Abstract
When mitochondria isolated from etiolated pea (Pisum sativum cv. Alaska) epicotyls were exposed briefly to red light, their ability to reduce exogenous NADP was enhanced. The red light effect was reversed by far red light. Photoreversible absorbance changes between 730 nm and 800 nm were spectrophotometrically detected in the purified mitochondria and its membrane fraction. The dehydrogenase activity in the mitochondria was heat-labile and was dependent on the presence of magnesium ion and appropriate substrates such as glucose 6-phosphate, isocitrate, pyruvate, 6-phosphogluconate, and succinate. The photoreversible effect was seen only for a few minutes after the irradiation, and was cancelled by hypotonic treatment or addition of Triton X-100. A similar but lesser effect was observed in the pea microsome fraction, whereas no photoreversible response was seen with a supernatant fraction resulting from centrifugation at 105g for 30 minutes.
Full text
PDF
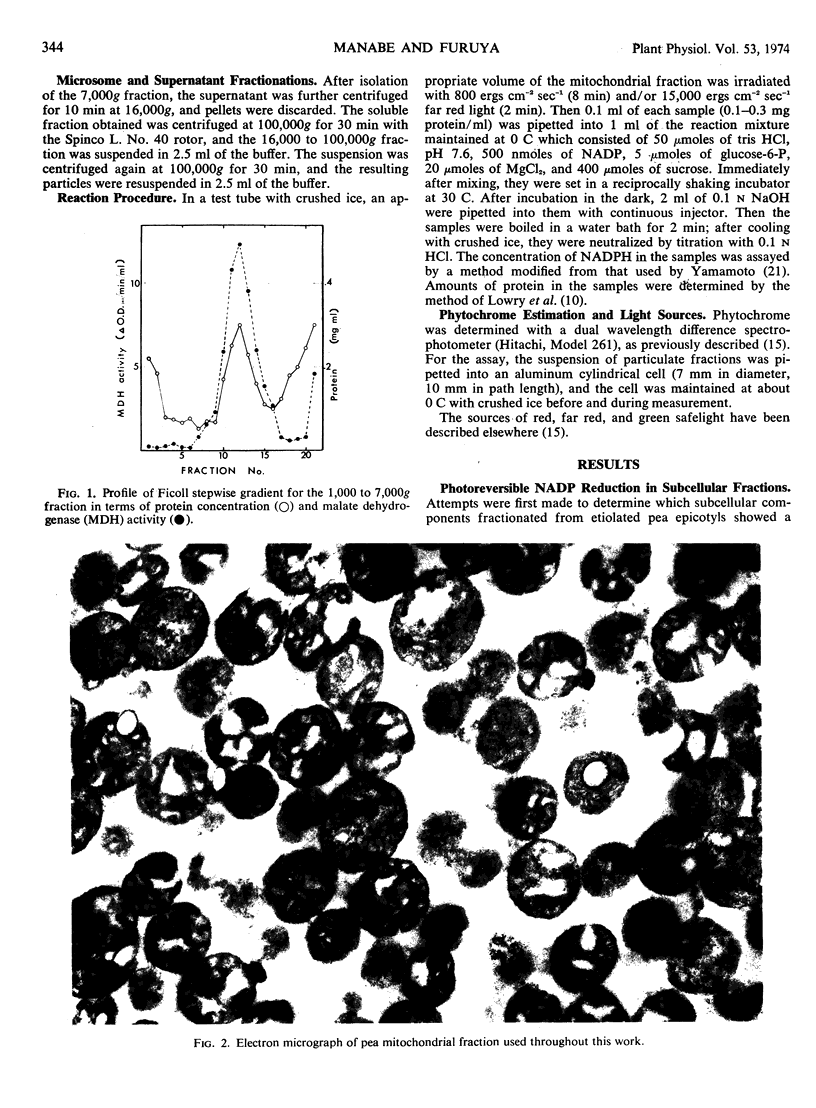

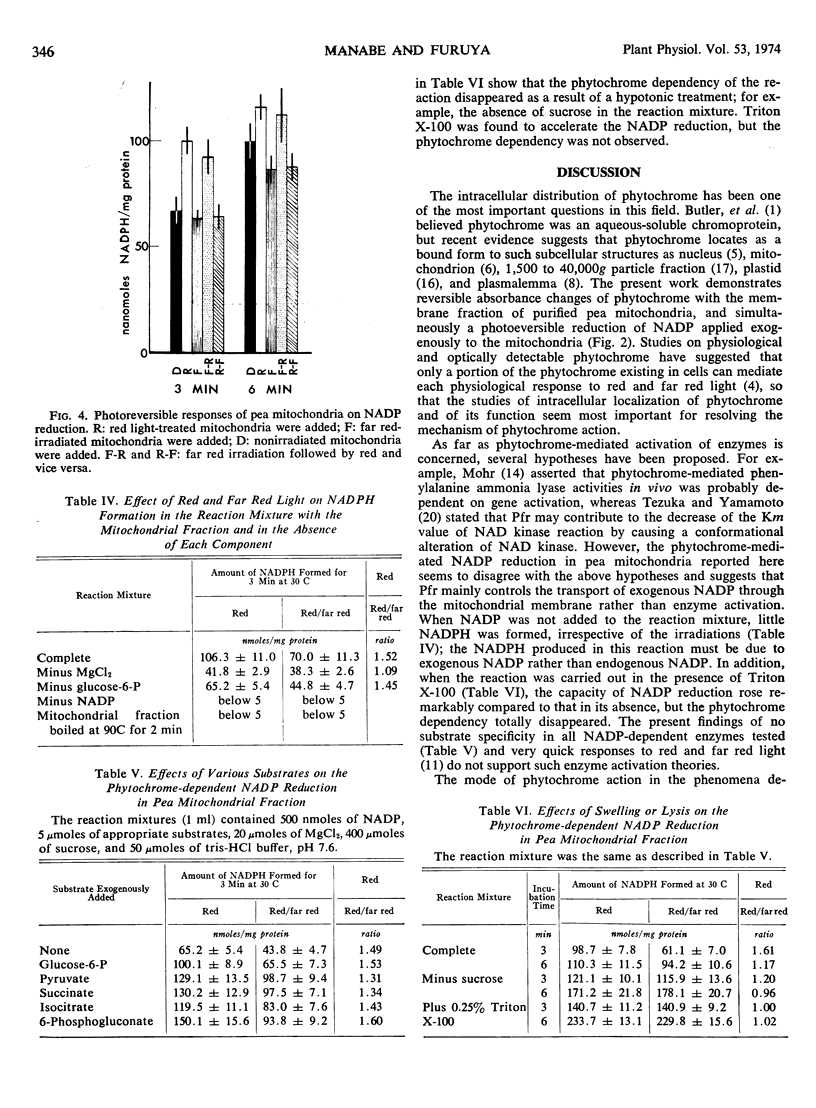

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler W. L., Norris K. H., Siegelman H. W., Hendricks S. B. DETECTION, ASSAY, AND PRELIMINARY PURIFICATION OF THE PIGMENT CONTROLLING PHOTORESPONSIVE DEVELOPMENT OF PLANTS. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1703–1708. doi: 10.1073/pnas.45.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON S. A., SURREY K. Red and far-red action on oxidative phosphorylation. Radiat Res. 1960 Apr;12:325–339. [PubMed] [Google Scholar]
- Galston A. W. Microspectrophotometric evidence for phytochrome in plant nuclei. Proc Natl Acad Sci U S A. 1968 Oct;61(2):454–460. doi: 10.1073/pnas.61.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manabe K. A Rapid Phytochrome-dependent Reduction of Nicotinamide Adenine Dinucleotide Phosphate in Particle Fraction from Etiolated Bean Hypocotyl. Plant Physiol. 1973 May;51(5):982–983. doi: 10.1104/pp.51.5.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A. Photocontrol of Formation of Red Kidney Bean Leaf Triphosphopyridine Nucleotide Linked Triosephosphate Dehydrogenase. Plant Physiol. 1960 Jan;35(1):126–128. doi: 10.1104/pp.35.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M. M. Effect of Chloramphenicol on Light-Dependent Synthesis of Proteins and Enzymes of Leaves and Chloroplasts of Phaseolus vulgaris. Plant Physiol. 1964 Jul;39(4):579–585. doi: 10.1104/pp.39.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H., Coleman R. A. Immunocytochemical localization of phytochrome. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2431–2435. doi: 10.1073/pnas.68.10.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B., Drury K. S., Park R. B. Evidence for bound phytochrome in oat seedlings. Plant Physiol. 1969 Jan;44(1):105–109. doi: 10.1104/pp.44.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin J. E., Furuya M. A red-far red reversible effect on uptake of exogenous indoleacetic Acid in etiolated rice coleoptiles. Plant Physiol. 1973 Feb;51(2):295–298. doi: 10.1104/pp.51.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanada T. A rapid photoreversible response of barley root tips in the presence of 3-indoleacetic Acid. Proc Natl Acad Sci U S A. 1968 Feb;59(2):376–380. doi: 10.1073/pnas.59.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka T., Yamamoto Y. Photoregulation of Nicotinamide Adenine Dinucleotide Kinase Activity in Cell-free Extracts. Plant Physiol. 1972 Oct;50(4):458–462. doi: 10.1104/pp.50.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]