinical relapse. Patients were considered to have achieved clinical resolution if their presenting signs of pneumonia resolved and no relapse was identified based on the study criteria within 3 mo of diagnosis.
Additional diagnostic data such as complete blood (cell) count (CBC), trans- or endo-tracheal wash cytology and culture data, were collected when available, but were not required for inclusion. The study was approved by Angell Animal Medical Center’s Clinical Studies Committee and owner consent was obtained.
Median, interquartile ranges (IQR), and range were reported for antibiotic therapy duration as the distribution was non-parametric based on visual inspection. Fisher’s exact test was used to compare 3 outcome points in dogs treated with short versus long course: radiographic resolution at 2-week, 4-week, and 3-month relapse. This test was chosen over the Chi-squared test due to the low frequency of certain events (relapse of pneumonia and persistent evidence of pneumonia at both radiographic rechecks) (7).
Forty-seven dogs were included in the final analysis. Selected demographic data are presented in Table 1. Breeds included 4 golden retrievers, 3 German shepherd dogs, and the remainder consisted of a variety of breeds with less than 2 representatives each.
Table 1.
Demographic composition of the study population (n = 47)
| Frequency (%), short course | Frequency (%), long course | P-valuea | |
|---|---|---|---|
| Gender | |||
| Female, spayed | 9 | 11 | |
| Female, intact | 0 | 0 | 1.00 |
| Male, castrated | 11 | 11 | |
| Male, intact | 4 | 1 | 0.34 |
| Age | |||
| < 1 y | 7 | 3 | |
| 1 to 5 y | 6 | 7 | |
| 5.1 to 8 y | 7 | 4 | |
| > 8 y | 4 | 9 | 0.23 |
| Airway status | |||
| Brachycephalic | 7 | 6 | |
| Non-brachycephalic | 17 | 17 | 1.00 |
Fisher’s exact test used to determine difference between groups, for age groups the Freeman-Halton extension of the Fisher exact probability test was used.
Comparison between short and long course groups for selected physical examination, biochemical and treatment features are summarized in Table 2. The most commonly used single agent antibiotic was ampicillin-sulbactam and/or amoxicillin-clavulanic acid (32%). The most common combination was amoxicillin-sulbactam and/or amoxicillin-clavulanic acid and enrofloxacin (36%). Standard doses at our hospital for ampicillin-sulbactam is 50 mg/kg body weight (BW), IV, q8h; 14 mg/kg BW, PO, q12h for amoxicillin-clavulanix acid and 10 mg/kg BW, IV, q24h for enrofloxacin.
Table 2.
Comparison between selected physical examination, biochemical and treatment features of the study population and P-value between groups
| Frequency (%), short course | Frequency (%), long course | P-valuea | |
|---|---|---|---|
| Hospital admission | |||
| n = 47 | |||
| Yes | 17 | 20 | |
| No | 7 | 3 | 0.29 |
| Body temperature | |||
| n = 46 | |||
| > 39°C | 12 | 12 | |
| ≤ 39°C | 11 | 11 | 1.00 |
| Lung auscultation | |||
| n = 47 | |||
| Normal | 12 | 11 | |
| Abnormal | 12 | 12 | 1.00 |
| CBC results | |||
| n = 30 | |||
| Leukocytosis | 9 | 11 | |
| No leukocytosis | 4 | 6 | 1.00 |
| Transtracheal wash | |||
| n = 3b | 2 | 1 | |
| Positive for growth | 1 | 1 | |
| Negative for growth | 1 | 0 | N/A |
| Antibiotic treatment | |||
| n = 47 | |||
| Single agent | 11 | 11 | |
| Multiple drug combination | 13 | 12 | 1.00 |
Fisher’s exact test used to determine difference between groups.
Difference not calculated due to low sample size.
N/A — Not available.
Twenty-four of 47 (51.1%) dogs were treated with short course antibiotics and 23 of 47 (48.9%) were treated with long course antibiotics. In 12 of 47 cases the attending clinician stated they elected to extend the antibiotic course based on radiographic evidence of pneumonia on recheck radiographs. Median duration for short course was 11 d (IQR: 9.5 to 14 d; range: 7 to 14 d). The median duration for long course was 21 d (IQR: 16 to 21 d; range: 15 to 28 d).
Forty-two dogs had recheck radiographs at 2 wk. Eleven of 20 (55.0%) treated with short course antibiotics had radiographic resolution of pneumonia. Twelve of 22 (54.5%) treated with long course antibiotics achieved radiographic resolution. There was no statistically significant difference between groups (P = 1.0). One dog was euthanized due to pericardial effusion after the 2-week follow-up radiographs revealed radiographic resolution of pneumonia.
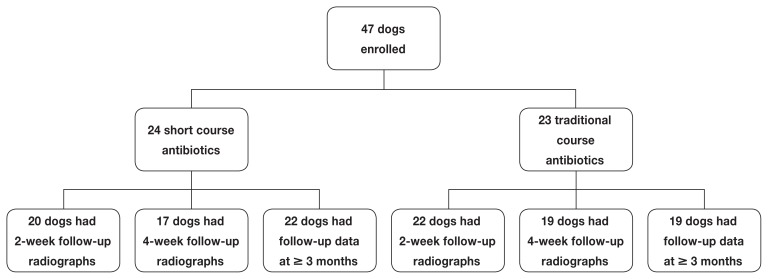
Thirty-six dogs had recheck radiographs at 4 wk. Fourteen of 17 (82.4%) dogs treated with short course antibiotics had radiographic resolution of pneumonia. Sixteen of 19 (84.2%) treated with long course antibiotics achieved radiographic resolution. There was no statistically significant difference between groups (P = 1.0) (Figure 1).
Figure 1.
Flowchart showing the number of dogs in each of the 2 groups at each follow-up period. All dogs must have had at least 1 follow-up data point (2-week radiograph, 4-week radiograph, or 3-month contact) to be included in statistical analysis.
Forty-one dogs had follow-up data at 3 mo or more. Four of 22 (18.2%) treated with short course antibiotics had relapse of pneumonia while 3 of 19 (15.7%) treated with long course antibiotics experienced relapse. There was no significant difference between groups (P = 1.0). Overall relapse rate for all dogs was 7/41 (17.1%).
In this prospective study of 47 dogs with uncomplicated, presumptive bacterial pneumonia, there was no significant difference between radiographic resolution rates at 2 wk, 4 wk, nor between relapse rates at 3 mo or longer in dogs treated with short course (≤ 14 d) or long course (> 14 d) antibiotics. Overall relapse rates (lack of resolution of radiographic or clinical signs) of dogs treated for uncomplicated pneumonia was 17.1%.
To the authors’ knowledge, no previously published studies examined duration of treatment or relapse rates in dogs with pneumonia. Several previous veterinary studies have raised the question of whether longer course of antibiotics are required in other disease states. A 2013 study of shelter cats with mycoplasma that were treated with either a 7- or a 14-day course of doxycycline did not find improved clinical results with the longer course of treatment (8). A 2012 study of dogs with uncomplicated urinary tract infections found that high dose treatment with enrofloxacin for 3 d was as efficacious as a conventional 14-day treatment with amoxicillin-clavulanic acid (9).
The recommendation to treat dogs with pneumonia for 3 to 6 wk, with the commonly cited suggestion of treating for 1 to 2 wk beyond radiographic resolution does not seem to be evidence-based, but rather expert opinion with multiple sources cross-referencing each other’s expert opinion (4–6,10–12). The 2015 ACVIM Consensus Statement on Therapeutic Antimicrobial Use in Animals and Antimicrobial Resistance states that “treatment durations are typically shorter in humans compared to corresponding veterinary recommendations with little apparent justification for longer therapy in animals (13).” While the evidence presented here must be interpreted as preliminary, these data call into question both the necessity of several weeks of antibiotic therapy for pneumonia in dogs and the utility of using radiographic resolution to determine the duration of antimicrobial therapy.
Ideally, diagnosis of bacterial pneumonia is based on positive culture results; however, diagnosis based on clinical signs (cough, tachypnea, hypoxemia) and radiographic changes showing alveolar infiltrates is common (6,14–16). Recruitment criteria were based on clinical signs and radiographic evidence to enroll a maximum of dogs. Although we cannot be certain all dogs that were included had bacterial pneumonia based on these inclusion criteria, we hope these findings serve as a starting point for re-evaluating the pervasive but unproven traditional guidelines. It is hoped that these data may provide some measure of encouragement to those designing clinical trials evaluating antibiotic courses for pneumonia in dogs that including a short-course arm (7 to 14 d) is not reckless.
In this study, only 3 of 47 dogs had trans- or endo-tracheal washes performed. Since all dogs had uncomplicated pneumonia with no antibiotic history in the last 3 mo there was a low expectation of antimicrobial resistance and this likely influenced clinician and client decisions to not pursue this diagnostic. The cornerstone of antibiotic stewardship is targeted therapy using culture results to guide antibiotic therapy. However, because of the observational nature of the study, cultures were not mandated.
The main limitation of this study is that the duration of treatment was not randomized. Clinicians had complete discretion over drug choice and treatment duration, thus the potential for bias is high. While not ideal, it was deemed a necessary step at this institution to allow maximal enrollment. Control bias was attempted by only including cases classified as uncomplicated; however, there is still a spectrum of patients within this population. Because pneumonia is a life-threatening disease, it was felt a non-randomized, observational proof-of-concept study was the most ethical way to begin to answer the question of the appropriate antibiotic duration. The authors hope that this study will encourage future participation in a randomized, double-blinded, non-inferiority study, which would be the most definitive way to identify the ideal duration of antibiotic therapy in uncomplicated pneumonia in dogs.
No previous studies have evaluated relapse rate in dogs with pneumonia, thus an accurate power calculation could not be used to design this study. While it is possible that the sample size may have been too small to detect a difference between short course and standard antibiotic therapy, if a difference exists it is small enough to indicate that future investigations into short-course antibiotic therapy for pneumonia do not represent reckless medicine. This study was insufficiently powered to evaluate whether radiographic resolution decreased the risk of clinical relapse.
In this prospective study of 47 dogs with uncomplicated pneumonia, there was no significant difference between dogs treated with a short course (≤ 14 d) as compared to long course (> 14 d) of antibiotics in radiographic resolution at 2 or 4 wk, or relapse rate at 3 mo or more. Based on these data, short course antibiotics for dogs with uncomplicated pneumonia defined as clinical signs referable to pneumonia with radiographic evidence may result in similar clinical resolution rates when compared to long courses of antibiotics and can be considered by clinicians in light of the complete clinical picture on a case-by-case basis. A sufficiently powered, randomized, double-blinded, non-inferiority study is required before broad clinical recommendations can be made regarding antibiotic course in uncomplicated pneumonia. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: A meta-analysis. Am J Med. 2007;120:783–790. doi: 10.1016/j.amjmed.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Mandell LA, Marrie TJ, Grossman RF, Chow AW, Hyland RH. Summary of Canadian Guidelines for the Initial Management of Community-acquired Pneumonia: An evidence-based update by the Canadian Infectious Disease Society and the Canadian Thoracic Society. Can Respir J. 2000;7:371–382. doi: 10.1155/2000/412616. [DOI] [PubMed] [Google Scholar]
- 4.Brady CA. Bacterial pneumonia in dogs and cats. In: King LG, editor. Textbook of Respiratory Disease in Dogs and Cats. St. Louis, Missouri: Elsevier; 2004. pp. 412–413. [Google Scholar]
- 5.Hanel RM, Hansen B. Pneumonia. In: Bonagura JD, Twedt DC, editors. Kirk’s Current Veterinary Therapy XV. St. Louis, Missouri: Elsevier; 2014. pp. 687–688. [Google Scholar]
- 6.Dear JD. Bacterial pneumonia in dogs and cats. Vet Clin Small Anim Pract. 2014;44:143–159. doi: 10.1016/j.cvsm.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitrie A, Watson PF. Statistics for Veterinary and Animal Science. 2nd ed. Oxford, England: Blackwell Publishing; 2010. pp. 110–111. [Google Scholar]
- 8.Kompare B, Litster AL, Leutenegger CM, Weng HY. Randomized masked controlled clinical trial to compare 7-day and 14-day course length of doxycycline in the treatment of Mycoplasma felis in shelter cats. Comp Immunol Microbiol Infect Dis. 2013;36:129–135. doi: 10.1016/j.cimid.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Westropp JL, Sykes JE, Irom S, et al. Evaluation of the efficacy and safety of high dose short duration enrofloxacin treatment regimen for uncomplicated urinary tract infections in dogs. J Vet Intern Med. 2012;26:506–512. doi: 10.1111/j.1939-1676.2012.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sykes JE. Canine and Feline Infectious Disease. St. Louis, Missouri: Elsevier; 2014. Bacterial bronchopneumonia and pyothorax; pp. 847–857. [Google Scholar]
- 11.Fowler TL, Reinero C. Bacterial respiratory infections. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 4th ed. Athens, Georgia: Elsevier; 2012. [Google Scholar]
- 12.Cohn L. Pulmonary parenchymal disease. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. St. Louis, Misouri: Elsevier Saunders; 2005. pp. 1259–1260. [Google Scholar]
- 13.Weese JS, Giguere S, Guardabassi L, et al. ACVIM Consensus statement on therapeutic antimicrobial use in animals and antimicrobial resistance. J Vet Intern Med. 2015;29:487–498. doi: 10.1111/jvim.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kogan DA, Johnson LR, Sturges BK, Jandry KE, Pollard RE. Etiology and clinical outcome in dogs with aspiration pneumonia: 88 cases (2004–2006) J Am Vet Med Assoc. 2008;233:1748–1755. doi: 10.2460/javma.233.11.1748. [DOI] [PubMed] [Google Scholar]
- 15.Kogan DA, Johnson LR, Jandry KE, Pollard RE. Clinical, clinicopathologic, and radiographic findings in dogs with aspiration pneumonia: 88 cases (2004–2006) J Am Vet Med Assoc. 2008;233:1742–1747. doi: 10.2460/javma.233.11.1742. [DOI] [PubMed] [Google Scholar]
- 16.Radhakrishnan A, Drobatz KJ, Culp WTN, King LG. Community-acquired infectious pneumonia in puppies: 65 cases (1993–2002) J Am Vet Med Assoc. 2007;230:1493–1497. doi: 10.2460/javma.230.10.1493. [DOI] [PubMed] [Google Scholar]