Abstract
Background and Purpose
This study evaluated the use of an artificial intelligence (AI) platform on mobile devices in measuring and increasing medication adherence in stroke patients on anticoagulation therapy. The introduction of direct oral anticoagulants (DOACs), while reducing the need for monitoring, have also placed pressure on patients to self-manage. Suboptimal adherence goes undetected as routine laboratory tests are not reliable indicators of adherence, placing patients at increased risk of stroke and bleeding.
Methods
A randomized, parallel-group, 12-week study was conducted in adults (n = 28) with recently diagnosed ischemic stroke receiving any anticoagulation. Patients were randomized to daily monitoring by the AI Platform (intervention) or to no daily monitoring (control). The AI application visually identified the patient, the medication and confirmed ingestion. Adherence was measured by pill counts and plasma sampling in both groups.
Results
For all patients (n = 28), mean (standard deviation [SD]) age was 57 (13.2) years and 53.6% were female. Mean (SD) cumulative adherence based on the AI Platform was 90.5% (7.5%). Plasma drug concentration levels indicated that adherence was 100% (15 of 15) and 50% (6 of 12) in the intervention and control groups, respectively.
Conclusions
Patients, some with little experience using a smartphone, successfully used the technology and demonstrated a 50% improvement in adherence based on plasma drug concentration levels. For patients receiving DOACs, absolute improvement increased to 67%. Real-time monitoring has the potential to increase adherence and change behavior, particularly in patients on DOAC therapy.
Clinical Trial Registration-URL: http://www.clinicaltrials.gov. Unique identifier: NCT02599259.
Keywords: Patient Adherence, Artificial Intelligence, Anticoagulants, Patient Outcome Assessment, Compliance/Adherence, Quality and Outcomes, Ischemic Stroke
Introduction
Treatment adherence is a critical component of anticoagulation therapy. The recent introduction of direct oral anticoagulants (DOACs) offers a more convenient alternative to warfarin, including a wide therapeutic window, few drug and food interactions, and fixed dosing without the need for laboratory monitoring. While DOACs have reduced the need for regular monitoring, they have also placed pressure on patients to self-manage. At the same time, the shorter half-life of DOACs makes medication adherence a significant concern (1). Laboratory tests currently used to monitor Vitamin K antagonists (VKAs) are either too sensitive or too insensitive to DOACs to act as reliable measures of adherence, making dose titration and the determination of failure of therapy versus poor adherence challenging in routine clinical practice (2, 3). As a result, suboptimal rates of adherence (4,5,6) to DOACs go undetected, placing patients at increased risk of stroke and bleeding.
Most studies rely on claims data and patient self-reports to measure adherence: both are unreliable (7,8). Other measures, such as electronic medication packaging (EMP), while providing a date and time stamp, are largely limited to adherence studies and have limited effect on adherence (9). Adherence interventions (counseling, educational, text messages, electronic monitoring) have demonstrated mixed results (10, 11). The major limitation of these approaches is that they do not verify drug administration. While blood levels are considered the gold standard, inter-person variation, logistics, and cost make them impractical in routine clinical practice (12).
Because of its ability to ensure treatment adherence, directly observed therapy (DOT) has been used for decades to measure and maximize adherence for treatment of tuberculosis, HIV and in inpatient settings. The AI Platform (AiCure, New York, NY) automates DOT using artificial intelligence to visually confirm medication ingestion on smartphones (Supplemental Figure I).
Methods
Eligible patients diagnosed with ischemic stroke (with or without preceding transient ischemic attack and with a score between 1 and 20 on the NIH Stroke Scale [NIHSS]) and receiving oral anticoagulation therapy, were randomized to daily monitoring by the AI Platform (intervention) or to no daily monitoring (control) in this 12-week, randomized, parallel-group, controlled, single-site study. All patients were prescribed warfarin, dabigatran, rivaroxaban, or apixaban. Patients attended 4 clinic visits (baseline and Weeks 4, 8, and 12); prothrombin time(PT)/International Normalized Ratio (INR) and the activated partial thromboplastin time (APTT) were regularly measured. Medication adherence was measured by pill counts, plasma sampling, and the AI Platform. Informed consent was obtained prior to entering the study. The study was approved by an institutional review board.
Exploratory, hypothesis-generating analyses were performed for all randomized patients who took at least 1 dose and included data through Week 12.
AI Platform
Patients randomized to the intervention group were provisioned mobile devices with the Health Insurance Portability and Accountability Act (HIPAA)-compliant application installed. Software algorithms identified the patient, the medication, and confirmed ingestion. The software provided medication reminders and dosing instructions. Late doses triggered notifications within the hour and prior to the end of the dosing window. Real-time data were encrypted and transmitted to web-based dashboards for review. Clinic staff received automated text messages or emails if doses were missed, late, or based on incorrect usage. AI app data fell into 5 categories: 1) visual confirmation of ingestion; 2) self-reported dose via the AI app; 3) self-reported dose by clinic staff, 4) missed dose, and 5) dose taken in clinic (Supplemental Figure I).
Results
A total of 117 patients were screened, with 28 patients randomized; 15 to the AI Platform and 13 to the control group. One patient randomized to the control group withdrew from the study prior to the first dose taken and is excluded from further analysis. Baseline demographics were similar across both groups (Table 1). Mean (standard deviation [SD]) age was 57.0 (13.17) years and 53.6% of patients were female. Patients receiving DOACs (n = 20) outnumbered patients on warfarin (n = 8). Prior smartphone usage and comfort with smartphones were comparable between the 2 groups.
Table 1.
Subject Disposition and Demographics (All Randomized Subjects)
| Overall (N = 28) |
Control (N = 13) |
AI Platform (N = 15) |
||
|---|---|---|---|---|
| Subject Disposition, n (%) | Completed | 27 (96) | 12 (92) | 15 (100) |
| Did not complete | 1 (4) | 1 (8) | 0 | |
| Demographic Characteristics | ||||
| Age, years | Mean (SD) | 57.0 (13.17) | 55.5 (16.55) | 58.3 (9.79) |
| Median (range) | 59 (30, 79) | 57 (30, 79) | 61 (38, 71) | |
| Sex, n (%) | Female | 15 (54) | 9 (69) | 6 (40) |
| Male | 13 (46) | 4 (31) | 9 (60) | |
| Race, n (%) | White | 3 (11) | 3 (23) | 0 |
| Black | 13 (46) | 4 (31) | 9 (60) | |
| Hispanic | 12 (43) | 6 (46) | 6 (40) | |
| Medication Type, n (%) | Warfarin | 8 (29) | 3 (23) | 5 (33) |
| Apixaban | 10 (36) | 5 (39) | 5 (33) | |
| Rivaroxaban | 7 (25) | 3 (23) | 4 (27) | |
| Dabigatran | 3 (11) | 2 (15) | 1 (7) | |
SD = standard deviation
Adherence for All Subjects
A total of 2234 adherence parameters were collected over 12 weeks for patients monitored with the AI app. Mean (SD) cumulative adherence (visual confirmation of drug administration using the AI app) was 90.5% (7.5%) (Figure 1).
Figure 1.
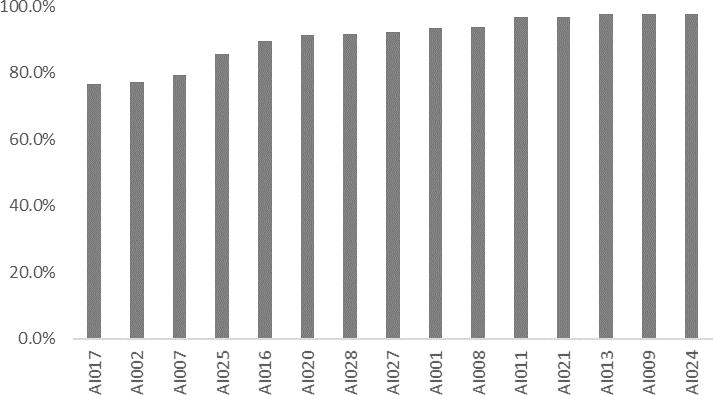
Mean Cumulative Adherence Per Patient Based on AI Platform (Intervention Group)
Mean (SD) cumulative adherence based on pill count was 97.2 (4.4%)for the AI Platform group and 90.6% (5.8%) for the control group. A total of 108 plasma samples (4 per patient) were collected across both groups (3 samples were clotted). Plasma samples were marked as adherent if drug concentration levels were above the minimum required therapeutic range (Cmin). Fifty percent (6 out of 12) and 100% (15 out of 15) of patients in the control and intervention groups, respectively, had all samples above Cmin. Among patients deemed nonadherent (n = 6), all were in the control group and all were prescribed DOACs. At all clinic visits, the intervention group had a higher percentage of samples above Cmin than the control group (Figure 2).
Figure 2.
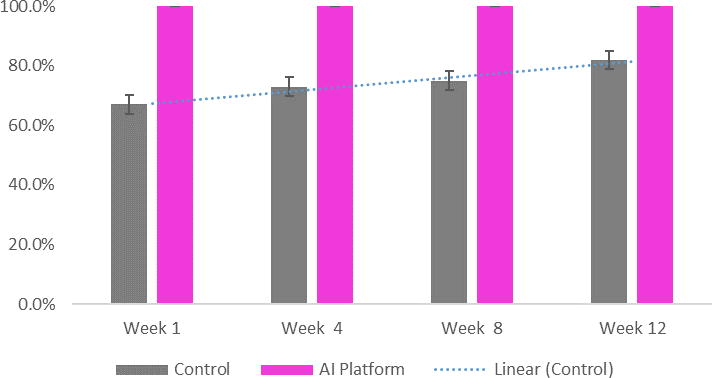
Mean Percentage of Samples Marked as Adherent Over Time (Above Cmin)
Substudy - Adherence for Patients Receiving DOACs
More than half of the patients (n = 19) received DOACs. Mean (SD) cumulative adherence (visual confirmation of drug administration using the AI app) was 90.1% (7.3%). Mean (SD) cumulative adherence based on pill count was 96.4% (5.1%) for the AI Platform group and and 90.9% (6.0%) for the control group. Mean (SD) cumulative adherence based on pill count for subjects with plasma samples below therapeutic range was 90.5% (5.6%) compared to 95.3% (5.9%) for subjects with plasma samples above therapeutic range. Based on drug concentration levels, 33% (3 out of 9) in the control and 100% (10 of 10) in the intervention group had all samples above Cmin.
APTT, PT/INR
Averages for APTT and PT/INR were similar across the control and intervention groups: PTT (48.4 and 41.7, respectively); PT (32.9 and 35.1, respectively); INR (3.1 and 3.4, respectively).
Usability and Feasibility of the AI Platform
Patients randomized to the intervention group were asked to complete pre- and post-study usability questionnaires. In the pre- and post-study questionnaire, overall 73.3% and 83.3% of patients, respectively, answered ‘extremely good’ when asked four questions to rate the AI Platform as a medication management tool and as a means to improve the doctor/patient relationship.
Discussion
This study utilized a novel artificial intelligence platform to assess and increase medication adherence in patients with a recently diagnosed ischemic stroke. Unlike most studies, which rely on indirect measures, to our knowledge, this study was the first RCT to compare adherence rates of all 3 DOACs (dabigatran, rivaroxaban, and apixaban) and warfarin together based on daily real-time monitoring against a control group, and verified by plasma sampling. Suboptimal adherence across all DOACs confirms previous findings although this study suggests adherence might be lower than previously recognized; high adherence to warfarin underlines the value of routine laboratory tests to in effect ensure adherence. Absolute improvement of 67% in patients taking DOACs and monitored by the AI app – and confirmed by drug levels – demonstrates the potential value of daily real-time monitoring to measure and maximize adherence. Few studies have deployed smartphone apps in middle-aged or elderly populations (13). Consistent use and general likability of the AI app over 12 weeks underscores the possibility of harnessing new technologies to optimize adherence in patients on DOACs for whom routine laboratory tests are not good indicators of adherence. Artificial Intelligence platforms have the potential to accurately monitor medication ingestion and change patient behavior.
Supplementary Material
Acknowledgments
The authors acknowledge Aubri Charboneau of Sage Scientific Writing, LLC for editorial support and John E. Hinkle of EarlyPhase Sciences, Inc. for database support and statistical analyses.
Sources of Funding
Funding was received from the National Center for Advancing Translational Sciences (NCATS), grant number: 9R44 TR000873-02.
Sponsored by AiCure, New York, NY.
Footnotes
Conflict(s)-of-Interest/Disclosure(s)
Daniel L. Labovitz, Deepti Virmani, and Morayma Reyes Gil are employees of Montefiore Medical Center, Bronx, NY. Laura Shafner and Adam Hanina are employees of AiCure, New York, NY, USA.
References
- 1.Bauer KA. Pros and Cons of New Oral Anticoagulants. Hematology Am Soc Hematol Educ Program. 2013;2013:464–470. doi: 10.1182/asheducation-2013.1.464. [DOI] [PubMed] [Google Scholar]
- 2.Ten Cate H. New Oral Anticoagulants: Discussion on Monitoring and Adherence Should Start Now! Thromb J. 2013;11:8. doi: 10.1186/1477-9560-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Favaloro EJ, Lippi G. The New Oral Anticoagulants and the Future of Haemostasis Laboratory Testing. Biochem Med (Zagreb) 2012;22:329–341. doi: 10.11613/bm.2012.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao X, Abraham NS, Alexander GC, Crown W, Montori VM, Sangaralingham LR, et al. Effect of Adherence to Oral Anticoagulants on Risk of Stroke and Major Bleeding Among Patients With Atrial Fibrillation. J Am Heart Assoc. 2016;5:e003074. doi: 10.1161/JAHA.115.003074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song X, Sander SD, Varker H, Amin A. Patterns and Predictors of Use of Warfarin and Other Common Long-term Medications in Patients with Atrial Fibrillation. Am J Cardiovasc Drugs. 2012;12:245–253. doi: 10.1007/BF03261833. [DOI] [PubMed] [Google Scholar]
- 6.Zhou M, Chang HY, Segal JB, Alexander GC, Singh S. Adherence to a Novel Oral Anticoagulant Among Patients with Atrial Fibrillation. J Manag Care Spec Pharm. 2015;21:1054–1062. doi: 10.18553/jmcp.2015.21.11.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaschke TF, Osterberg L, Vrijens B, Urquhart J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu Rev Pharmacol Toxicol. 2012;52:275–301. doi: 10.1146/annurev-pharmtox-011711-113247. [DOI] [PubMed] [Google Scholar]
- 9.Checchi KD, Huybrechts KF, Avorn J, Kesselheim AS. Electronic Medication Packaging Devices and Medication Adherence: A Systematic Review. JAMA. 2014;312:1237–1247. doi: 10.1001/jama.2014.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei J, Hollin I, Kachnowski SA. Review of the Use of Mobile Phone Text Messaging in Clinical and Healthy Behaviour Interventions. J Telemed Telecare. 2011;17:41–48. doi: 10.1258/jtt.2010.100322. [DOI] [PubMed] [Google Scholar]
- 11.Wu YP, Pai AL. Health Care Provider-delivered Adherence Promotion Interventions: A Meta-analysis. Pediatrics. 2014;133:e1698–707. doi: 10.1542/peds.2013-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shemesh E, Fine RN. Is Calculating the Standard Deviation of Tacrolimus Blood Levels the New Gold Standard for Evaluating Non-adherence to Medications in Transplant Recipients? Pediatr Transplant. 2010;14:940–943. doi: 10.1111/j.1399-3046.2010.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mira JJ, Navarro I, Botella F, Borrás F, Nuño-Solinís R, Orozco D, et al. A Spanish Pillbox App for Elderly Patients Taking Multiple Medications: Randomized Controlled Trial. J Med Internet Res. 2014;16:e99. doi: 10.2196/jmir.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


