Abstract
We assessed the association of erythrocyte indices on mean blood glucose-independent racial disparity in hemoglobin A1c (HbA1c) in youth with type 1 diabetes. Blacks still had higher HbA1c after adjustment for mean blood glucose, red blood cell indices, age, and sex. Such differences need to be taken into account when interpreting HbA1c in Black patients.
Hemoglobin A1c (HbA1c) is the most widely used indirect metric of glycemic control in patients with diabetes, and has been shown to be predictive of risk for long-term diabetes complications.1 Pediatric HbA1c goals for diabetes management have been endorsed and disseminated by professional groups; however, these recommendations do not take into account possible variation in HbA1c related to ethnicity or race.2 Evidence is accumulating that Black youth with type 1 diabetes (T1D) consistently have higher HbA1c than Whites.3–6 This has been the case even when the HbA1c data have been adjusted for concurrently measured blood glucose levels.5 Thus, factors besides mean blood glucose (MBG) appear to play a role in racial disparity of HbA1c and likely have relevance for diagnosis and management of diabetes across racial groups.5,7,8
Red blood cell (RBC) indices have been found to be correlated with HbA1c.9,10 We hypothesized that differences in RBC indices could underlie racial disparity in HbA1c. Therefore, we analyzed HbA1c data in a biracial population of children with T1D. The data were adjusted for the influence of MBG, RBC indices and age.
Methods
Youth with T1D from the Children’s Diabetes Center at Children’s Hospital of New Orleans, Louisiana, who self-identified as either Black or White, were recruited for the study. At the time of clinic visit, blood was drawn for HbA1c and complete blood count (CBC). MBG was derived from the average of self-monitored capillary glucoses from the patient’s home glucose meter, collected during the 30 days prior to the clinic visit as previously described.5 HbA1c was assayed by The National Glycohemoglobin Standardization Program approved immunoassay in the Vista automated system through the Children’s Hospital clinical lab. Hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), MCH concentration, platelet number, white blood cell (WBC) count, red cell distribution width, red cell distribution width coefficient of variation (RDW-CV), and red cell distribution width SD (RDW-SD) were determined on a Sysmex XN-1000 Hematology Analyzer in the Children’s Hospital of New Orleans clinical lab. The institutional review board of the Louisiana State University Health Sciences Center–New Orleans approved this study.
Pearson correlations between HbA1c and each of the other variables were calculated. Black vs White differences in means of variables were assessed by t-test. The influence of CBC indices, sex, race, chronological age, and MBG as independent variables on HbA1c was tested by multiple variable regression modeling (using PROC GLM, PROC STEPWISE of Statistical Analysis System; SAS Institute Inc, Cary, North Carolina). In the regression models the difference in HbA1c between Black and White patients was tested on the least squares means adjusted for race, age, sex, and RBC as main effects. Results were considered to be statistically significant at P = .05 level.
Results
Our study population was comprised of 83 patients (32 Blacks and 51 Whites; 42 male, 41 female). Among all patients, HbA1c was correlated with MBG, MCH, MCV, MCH concentration, RDW-CV and RDW-SD, WBC, and platelet number for patient chronological age was correlated with hematocrit, MCV, MCH, and RDW-SD.
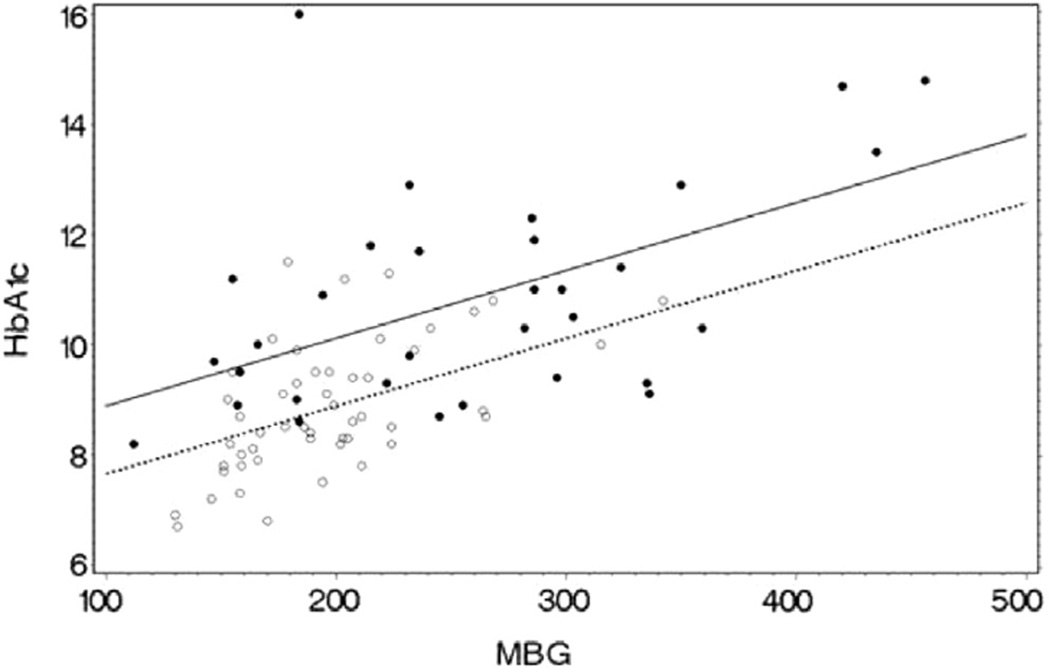
Variables by racial group are presented in the Table. There was no statistical difference in age or diabetes duration between Black and White patients. Except for RDW-SD and WBC, there were statistically significant differences in the means of all other CBC variables between the races (Table). The Figure depicts the relationship of HbA1c vs MBG by race. In the subsequent multiple variable regression model of HbA1c (dependent variable) with race, MBG, RDW-CV, sex, and age as independent variables, the overall model was statistically significant (R2 = 0.545, P < .0001). Race (P = .003), MBG (P < .0001), RDW-CV (P < .026), and age (P = .0211), were statistically significant, but sex (P = .74) was not. The least squares mean HbA1c for Blacks and Whites adjusted for the other variables in the model was statistically different at 10.3% and 9.2%, respectively (P = .003). Substitution of the other CBC indices in the model for RDW-CV as an independent variable were not statistically significant, but both race and MBG remained significant.
Table.
Differences in CBC indices between Black and White patients n = 83
| Index | Black n = 32 | White n = 51 | P |
|---|---|---|---|
| HbA1c (%) | 10.8 ± 1.7 | 8.9 ± 1.2 | <.0001 |
| Age (y) | 13.3 ± 3.8 | 14.5 ± 3.5 | .1573 |
| Duration (y) | 8.5 ± 3.8 | 8.3 ± 3.7 | .85 |
| MBG (mg/dL) | 260 ± 86 | 197 ± 43 | .0003 |
| Hemoglobin | 13.3 ± 1.5 | 14.3 ± 1.1 | .0016 |
| HCT | 40 ± 3.7 | 41.6 ± 3. | .0177 |
| MCV | 80.8 ± 5.7 | 84.5 ± 4.6 | .0028 |
| MCH | 27.1 ± 2.4 | 28.9 ± 1.8 | .0003 |
| MCH concentration | 33.5 ± 1.5 | 34.3 ± 0.9 | .0060 |
| RDW-CV | 13.5 ± 1.1 | 12.6 ± 0.6 | .0001 |
| RDW-SD | 39.5 ± 2.8 | 38.6 ± 2.2 | .118 |
| WBC | 6.7 ± 2.6 | 6.5 ± 1.9 | .66 |
| Platelet number | 289.8 ± 66.9 | 259.1 ± 46.2 | .0267 |
HCT, hematocrit.
Figure.
The relationship of HbA1c with MBG by race in youth with T1D. Black dots and unbroken regression line represent Black patients. Open circles and broken regression line represent White patients. Blacks have a higher HbA1c than Whites at similar levels of MBG. HbA1c (%) and MBG in mg/dL. Both MBG (P < .0001) and race (P = .003) have statistically significant independent influence on HbA1c when also adjusted for the influence of age and RDW-CV.
Discussion
Higher HbA1c in Blacks compared with Whites, independent of glucose levels, also has been reported from other populations.8 Thus, it appears thatHbA1cmay generally overestimate MBG for many Black individuals.5,7 The mechanisms leading to higher HbA1c not due to higher MBG in Black individuals are unclear.8 RBC factors such as RBC longevity and glucose permeability have been proposed as sources of this difference.11,12 However, current methods of direct measurement of RBC longevity and glucose permeability are quite expensive, time consuming, and impractical for general clinical research especially in children. RBC indices such as RDW-CV are readily obtainable and have been correlated with RBC longevity, iron status, andHbA1c.9,10,13 In this study, we found that even though multiple RBC indices were correlated with HbA1c, and the means of these variables differed between the races, none of these factors eliminated race as a statistically significant independent covariate in multiple variable models.
These findings provide additional evidence that higher HbA1c in Blacks compared with White patients with T1D is not completely explainable by differences in MBG, age, or RDW-CV. Increased RDW-CV and decreased hemoglobin may be associated with decreased iron status,14 which has been linked with higher HbA1c. Therefore, iron status should be explored more fully as a possible source of HbA1c racial disparity. A limitation of this study and other similar studies in the past5,8 has been the small number of glucose samples used to derive MBG and may contribute in part to greater variation in the HbA1c/MBG relationship. Future studies may strive to reduce such variation by deriving MBG from protocols using continuous glucose monitoring. Recognition of MBG-independent racial difference in HbA1c and determining its cause/s is important to making diabetes management safer and more effective for all children.
Acknowledgments
Supported by the Mid-South Transdisciplinary Collaborative Center for Health Disparities Research through the National Institute on Minority Health and Health Disparities of the National Institutes of Health (NIH; U54MD008176). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Glossary
- CBC
Complete blood count
- HbA1c
Hemoglobin A1c
- MBG
Mean blood glucose
- MCH
Mean corpuscular hemoglobin
- MCV
Mean corpuscular volume
- RBC
Red blood cell
- RDW-CV
Red cell distribution width coefficient of variation
- RDW-SD
Red cell distribution width SD
- T1D
Type 1 diabetes
- WBC
White blood cell
Footnotes
The authors declare no conflicts of interest.
References
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Rewers MJ, Pillay K, de Beaufort C, Craig ME, Hanas R, Acerini CL, et al. ISPAD Clinical Practice Consensus Guidelines 2014. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes. 2014;15:102–114. doi: 10.1111/pedi.12190. [DOI] [PubMed] [Google Scholar]
- 3.Delamater AM, Albrecht DR, Postellon DC, Gutai JP. Racial differences in metabolic control of children and adolescents with type I diabetes mellitus. Diabetes Care. 1991;14:20–25. doi: 10.2337/diacare.14.1.20. [DOI] [PubMed] [Google Scholar]
- 4.Chalew SA, Gomez R, Butler A, Hempe J, Compton T, Mercante D, et al. Predictors of glycemic control in children with type 1 diabetes: the importance of race. J Diabetes Complications. 2000;14:71–77. doi: 10.1016/s1056-8727(00)00072-6. [DOI] [PubMed] [Google Scholar]
- 5.Kamps JL, Hempe JM, Chalew SA. Racial disparity in A1C independent of mean blood glucose in children with type 1 diabetes. Diabetes Care. 2010;33:1025–1027. doi: 10.2337/dc09-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willi SM, Miller KM, DiMeglio LA, Klingensmith GJ, Simmons JH, Tamborlane WV, et al. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135:424–434. doi: 10.1542/peds.2014-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalew SA. The continuing challenge of outcome disparities in children with diabetes. Pediatrics. 2015;135:552–553. doi: 10.1542/peds.2014-4136. [DOI] [PubMed] [Google Scholar]
- 8.Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. J Clin Endocrinol Metab. 2012;97:1067–1072. doi: 10.1210/jc.2011-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veeranna V, Zalawadiya SK, Panaich SS, Ramesh K, Afonso L. The association of red cell distribution width with glycated hemoglobin among healthy adults without diabetes mellitus. Cardiology. 2012;122:129–132. doi: 10.1159/000339225. [DOI] [PubMed] [Google Scholar]
- 10.English E, Idris I, Smith G, Dhatariya K, Kilpatrick ES, John WG. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review. Diabetologia. 2015;58:1409–1421. doi: 10.1007/s00125-015-3599-3. [DOI] [PubMed] [Google Scholar]
- 11.Cohen RM, Franco RS, Khera PK, Smith EP, Lindsell CJ, Ciraolo PJ, et al. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood. 2008;112:4284–4291. doi: 10.1182/blood-2008-04-154112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khera PK, Joiner CH, Carruthers A, Lindsell CJ, Smith EP, Franco RS, et al. Evidence for interindividual heterogeneity in the glucose gradient across the human red blood cell membrane and its relationship to hemoglobin glycation. Diabetes. 2008;57:2445–2452. doi: 10.2337/db07-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith EP, Cohen RM. Physiologic concepts that may revise the interpretation and implications of HbA1C in clinical medicine: an American perspective. J Diabetes Sci Technol. 2015;9:696–700. doi: 10.1177/1932296815572255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dugdale AE. Predicting iron and folate deficiency anaemias from standard blood testing: the mechanism and implications for clinical medicine and public health in developing countries. Theor Biol Med Model. 2006;3:34. doi: 10.1186/1742-4682-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]