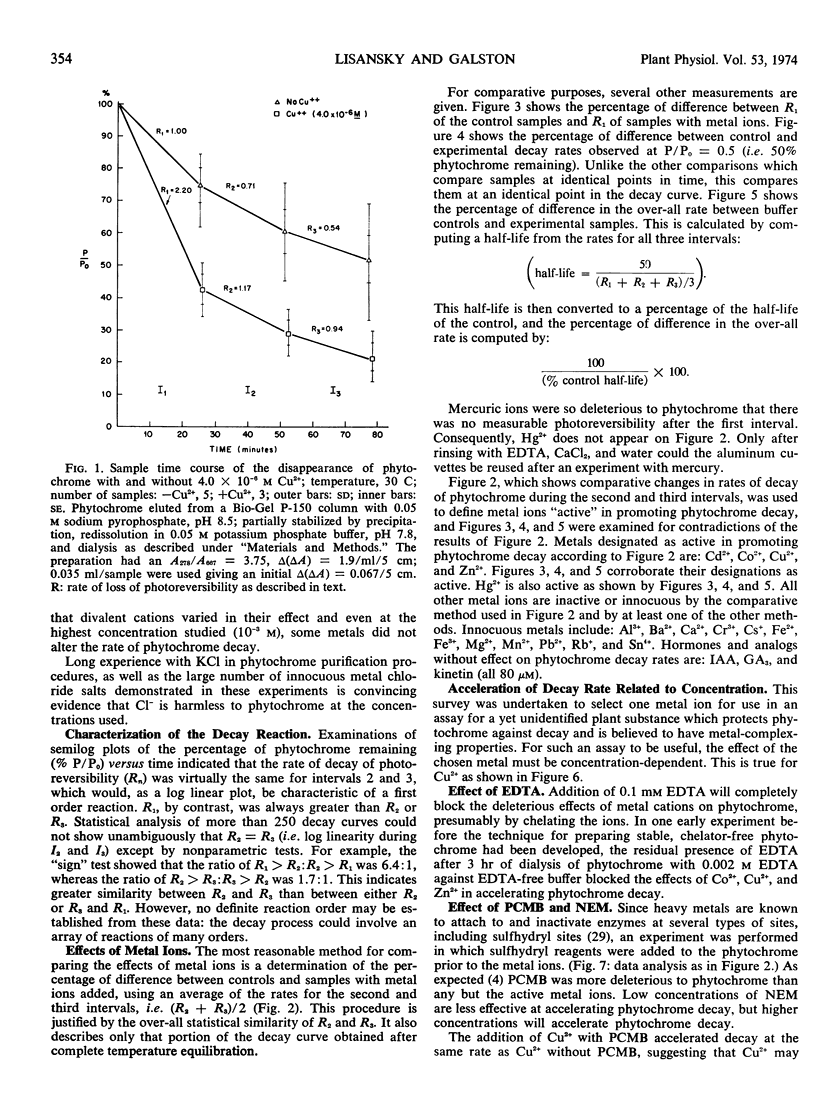
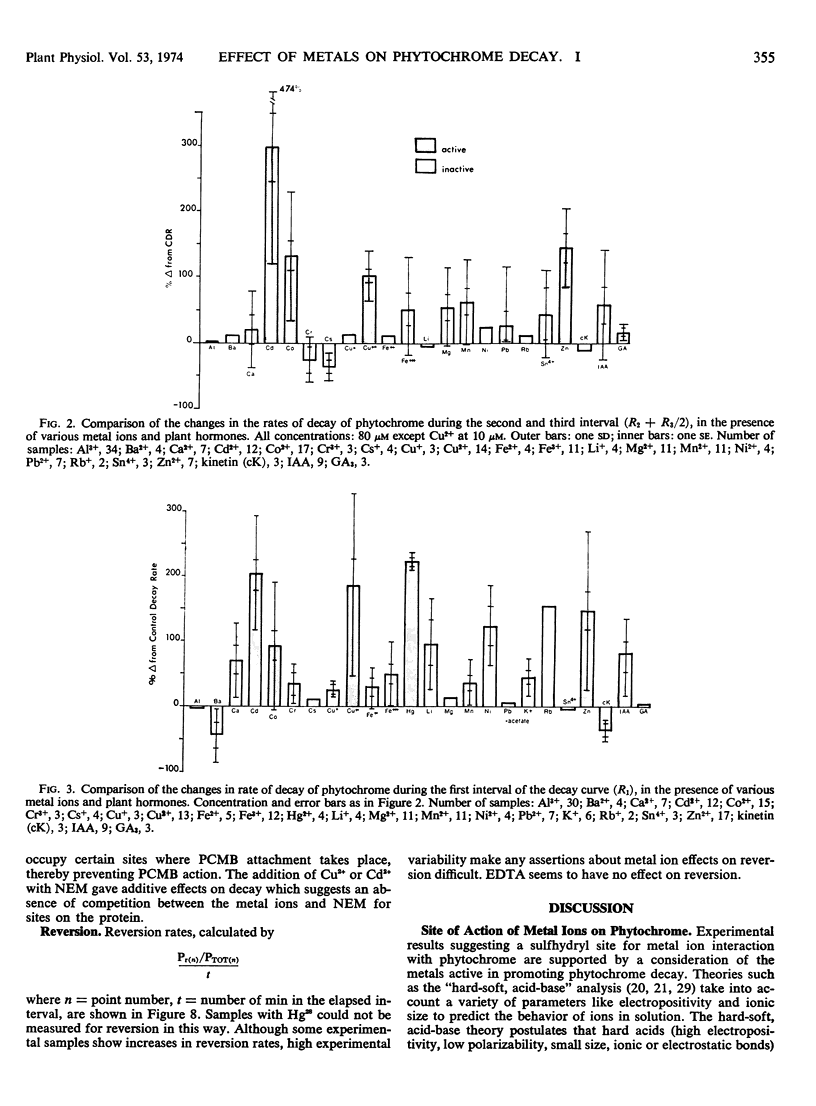
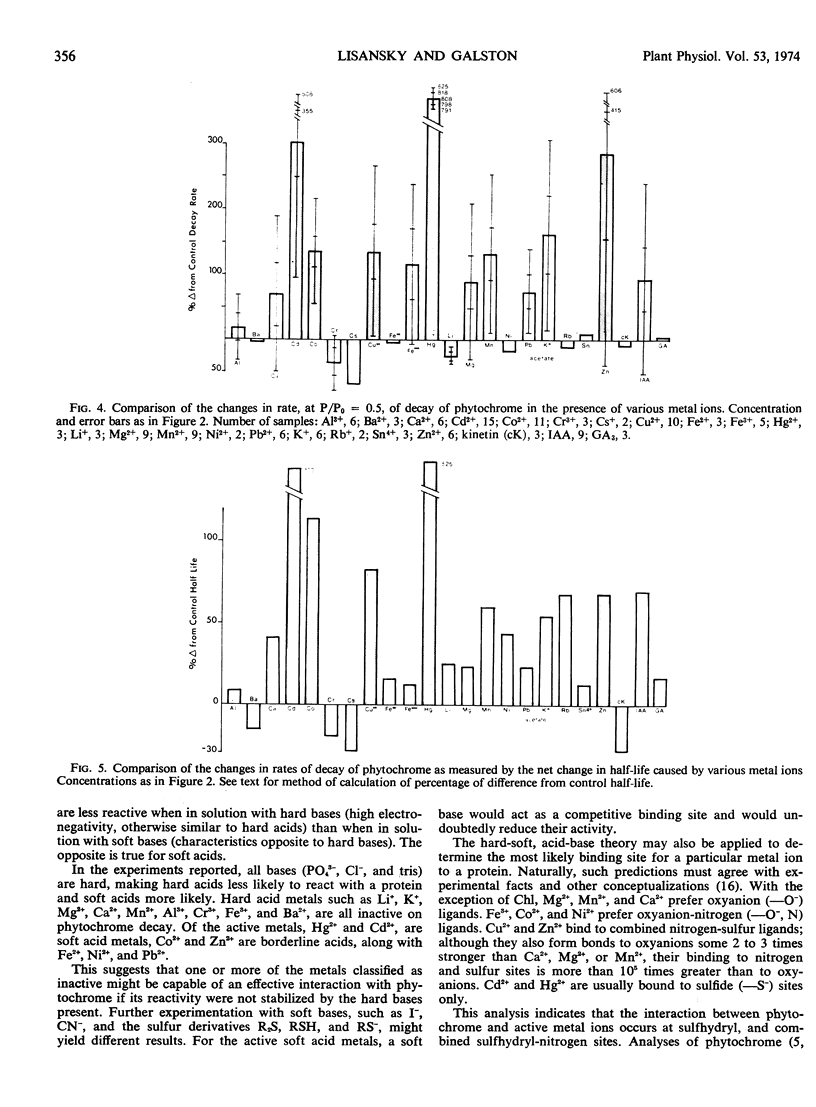
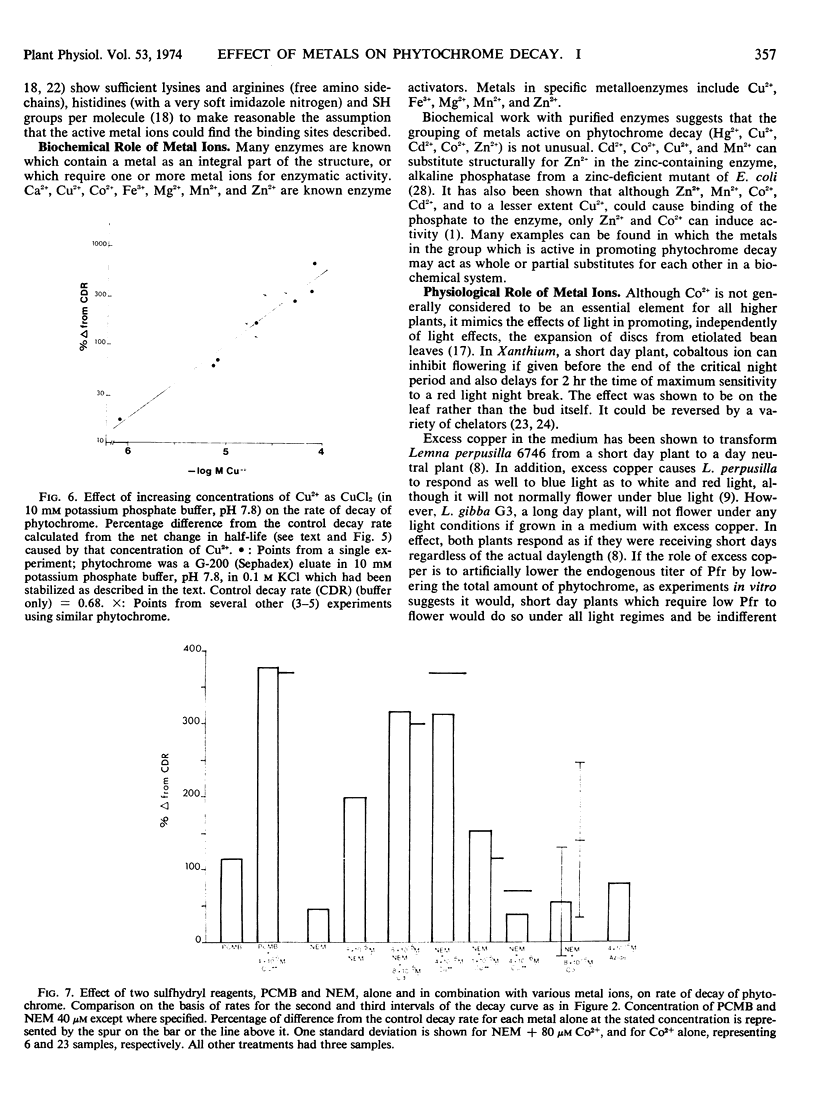
Abstract
Photoreversible phytochrome disappears from etiolated tissue upon actinic irradiation. Such disappearance, of possible physiological importance, involves several processes, at least one of which is accelerated by metals in vivo. Purified phytochrome from oat (Avena sativa L. cv. Garry) coleoptiles is greatly stabilized in vitro by scrupulous removal of metal impurities via chelating agents. Such stabilized phytochrome decays rapidly upon the addition of about 10 μm Hg2+, Cd2+, Cu2+, and Zn2+, all of which probably act on sulfhydryl groups. Other tested metals and growth factors were much less active or inactive. The metals effective in promoting decay do not affect the Pfr → Pr reversion process. This supports other evidence indicating the possible physiological importance of phytochrome “decay.”
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER W. L., SIEGELMAN H. W., MILLER C. O. DENATURATION OF PHYTOCHROME. Biochemistry. 1964 Jun;3:851–857. doi: 10.1021/bi00894a022. [DOI] [PubMed] [Google Scholar]
- Butler W. L., Lane H. C. Dark Transformations of Phytochrome in vivo. II. Plant Physiol. 1965 Jan;40(1):13–17. doi: 10.1104/pp.40.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll D. L., Steers E., Jr, Towe K. M., Shropshire W., Jr Phytochrome in etiolated annual rye. IV. Physical and chemical characterization of phytochrome. Biochim Biophys Acta. 1968 Sep 10;168(1):46–57. doi: 10.1016/0005-2795(68)90232-8. [DOI] [PubMed] [Google Scholar]
- Furuya M., Hopkins W. G., Hillman W. S. Effects of metal-complexing and sulfhydryl compounds on nonphotochemical phytochrome changes in vivo. Arch Biochem Biophys. 1965 Oct;112(1):180–186. doi: 10.1016/0003-9861(65)90026-3. [DOI] [PubMed] [Google Scholar]
- Kendrick R. E., Hillman W. S. Dark Reversion of Phytochrome in Sinapis alba L. Plant Physiol. 1970 Oct;46(4):596–598. doi: 10.1104/pp.46.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. O. Relationship of the Cobalt and Light Effects on Expansion of Etiolated Bean Leaf Disks. Plant Physiol. 1952 Apr;27(2):408–412. doi: 10.1104/pp.27.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford F. E., Jenner E. L. Catalysis of the phytochrome dark reaction by reducing agents. Biochemistry. 1971 Jan 5;10(1):98–101. doi: 10.1021/bi00777a015. [DOI] [PubMed] [Google Scholar]
- Roux S. J. Chemical evidence for conformational differences between the red- and far-red-absorbing forms of oat phytochrome. Biochemistry. 1972 May 9;11(10):1930–1936. doi: 10.1021/bi00760a030. [DOI] [PubMed] [Google Scholar]
- Salisbury F. B. Growth Regulators and Flowering. II. The Cobaltous Ion. Plant Physiol. 1959 Nov;34(6):598–604. doi: 10.1104/pp.34.6.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. J., Stimson W. R. Contributions of photosynthesis and phytochrome to the formation of anthocyanin in turnip seedlings. Plant Physiol. 1971 Sep;48(3):312–315. doi: 10.1104/pp.48.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotman C. N., Greenwood C. Effects of zinc and other metal ions on the stability and activity of Escherichia coli alkaline phosphatase. Biochem J. 1971 Aug;124(1):25–30. doi: 10.1042/bj1240025. [DOI] [PMC free article] [PubMed] [Google Scholar]


