Abstract
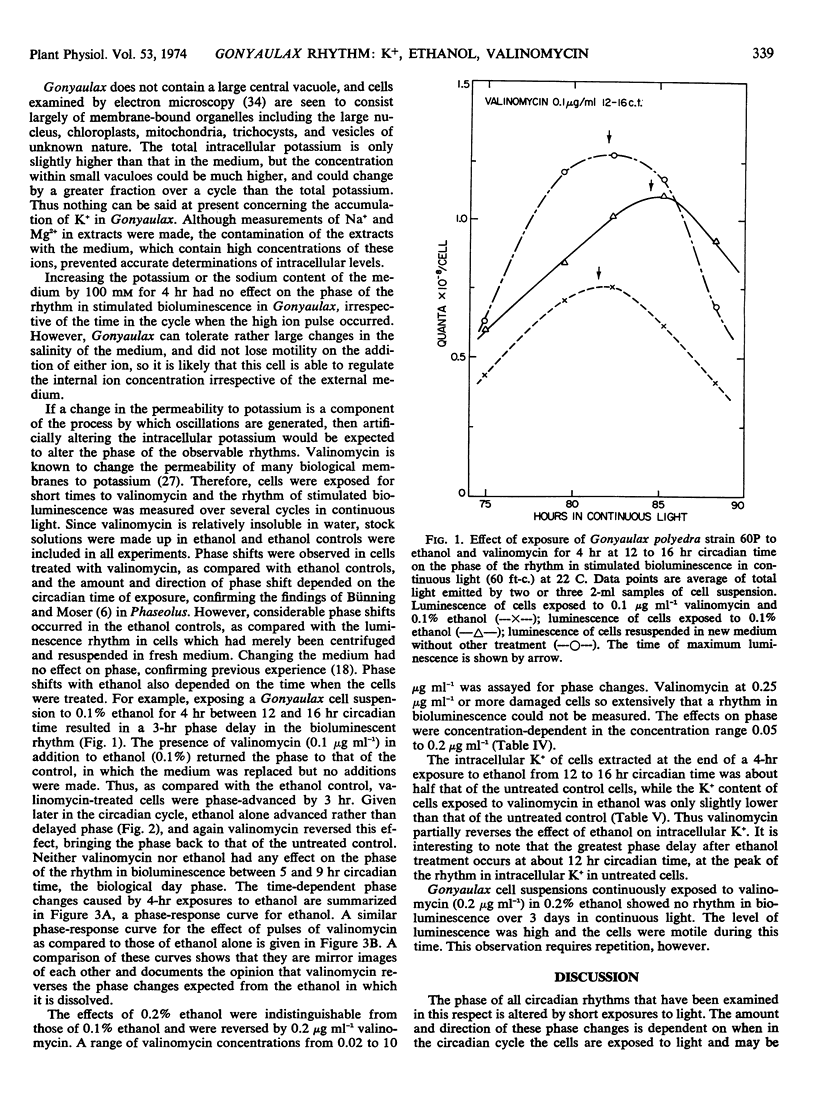
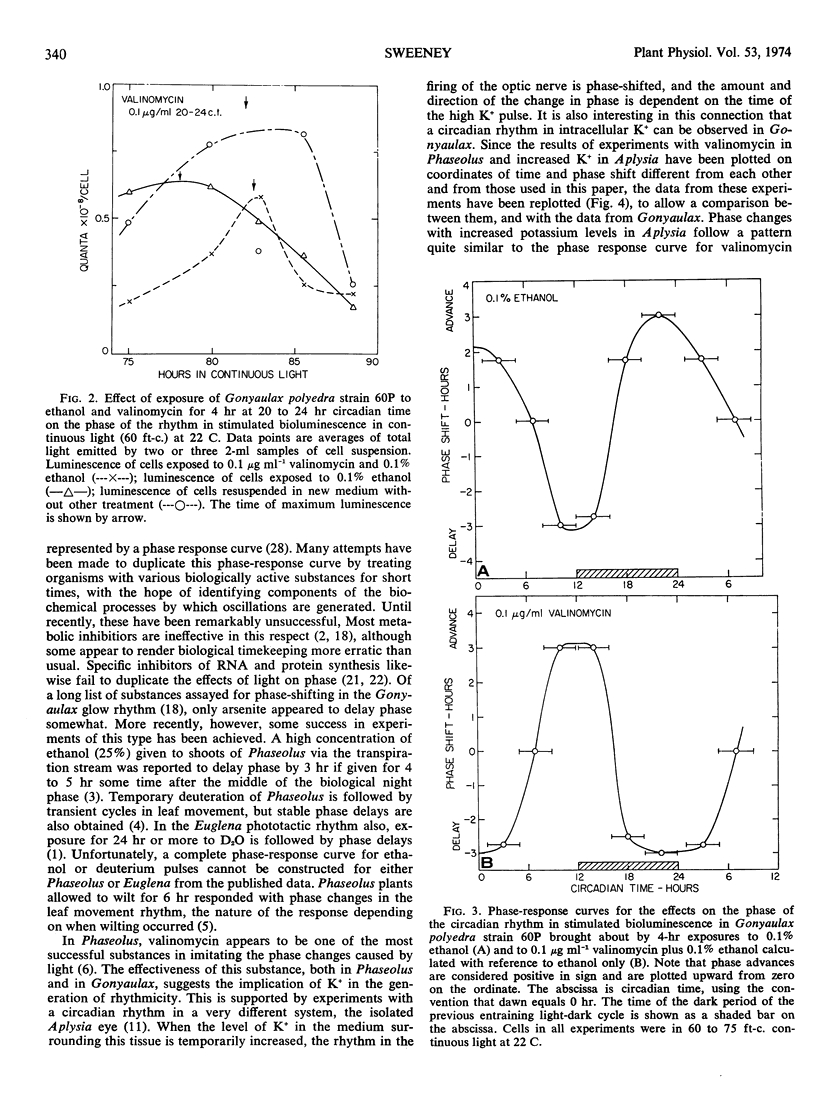
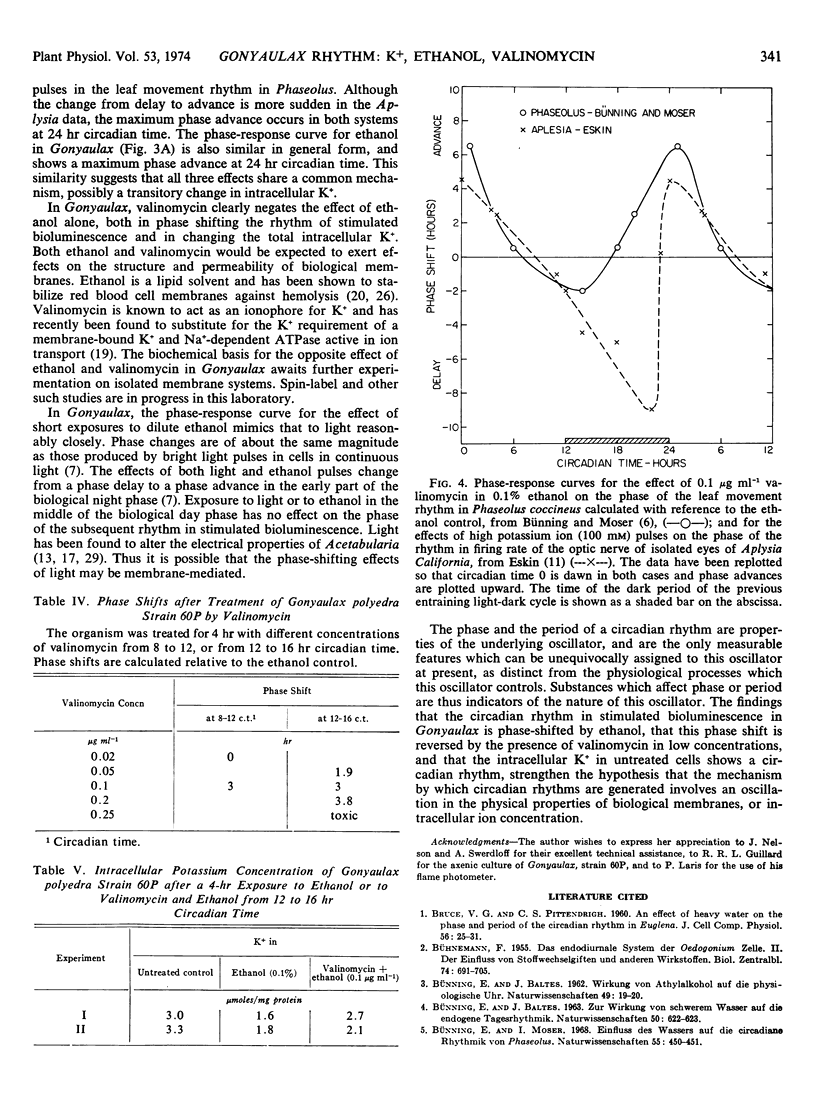
A circadian rhythm in the intracellular level of K+ in Gonyaulax polyedra is reported. When axenic cultures of Gonyaulax in continuous light (60-75 fot candles) are exposed for 4 hours to 0.1 or 0.2% ethanol, the subsequent free-running rhythm in stimulated bioluminescence is phase-shifted, the amount and direction of the shift being dependent on the time in the circadian cycle when cells are treated. The phase-response curve for ethanol closely resembles that for light in similarly maintained cells. When valinomycin (0.1 or 0.2 μg ml−1) is present in addition to ethanol, the phase of the bioluminescence rhythm is returned to that of an untreated cell suspension. Valinomycin thus negates the effect of ethanol on phase. The intracellular K+ level immediately after treatment of a cell suspension for 4 hours with ethanol (0.1%) is about half that of untreated cells. If valinomycin (0.1 μg ml−1) is also present during the 4-hour treatment, the intracellular K+ is only slightly lower than in untreated cells. Increasing the external concentration of K+ or Na+ for 4 hours has no effect on the rhythm of stimulated bioluminescence. These results are interpreted as support for the hypothesis that the mechanism by which circadian oscillations are generated involves changes in membrane properties.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bünning E., Moser I. Einfluss des Wassers auf die circadiane Rhythmik von Phaseolus. Naturwissenschaften. 1968 Sep;55(9):450–451. doi: 10.1007/BF00602680. [DOI] [PubMed] [Google Scholar]
- Bünning E., Moser I. Influence of valinomycin on circadian leaf movements of Phaseolus. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2732–2733. doi: 10.1073/pnas.69.9.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson R., Sweeney B. M. The dependence of the phase response curve for the luminescence rhythm in gonyaulax on the irradiance in constant conditions. Int J Chronobiol. 1973;1(1):95–100. [PubMed] [Google Scholar]
- Ehret C. F., Trucco E. Molecular models for the circadian clock. I. The chronon concept. J Theor Biol. 1967 May;15(2):240–262. doi: 10.1016/0022-5193(67)90206-8. [DOI] [PubMed] [Google Scholar]
- Feldman J. F. Lengthening the period of a biological clock in Euglena by cycloheximide, an inhibitor of protein synthesis. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1080–1087. doi: 10.1073/pnas.57.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUILLARD R. R., RYTHER J. H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol. 1962 Apr;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Grell E., Funck T. Carbon- 13 nuclear-magnetic-resonance and infrared-absorption spectroscopy of valinomycin and its alkali-ion complexes. Eur J Biochem. 1973 May 2;34(3):415–424. doi: 10.1111/j.1432-1033.1973.tb02774.x. [DOI] [PubMed] [Google Scholar]
- HASTINGS J. W. Biochemical aspects of rhythms: phase shifting by chemicals. Cold Spring Harb Symp Quant Biol. 1960;25:131–143. doi: 10.1101/sqb.1960.025.01.012. [DOI] [PubMed] [Google Scholar]
- Hamman J. P., Seliger H. H. The mechanical triggering of bioluminescence in marine dinoflagellates: chemical basis. J Cell Physiol. 1972 Dec;80(3):397–408. doi: 10.1002/jcp.1040800310. [DOI] [PubMed] [Google Scholar]
- Hegyvary C. Effects of some organic solvents on the reactivity of sodium plus potassium ion-transport ATPase. Biochim Biophys Acta. 1973 Jun 22;311(2):272–291. doi: 10.1016/0005-2736(73)90274-5. [DOI] [PubMed] [Google Scholar]
- KARAKASHIAN M. W., HASTINGS J. W. THE EFFECTS OF INHIBITORS OF MACROMOLECULAR BIOSYNTHESIS UPON THE PERSISTENT RHYTHM OF LUMINESCENCE IN GONYAULAX. J Gen Physiol. 1963 Sep;47:1–12. doi: 10.1085/jgp.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARAKASHIAN M. W., HASTINGS J. W. The inhibition of a biological clock by actinomycin D. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2130–2137. doi: 10.1073/pnas.48.12.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PITTENDRIGH C. S. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol. 1960;25:159–184. doi: 10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- Paterson S. J., Butler K. W., Huang P., Labelle J., Smith I. C., Schneider H. The effects of alcohols on lipid bilayers: a spin label study. Biochim Biophys Acta. 1972 Jun 20;266(3):597–602. doi: 10.1016/0006-3002(72)90003-0. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Induced active transport of ions in mitochondria. Proc Natl Acad Sci U S A. 1965 May;53(5):1076–1083. doi: 10.1073/pnas.53.5.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWEENEY B. M. The photosynthetic rhythm in single cells of Gonyaulax polyedra. Cold Spring Harb Symp Quant Biol. 1960;25:145–148. doi: 10.1101/sqb.1960.025.01.013. [DOI] [PubMed] [Google Scholar]
- Satter R. L., Galston A. W. Potassium flux: a common feature of albizzia leaflet movement controlled by phytochrome or endogenous rhythm. Science. 1971 Oct 29;174(4008):518–520. doi: 10.1126/science.174.4008.518. [DOI] [PubMed] [Google Scholar]
- Sweeney B. M., Tuffli C. F., Jr, Rubin R. H. The circadian rhythm in photosynthesis in Acetabularia in the presence of actinomycin D, puromycin, and chloramphenicol. J Gen Physiol. 1967 Jan;50(3):647–659. doi: 10.1085/jgp.50.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Driessche T., Bonotto S., Brachet J. Inability of rifampicin to inhibit circadian rhythmicity in Acetabularia despite inhibition of RNA synthesis. Biochim Biophys Acta. 1970 Dec 14;224(2):631–634. doi: 10.1016/0005-2787(70)90599-x. [DOI] [PubMed] [Google Scholar]


