Abstract
Aim
Higher haemoglobin levels and differences in glucose metabolism have been reported among high‐altitude residents, which may influence the diagnostic performance of HbA1c. This study explores the relationship between HbA1c and fasting plasma glucose (FPG) in populations living at sea level and at an altitude of > 3000 m.
Methods
Data from 3613 Peruvian adults without a known diagnosis of diabetes from sea‐level and high‐altitude settings were evaluated. Linear, quadratic and cubic regression models were performed adjusting for potential confounders. Receiver operating characteristic (ROC) curves were constructed and concordance between HbA1c and FPG was assessed using a Kappa index.
Results
At sea level and high altitude, means were 13.5 and 16.7 g/dl (P > 0.05) for haemoglobin level; 41 and 40 mmol/mol (5.9% and 5.8%; P < 0.01) for HbA1c; and 5.8 and 5.1 mmol/l (105 and 91.3 mg/dl; P < 0.001) for FPG, respectively. The adjusted relationship between HbA1c and FPG was quadratic at sea level and linear at high altitude. Adjusted models showed that, to predict an HbA1c value of 48 mmol/mol (6.5%), the corresponding mean FPG values at sea level and high altitude were 6.6 and 14.8 mmol/l (120 and 266 mg/dl), respectively. An HbA1c cut‐off of 48 mmol/mol (6.5%) had a sensitivity for high FPG of 87.3% (95% confidence interval (95% CI) 76.5 to 94.4) at sea level and 40.9% (95% CI 20.7 to 63.6) at high altitude.
Conclusion
The relationship between HbA1c and FPG is less clear at high altitude than at sea level. Caution is warranted when using HbA1c to diagnose diabetes mellitus in this setting.
What's new?
Haemoglobin levels and differences in glucose metabolism at high altitude may influence the diagnostic performance of testing for diabetes using HbA1c.
We found that the relationship between HbA1c and fasting plasma glucose (FPG) differed markedly between high‐altitude and sea‐level areas.
The relationship between HbA1c and FPG was quadratic at sea level and linear at high altitude.
Corresponding FPG values for an HbA1c ≥ 48 mmol/mol (≥ 6.5%) cut‐off point, used for the diagnosis of diabetes, were 6.6 and 14.8 mmol/l (120 and 266 mg/dl) at sea level and high altitude, respectively.
The sensitivity of HbA1c to detect abnormal FPG was 87.3% at sea level and 40.9% at high altitude. This suggests a limitation in the performance of HbA1c to diagnose diabetes at altitude.
What's new?
Haemoglobin levels and differences in glucose metabolism at high altitude may influence the diagnostic performance of testing for diabetes using HbA1c.
We found that the relationship between HbA1c and fasting plasma glucose (FPG) differed markedly between high‐altitude and sea‐level areas.
The relationship between HbA1c and FPG was quadratic at sea level and linear at high altitude.
Corresponding FPG values for an HbA1c ≥ 48 mmol/mol (≥ 6.5%) cut‐off point, used for the diagnosis of diabetes, were 6.6 and 14.8 mmol/l (120 and 266 mg/dl) at sea level and high altitude, respectively.
The sensitivity of HbA1c to detect abnormal FPG was 87.3% at sea level and 40.9% at high altitude. This suggests a limitation in the performance of HbA1c to diagnose diabetes at altitude.
Introduction
Nowadays, Type 2 diabetes mellitus is a global epidemic, and the prevalence is far from levelling off. The prevalence of diabetes has almost doubled in the last three decades and the chances of achieving the global target of halting the increase in prevalence by 2025 are < 1% 1. Although originally identified by the presence of glucose in urine, glucose tests for the diagnosis of Type 2 diabetes have been developed over the last century. The oral glucose tolerance test has been used for the diagnosis of Type 2 diabetes over the last three decades. However, this test is laborious for individuals, and thus, has been replaced by fasting plasma glucose (FPG) for use in both clinical settings and epidemiological studies.
HbA1c had been established as the monitoring test of choice to evaluate medium‐term diabetic control 2. Several international societies, including the American Diabetes Association and World Health Organization (WHO) recommend using HbA1c as a diagnostic criterion for Type 2 diabetes in stable haematological circumstances, because it has several advantages over glucose tests, such as low intra‐individual variation and the convenience of taking the test without fasting. However, this recommendation has been criticized because of the observed discordance between HbA1c and glucose tests, and biological variation in certain ethnic groups 3, 4. As such, alternative population‐specific HbA1c cut‐off points for the diagnosis of Type 2 diabetes have recently been proposed 5.
Changes in erythrocytes states, for example, due to folic acid deficiency and renal disease, erythrocyte lifespan and levels of haemoglobin can also influence HbA1c levels 6. One of the mechanisms of adaptation in high‐altitude settings is secondary polycythaemia (increase in haemoglobin levels) 7. A common feature of many Latin American countries, where over the last two decades Type 2 diabetes‐related mortality has been the highest worldwide 8, is a significant proportion of people living at high altitude. Over 30 million people currently reside in the Central American highlands, and in Peru, one third of the population live at altitude 9. Indeed, secondary polycythaemia has been largely reported among Andean natives 10, 11. Additionally, differences in glucose metabolism have been reported among people residing at high altitude 12.
In this study, we aim to explore and compare the relationship between HbA1c and FPG in populations living at high altitude and sea level.
Methods
Study settings and participants
We identified eligible individuals from two Peruvian longitudinal population‐based studies: the CRONICAS Cohort Study (n = 3601, baseline conducted in 2010–2011), and the rural Ayacucho population of the PERU MIGRANT Study (n = 200, baseline conducted in 2007–2008). The CRONICAS Cohort Study aimed to assess the prevalence and incidence of cardiometabolic and pulmonary conditions at four sites: Lima, highly urban, sea level; Tumbes, semi‐urban, sea level; and two high‐altitude locations (3825 m above sea level), rural and urban Puno. All participants were aged 35 years or older and full‐time residents in the area. The PERU MIGRANT Study was designed to investigate differences in cardiovascular disease risk factors between rural‐to‐urban migrant and non‐migrant groups. This study was performed in participants aged 30 years and over from a rural site in Ayacucho, located at 2900–3100 m above sea level, an urban site in Lima, and rural‐to‐urban migrants from Ayacucho currently residing in Lima. In both studies, participants were sex‐ and age‐stratified, a single‐stage random sampling was used, and only one participant per household was enrolled. The studies are described in detail elsewhere 13, 14.
The original pooled dataset had 3801 cases. We excluded 187 individuals with self‐reported diagnosis of diabetes or use of anti‐diabetic medications. In addition, one person was excluded during regression analysis because that individual was an influential point. The final number of people included in this analysis was 3613.
Participants were classified into two geography‐based categories: (1) sea‐level population (those from Lima and Tumbes), and (2) high‐altitude population (those from Ayacucho and Puno).
Study variables
We evaluated clinical variables, including BMI, hypertension (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or current use of antihypertensive medications), and current smoking status (self‐report of having smoked at least one cigarette in the last 30 days). We also explored sociodemographic variables, such as wealth index based on asset possessions 15 and educational level (primary or less, secondary and higher).
Besides FPG (mmol/l) and HbA1c (mmol/mol; %), additional laboratory variables included lipid measurements (total cholesterol, triglycerides, HDL‐C and Friedewald‐estimated LDL‐C, in mg/dl), and haematological parameters, such as total haemoglobin (g/dl), mean corpuscular volume (fl/red blood cell), mean corpuscular haemoglobin (pg/cell) and mean corpuscular haemoglobin concentration (g/dl). HbA1c was measured using high‐performance liquid chromatography (HPLC, D10‐BIORAD, Germany), which is traceable to the Diabetes Control and Complications Trials reference study as certified by the National Glycohemoglobin Standardization Program.
Statistical analysis
For descriptive purposes, study variables were compared between sea‐level and high‐altitude settings using analysis of variance, chi‐squared or Fisher's exact tests. Linear, quadratic and cubic regression models were performed to assess the relationship between HbA1c and FPG, crude and adjusted by age, sex, education, wealth, BMI and total haemoglobin. Models were performed separately for each sea‐level and high‐altitude subgroup.
Beta coefficients and 95% confidence intervals (95% CI) were calculated for FPG, squared‐FPG and/or cubic‐FPG. Maximum likelihood optimization (Newton–Raphson) and robust variance estimations 16 were used in these models to compensate for heteroscedasticity and non‐normality. Information from Wald's test and Bayesian information criteria helped select the best models.
We evaluated diagnostic performance for diabetes and prediabetes in the sea‐level and high‐altitude subgroups using FPG as the gold standard. We used the cut‐off points recommended by the American Diabetes Association for HbA1c (normal < 39 mmol/mol, < 5.7%; prediabetes 39 to < 48 mmol/mol, 5.7 to < 6.5%; diabetes ≥ 48 mmol/mol, ≥ 6.5%) and FPG (normal < 5.6 mmol/l; prediabetes 5.6–6.9 mmol/l; diabetes ≥ 7.0 mmol/l). We evaluated sensitivity, specificity, positive predictive value, negative predictive value and likelihood ratios of HbA1c, and receiver operating characteristic (ROC) curves.
Finally, we compared concordance between diagnosis by HbA1c and FPG using a Kappa index. All analyses were conducted using Stata/IC v. 12 (Stata Corp, College Station, TX, USA). Ethical approval was obtained for the original studies.
Results
In total, 3613 individuals were included in the analysis: Lima, n = 1036; Tumbes, n = 963; Ayacucho, n = 200; and Puno, n = 1414. Haemoglobin levels were significantly lower in individuals at sea level (13.5 ± 1.4 g/dl) than those at high altitude (16.7 ±1.9 g/dl) (P < 0.001). Mean HbA1c was 41 mmol/mol (5.9 ± 0.88%) at sea level, and 40 mmol/mol (5.8 ± 0.48%) at high altitude. Individuals at sea level had higher mean FPG (5.3 ± 1.4 mmol/l) compared with those from high altitude (4.9 ± 0.9 mmol/l) (P < 0.001). The cardiovascular risk factor profile, in terms of adiposity, lipid markers, hypertension and smoking status, was poorer among those living at sea level. Diabetes, defined both by HbA1c and FPG, was more prevalent at sea level than high altitude. In both high‐altitude and sea‐level settings, the estimates of HbA1c‐defined diabetes were three times higher than those based on FPG. We had haematological parameters from one site at high altitude (Ayacucho, n = 167). Levels of mean haematocrit were 48.5 ± 4.1%; mean corpuscular volume was 94.9 ± 4.9 fl/red blood cell; mean corpuscular haemoglobin was 31.2 ± 1.7 pg/cell; and mean corpuscular haemoglobin concentration was 32.8 ± 1.2 g/dl (Table 1).
Table 1.
Characteristics of study participants at sea‐level and high‐altitude settings
| Variable | Total (n = 3613) | Sea level (n = 1999) | High altitude (n = 1614) | P a |
|---|---|---|---|---|
| Sociodemographic | ||||
| Age (mean ± sd) | 3611 | 55.2 ± 12.7 | 55.0 ± 13.0 | 0.77 |
| Male, n (%) | 3610 | 986 (49.3) | 770 (47.8) | 0.36 |
| Wealth index (mean ± sd) | 3613 | 251.9 ± 153.8 | 167.9 ± 161.8 | < 0.001 |
| Education | ||||
| Primary or less, n (%) | 1709 | 971 (48.6) | 738 (45.7) | < 0.001 |
| Secondary, n (%) | 1137 | 707 (35.4) | 430 (26.7) | |
| Higher, n (%) | 764 | 319 (16.0) | 445 (27.6) | |
| Cardiovascular risk factors | ||||
| BMI (kg/m2, mean ± sd) | 3248 | 28.3 ± 4.6 | 25.9 ± 4.2 | < 0.001 |
| Waist circumference, cm (mean ± sd) | 3243 | 93.2 ± 10.4 | 86.6 ± 12.1 | < 0.001 |
| Total cholesterol, mg/dL (mean ± sd) | 2947 | 202.4 ± 38.7 | 194.7 ± 40.8 | < 0.001 |
| Triglycerides, mg/dL [median (IQR)] | 3147 | 139 (97) | 125 (83) | < 0.001 |
| HDL‐C, mg/dL (mean ± sd) | 2947 | 40.9 ± 11.5 | 43.0 ± 11.3 | < 0.001 |
| LDL‐C, mg/dL (mean ± sd)b | 200 | – | 85.7 ± 27.1 | |
| Hypertension, n (%) | 3047 | 291 (15.0) | 106 (9.6) | < 0.001 |
| Current smoker, n (%) | 3610 | 268 (13.4) | 130 (8.1) | < 0.001 |
| Haematological variables | ||||
| Haemoglobin, g/dL (mean ± sd) | 3146 | 13.5 ± 1.4 | 16.7 ± 1.9 | < 0.001 |
| Mean corpuscular volume, fl/red blood cellb | 167 | – | 94.9 ± 4.9 | |
| Mean corpuscular haemoglobin, pg/cellb | 167 | – | 31.2 ± 1.7 | |
| Mean corpuscular haemoglobin concentration, g/dlb | 167 | – | 32.8 ± 1.2 | |
| Diabetes‐related markers | ||||
| HbA1c (mean mmol/mol) | 3146 | 41 | 40 | 0.10 |
| HbA1c (mean % ± sd) | 5.9 ± 0.88 | 5.8 ± 0.48 | ||
| Fasting plasma glucose (mean mmol/l ± sd) | 3146 | 5.3 ± 1.4 | 4.9 ± 0.9 | < 0.001 |
| Diabetes diagnosed by HbA1c c | ||||
| Normal, n (%) | 1227 | 789 (40.9) | 438 (36.0) | < 0.001 |
| Prediabetes, n (%) | 1687 | 978 (50.6) | 709 (58.3) | |
| Diabetes, n (%) | 232 | 163 (8.5) | 69 (5.7) | |
| Diabetes diagnosed by FPGd | ||||
| Normal, n (%)a | 2493 | 1433 (74.3) | 1060 (87.2) | < 0.001 |
| Prediabetes, n (%)a | 568 | 434 (22.5) | 134 (11.0) | |
| Diabetes, n (%)a | 85 | 63 (3.3) | 22 (1.8) | |
ANOVA one‐way for mean differences; Kruskal–Wallis or median differences; chi square for distribution differences.
Only available for Ayacucho.
Prediabetes and diabetes were diagnosed using the American Diabetes Association recommended HbA1c cut‐off point: diabetes, HbA1c ≥ 48 mmol/mol (≥ 6.5%); prediabetes, ≥ 48 mmol/mol (6.5%) > HbA1c ≥ 39 mmol/mol (≥ 5.7%); normal, HbA1c < 39 mmol/mol (< 5.7%).
Prediabetes and diabetes were diagnosed using the American Diabetes Association recommended FPG cut‐off point: diabetes, FPG ≥ 7.0 mmol/l; prediabetes, 7.0 mmol/l > FPG ≥ 5.6 mmol/l; normal: FPG < 5.6 mmol/l.
FPG, fasting plasma glucose.
In both crude and adjusted models, we found differences between predictions of HbA1c by FPG at sea level and high altitude (Figs 1 and 2). Whereas HbA1c and FPG showed a non‐linear, quadratic relationship at sea level, we found a linear association at high altitude (Table S1). Differences in relationship patterns and intercept values (3.9 for high altitude, 4.6 for sea level) display notable differences in the shape of each curve (Fig. 1). This effect has a repercussion on values for diagnosis: to predict an HbA1c value of 48 mmol/mol (6.5%), mean FPG values of 6.6 and 14.8 mmol/l were needed at sea level and high altitude, respectively.
Figure 1.
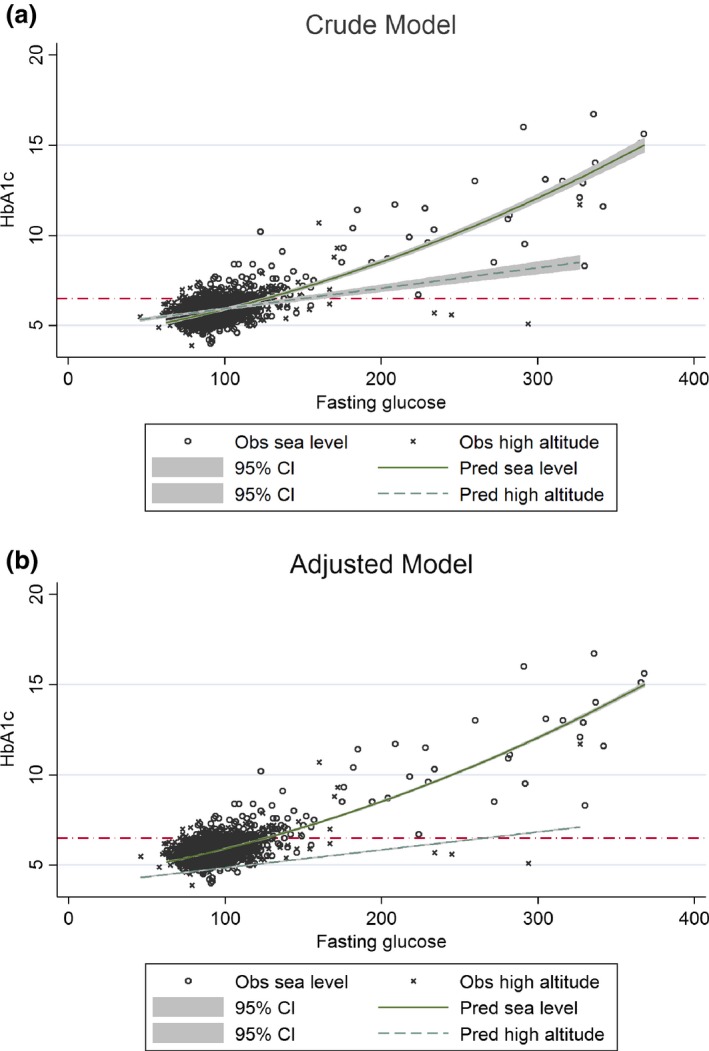
Graphical representation of the quadratic model (sea level) and linear model (high altitude) for HbA1c (dependent variable) and fasting plasma glucose (independent variable), crude and adjusted by age, sex, education, wealth, BMI and total haemoglobin levels. After comparison of linear, quadratic and cubic models of the relationship between HbA1c and fasting plasma glucose, a quadratic adjusted model was selected as the best for people at sea level, and a linear adjusted model was selected as the best for people at high altitude (Table S1). The red line was established at an HbA1c value of 48 mmol/mol (6.5%) to represent the current recommended diagnostic cut‐point for diabetes 17.
Figure 2.
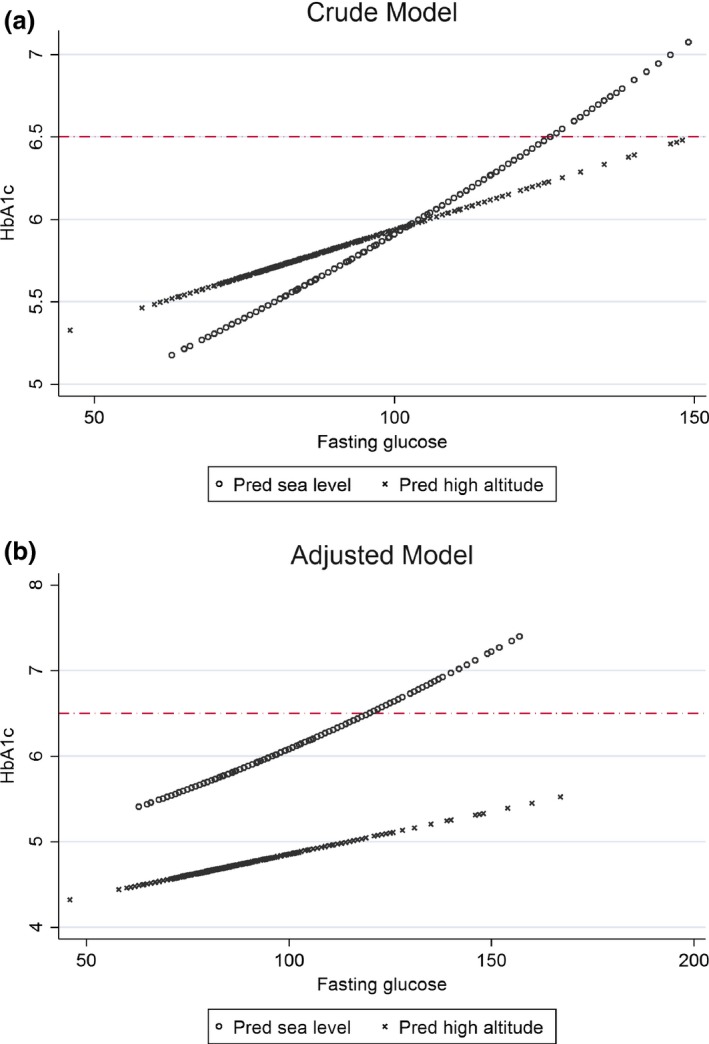
Amplification of the zone near to an HbA1c value of 48 mmol/mol (6.5%) in the graphical representation of the quadratic model (sea level) and linear model (high altitude) for HbA1c (dependent variable) and fasting plasma glucose (independent variable) both crude and adjusted by age, sex, education, wealth, BMI and total haemoglobin. The red line was established at a HbA1c value of 48 mmol/mol (6.5%) to indicate the standard diagnostic cut‐point for diabetes 17.
Among those with diabetes, at sea level, the number of individuals diagnosed by HbA1c was 13.5 times greater than diagnosed by FPG only (108 vs. 8), and this relationship was 4.6 times greater at high altitude (60 vs. 13). Individuals diagnosed by HbA1c only were older, but metabolically healthier at high altitude than at sea level. Similar results were found among individuals diagnosed by FPG, and by the combination of HbA1c and FPG (Table 2).
Table 2.
Clinical characteristics of individuals with diagnosis of diabetes by different criteriaa
| Overall | Sea‐level population | High‐altitude population | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | HbA1c only | HbA1c and FPG | FPG only | HbA1c only | HbA1c and FPG | FPG only | HbA1c only | HbA1c and FPG | FPG only |
| n b | 168 | 64 | 21 | 108 | 55 | 8 | 60 | 9 | 13 |
| Age (mean ± sd) | 60.4 ± 12.8 | 57.2 ± 10.5 | 58.4 ± 12.3 | 59.1 ± 12.9 | 57.0 ± 10.3 | 53.8 ± 14.1 | 62.7 ± 12.2 | 58.7 ± 12.0 | 61.3 ± 10.6 |
| Male, n (%) | 59 (35.1) | 26 (40.6) | 12 (57.1) | 42 (38.9) | 23 (41.8) | 5 (62.5) | 17 (28.3) | 3 (33.3) | 7 (53.9) |
| BMI (kg/m2) | 29.6 ± 6.0 | 30.6 ± 5.1 | 28.5 ± 6.1 | 30.7 ± 5.8 | 30.9 ± 5.0 | 33.0 ± 7.8 | 27.6 ± 5.9 | 28.9 ± 5.3 | 25.7 ± 2.4 |
| Waist circumference, cm (mean ± sd) | 95.1 ± 13.4 | 99.0 ± 9.2 | 94.4 ± 15.0 | 98.3 ± 11.0 | 99.7 ± 11.3 | 104.9 ± 17.5 | 89.3 ± 15.4 | 95.0 ± 13.5 | 87.9 ± 8.9 |
| Total cholesterol, mg/dL (mean ± sd) | 208.8 ± 42.9 | 218.0 ± 39.4 | 220.0 ± 42.3 | 211.8 ± 39.4 | 217.7 ± 40.8 | 236.6 ± 43.2 | 201.9 ± 49.7 | 219.5 ± 29.9 | 209.6 ± 39.9 |
| Triglycerides, mg/dL [median (IQR)] | 149 (101) | 184 (100) | 112 (81) | 148 (97) | 183 (108) | 138 (66) | 149 (105) | 185 (96) | 111 (84) |
| HDL‐C, mg/dL (mean ± sd) | 39.0 ± 10.3 | 38.6 ± 10.6 | 50.1 ± 15.3 | 38.7 ± 9.8 | 38.5 ± 11.1 | 48.1 ± 17.8 | 39.8 ± 11.6 | 39.4 ± 7.8 | 51.3 ± 14.2 |
| Hypertension, n (%) | 34 (22.1) | 20 (31.8) | 2 (9.5) | 28 (25.9) | 20 (36.4) | 2 (25.0) | 6 (13.0) | 0 (0) | 0 (0) |
| Current smoker, n (%) | 23 (13.7) | 11 (17.2) | 4 (19.1) | 16 (14.8) | 10 (18.2) | 2 (25.0) | 7 (11.7) | 1 (11.1) | 2 (15.4) |
| Haemoglobin, g/dL (mean ± sd) | 14.5 ± 2.6 | 14.3 ± 2.2 | 16.0 ± 2.0 | 13.2 ± 1.7 | 13.6 ± 1.1 | 14.3 ± 0.98 | 16.9 ± 2.3 | 18.5 ± 2.5 | 17.0 ± 1.8 |
Diagnosed by HbA1c only: HbA1c ≥ 48 mmol/mol (≥ 6.5%) and FPG < 7.0 mmol/l; diagnosed by FPG only: HbA1c < 48 mmol/mol (< 6.5%) and FPG ≥ 7.0 mmol/l; diagnosed by both: HbA1c ≥ 48 mmol/mol (≥ 6.5%) and FPG ≥ 7.0 mmol/l.
Of a total 3613 people in the study, we excluded those without diabetes (n = 2892) and those without complete data to evaluate diabetes status (HbA1c and FPG, n = 468), therefore data from n = 253 people is included.
Entries in bold represent P < 0.05 in comparisons between sea‐level and high‐altitude populations. For categorical variables (%) we used Fisher's exact test (2 × 3 cross table). For continuous variables (means) we used one‐way ANOVA, comparing each criterion by altitude (separately). For non‐normal variables (medians) we used Kruskal–Wallis test, comparing each criterion by altitude (separately).
FPG, fasting plasma glucose.
Using HbA1c instead of FPG to diagnose diabetes increased the number of cases by 159% at sea level and 215% at high altitude. Finally, when evaluating the agreement between diagnosis of diabetes and prediabetes by HbA1c and FPG, we found poor agreement at sea level (Kappa = 0.19) and at high altitude (Kappa = 0.04) (Table 3).
Table 3.
Concordance of diabetes and prediabetes diagnostics at sea level and high altitude settings considering HbA1c or FPG standard cut‐points
| Test | Sea‐level population | High‐altitude population | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HbA1c | HbA1c | ||||||||
| Normal | Prediabetes | Diabetes | Total | Normal | Prediabetes | Diabetes | Total | ||
| Fasting plasma glucose | Normal | 692 | 691 | 50 | 1433 | 402 | 619 | 38 | 1059 |
| Prediabetes | 95 | 281 | 58 | 434 | 32 | 80 | 22 | 134 | |
| Diabetes | 2 | 6 | 55 | 63 | 4 | 9 | 9 | 22 | |
| Totala | 789 | 978 | 163 | 1930 | 438 | 708 | 69 | 1215 | |
Of a total of 3613 people in the study, we excluded those without complete data to evaluate diabetes status (HbA1c and FPG, n = 468), therefore, data from n = 3145 people are included.
Diagnostic criteria for HbA1c: diabetes, HbA1c ≥ 48 mmol/mol (≥ 6.5%); prediabetes, ≥ 48 mmol/mol (6.5%) > HbA1c ≥ 39 mmol/mol (≥ 5.7%); normal: HbA1c < 39 mmol/mol (< 5.7%).
Diagnostic criteria for FPG: diabetes, FPG ≥ 7.0 mmol/l; prediabetes, 7.0 > FPG ≥ 5.6 mmol/l; normal, FPG < 5.6 mmol/l.
Concordance at sea‐level settings: kappa = 0.19, expected agreement = 42.0%; agreement = 53.3%.
Concordance at high‐altitude settings: kappa = 0.04, expected agreement = 38.0%; agreement = 40.4%.
FPG, fasting plasma glucose.
The sensitivity of an HbA1c cut‐off value 48 mmol/mol (6.5%) for diabetes diagnoses, using FPG as a gold standard, was much higher in the sea‐level groups (87.3%) than in the high‐altitude groups (40.9%), with specificities of 94.2% and 95.0%, respectively. Positive likelihood ratios were 15.1 and 8.1, respectively. Sensitivities for diagnosis of prediabetes were similar, 74.7% and 71.4% in the sea‐level and high‐altitude groups, respectively (Table 4). ROC areas for sea level (0.95) and high altitude (0.74) were significantly different (chi2(1) = 9, P < 0.01) using HbA1c standard cut‐points and FPG as the gold standard of diabetes (Fig. S1). ROC areas for sea level (0.68) and high altitude (0.57) were also significantly different (chi2(1) = 12, P < 0.001) using HbA1c standard cut‐points and FPG as the gold standard of prediabetes (Fig. S2).
Table 4.
Diagnostic test characteristics for HbA1c standard cut‐points using FPG as the gold standard
| Sea‐level population (n = 1930)a | High‐altitude population (n = 1215)a | ||||
|---|---|---|---|---|---|
| Test | % | 95% CI | % | 95% CI | |
| Diabetes, HbA1c ≥ 48 mmol/mol (≥ 6.5%) | Sensitivity | 87.3 | (76.5–94.4) | 40.9 | (20.7–63.6) |
| Specificity | 94.2 | (93.1–95.2) | 95.0 | (93.6–96.1) | |
| PPV | 51.2 | (46.1–56.3)b | 25.3 | (16.2–37.2)b | |
| NPV | 99.1 | (98.2–99.5)b | 97.5 | (96.5–98.2)b | |
| LR+ | 15.1 | (12.3–18.5) | 8.1 | (4.7–14.2) | |
| LR− | 0.14 | (0.07–0.26) | 0.62 | (0.44–0.88) | |
| Prediabetes, HbA1c ≥ 39 mmol/mol (≥ 5.7%) and < 48 mmol/mol (< 6.5%) | Sensitivity | 74.7 | (70.0–79.0) | 71.4 | (62.1–79.6) |
| Specificity | 50 | (47.4–52.7) | 39.4 | (36.4–42.4) | |
| PPV | 32.1 | (30.4–33.8)b | 13.8 | (12.4–15.4)b | |
| NPV | 86.2 | (83.9–88.3)b | 91.0 | (88.2–93.2)b | |
| LR+ | 1.5 | (1.4–1.6) | 1.2 | (1.1–1.3) | |
| LR− | 0.51 | (0.42–0.61) | 0.73 | (0.54–0.98) | |
Of a total 3613 people in the study, we excluded those without complete data to evaluate diabetes status (HbA1c and FPG, n = 468), therefore data from n = 3145 people are included.
Values and confidence intervals are based on likelihood ratios, using prevalence estimated in this study (using explained FPG cut‐off points). Diabetes sea level = 6.5%; diabetes high altitude = 4%; prediabetes sea level = 24%; prediabetes high altitude = 12%.
The gold standard for diabetes is defined as FPG ≥ 7.0 mmol/l and for prediabetes 7.0 mmol/l > FPG ≥ 5.6 mmol/l.
95% CI, 95% confidence interval; FPG, fasting plasma glucose; PPV, positive predictive value; NPV, negative predictive value; LR+, positive likelihood ratio; LR−, negative likelihood ratio.
The prevalence of diabetes is higher when HbA1c is used (≥ 48 mmol/mol; ≥ 6.5%) rather than FPG (≥ 7.0 mmol/l) for sea‐level populations (diabetes prevalence of 12.3% with HbA1c and 6.5% with FPG) and high‐altitude populations (diabetes prevalence of 7.9% with HbA1c and 3.8% with FPG) (Table S2).
Discussion
In this study, we found that the relationship between HbA1c and FPG differed markedly between populations living at high altitude and sea level. Using current recommended HbA1c cut‐off points for the diagnosis of diabetes (≥ 48 mmol/mol, ≥ 6.5%), our models showed a discrepancy of up to 8.2 mmol/l units of FPG. In other words, corresponding FPG values for such HbA1c cut‐off point were 6.6 and 14.8 mmol/l at sea level and high altitude, respectively. This translated into major discrepancies in diagnostic performance, as shown by differences in the sensitivity of HbA1c at sea level (89%) compared with at high altitude (41%). In terms of new cases of diabetes, greater discordance was observed in high‐altitude settings, which was confirmed by the poor agreement found. Taken together, our findings show that high altitude is another setting in which HbA1c might not be appropriate when used as a diagnostic tool for Type 2 diabetes.
Discordance between FPG and HbA1c has been reported in American, European and Asian populations, as well as in older and female individuals. However, this is the first study reporting discordance in Andean populations. Differences in the glycation process, of genetic or adaptive origin, have been shown to play a significant role in inter‐individual variance by causing abnormally high or low levels of HbA1c for a given plasma glucose level 18. However, other physiological or environmental pathways may contribute to discordance observed between FPG and HbA1c in our study settings.
We observed that FPG was, on average, 0.4 mmol/l higher at sea‐level sites than at high‐altitude sites, yet mean HbA1c was similar in both study groups. Glucose metabolism has been shown to differ at altitude; for instance, an association between polycythaemia and glucose intolerance has previously been described in an Andean population 12. A study in rats showed that exposure to hypobaric hypoxia is associated with reduced insulin release due to inhibition of corticotrophin‐releasing hormone 19. This has also been replicated in clinical research; a recent publication by our group found that a 5% decrease in oxyhaemoglobin saturation was strongly associated with a HbA1c value ≥ 48 mmol/mol (≥ 6.5%) (JC Bazo‐Alvarez, R Quispe, TD Pillay, A Bernabé‐Ortiz, L Smeeth, W Checkley, RH Gilman, G Málaga, JJ Miranda, personal communication). It is possible that these processes of relative intolerance lead to a serum glucose level that is not represented by a FPG test obtained in a fasting state, but is identified by HbA1c.
We hypothesize that the discrepancy observed in our study might be partially explained by an increase in haemoglobin production. An erythropoietin‐driven increase in haemoglobin production is the most important mechanism of adaptation and acclimatization seen at altitude, especially in the Andes 20, 21. This observation was further confirmed in our study, because we found significantly higher mean haemoglobin levels at high altitude than at sea level. Increased erythropoiesis due to other causes, such as intravenous iron or erythropoietin‐stimulating agents, has also been shown to influence HbA1c levels 22, 23, 24. In high‐altitude native populations, the utilization of iron appears to be 25% greater than in people from sea‐level settings 25, and higher HbA1c values have been reported in individuals with iron deficiency 26, 27. Haemoglobin levels may affect the extent of glycation, however, the effect of altitude on lifespan remains unclear 28. Our haematological data were limited and thorough evaluation of haematological parameters in relationship to glucose and other metabolic markers will add to this understanding. Another potential mechanism to explain our observations might be related to haemoglobin glycation itself at altitude, although the literature is limited in this field. Indeed, many of the mechanisms presented deserve to be fully studied in high‐altitude settings.
Mounting evidence from observational and controlled clinical trials has demonstrated a strong association between HbA1c levels and retinopathy and other microvascular complications of diabetes. Moreover, HbA1c is also associated with increased risk of cardiovascular disease, even in individuals without diabetes 29. As such, the role of HbA1c as a key biomarker is undeniable. Yet, the discordance between FPG and HbA1c at altitude observed in our study, particularly in high‐altitude settings, merits further and deeper scrutiny because the number of people classified as having diabetes would treble if HbA1c was used as a diagnosis tool. This discrepancy between HbA1c and FPG has recently been highlighted in a global data‐pooling study signalling difficulties for monitoring of diabetes targets at a policy level 30. Consequently, many individuals residing at high altitude, who were shown to have a more favourable cardiometabolic risk profile than those residing at sea level, would initiate glucose‐lowering medications and be exposed to the unnecessary harm associated with such treatments. Given the large populations living at high altitude and the rising prevalence of diabetes worldwide, especially in the southern hemisphere, inappropriate prescription of anti‐diabetic medications might lead to inefficient public health policies in countries with limited economic resources.
This study has benefited from leveraging data from well‐defined population‐based studies and relatively large sample sizes. Peru has a particular geographical distribution characterized by a large variety of climates and altitudes. The CRONICAS Cohort Study and the PERU MIGRANT Study cohorts are unique in that they have a relatively large proportion of individuals living at high altitude, > 3000 m above sea level, where increases in haemoglobin levels are mostly observed. Despite this, our study did not have data on oral glucose tolerance test, the gold standard test used for diabetes research, multiple FPG readings over time to more accurately represent glucose levels in people with diabetes, or a detailed evaluation of all haematological markers in all sites. The cross‐sectional approach of this study precludes the ascertainment of causal relationships; therefore, longitudinal studies are better placed to explore the long‐term consequences of the discordant patterns reported, particularly in terms of progression of diabetes‐related complications. Prospective evaluations are also required to evaluate clinical and economic consequences that may result from modification of current diagnostic criteria.
Conclusions
These findings provide unique evidence that the relationship between HbA1c and FPG differs considerably between sea‐level and high‐altitude settings. Our models show that an HbA1c of 48 mmol/mol (6.5%) would correspond to different FPG levels in each setting, as shown by a discrepancy of up to 7.8 mmol/l. Such a substantial difference hampers potential strategies for expanding diabetes diagnosis and public health planning in high‐altitude settings, and therefore FPG and the oral glucose tolerance test should be used as diagnostic criteria under these circumstances.
Funding sources
The CRONICAS Cohort Study was funded in whole with Federal funds from the United States National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract No. HHSN268200900033C. The PERU MIGRANT Study was funded by the Wellcome Trust (GR074833MA) and Universidad Peruana Cayetano Heredia (Fondo Concursable No. 20205071009). William Checkley was further supported by a Pathway to Independence Award (R00HL096955) from the National Heart, Lung and Blood Institute. AB‐O (103994/Z/14/Z) and LS (098504/Z/12/Z) are both funded by Wellcome Trust. JJM is also supported by Fogarty International Centre (R21TW009982), Grand Challenges Canada (0335‐04), International Development Research Center Canada (106887‐001), Inter‐American Institute for Global Change Research (IAI CRN3036), Medical Research Council UK (M007405), National Heart, Lung, and Blood Institute (U01HL114180), National Institutes of Mental Health (U19MH098780).
Competing interests
None declared.
Supporting information
Table S1. Linear, quadratic and cubic regression models for HbA1c using glucose‐like predictor (crude and adjusted models).
Table S2. Distribution of diabetes and prediabetes at sea level and high altitude considering HbA1c or FPG standard cut‐off points and including cases of diabetes diagnosed by physician and pharmacological treatment.
Figure S1. Comparison between ROC curves at sea level (blue) and high altitude (red), for HbA1c standard cut‐off points using FPG as the gold standard of diabetes.
Figure S2. Comparison between ROC curves at sea level (blue) and high altitude (red), for HbA1c standard cut‐off points using FPG as the gold standard of prediabetes.
Acknowledgements
We would like to thank Ian Bennett, Fabiola León‐Velarde and Miguel Villanueva for their useful and insightful comments to earlier drafts of this manuscript. Our gratitude is extended to all fieldworkers and participants for making this study possible.
Members of the PERU MIGRANT Study Group: Antonio Bernabé‐Ortiz, Lilia Cabrera, Héctor H. García, Robert H. Gilman, J. Jaime Miranda, Julio A. Poterico, Renato Quispe, Candice Romero, Juan F. Sánchez, Liam Smeeth.
Members of the CRONICAS Cohort Study Group (in alphabetic order): Cardiovascular Disease: Antonio Bernabé‐Ortiz, Juan P. Casas, George Davey Smith, Shah Ebrahim, Raúl Gamboa (R.I.P.), Héctor H. García, Robert H. Gilman, Luis Huicho, Germán Málaga, J. Jaime Miranda, Víctor M. Montori, Liam Smeeth; Chronic Obstructive Pulmonary Disease: William Checkley, Gregory B. Diette, Robert H. Gilman, Luis Huicho, Fabiola León‐Velarde, María Rivera, Robert A. Wise; Training & Capacity Building: William Checkley, Héctor H. García, Robert H. Gilman, J. Jaime Miranda, Katherine Sacksteder.
The PERU MIGRANT Study data are publicly available at: https://figshare.com/articles/PERU_MIGRANT_Study_Baseline_dataset/3125005. The CRONICAS Cohort Study data will be available at NHLBI's open repository (https://biolincc.nhlbi.nih.gov/home/).
Author contributions
JJM and GM conceived the original idea. JCBA led the statistical analysis. RQ, TDP and JCBA wrote the first draft of the manuscript. ABO, WC and JJM aided with conceptualizing the study and edited/reviewed the manuscript. GM supervised analytical work, provided clinical feedback and edited/reviewed the manuscript. LS and RHG also provided critical inputs to earlier versions of the manuscript.
Diabet. Med. 34, 804–812 (2017)
References
- 1. NCD Risk Factor Collaboration (NCD‐RisC) . Worldwide trends in diabetes since 1980: a pooled analysis of 751 population‐based studies with 4.4 million participants. Lancet 2016; 387: 1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sacks DB. A1C versus glucose testing: a comparison. Diabetes Care 2011; 34: 518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herman WH, Dungan KM, Wolffenbuttel BH, Buse JB, Fahrbach JL, Jiang H et al Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 1,5‐anhydroglucitol in over 2000 patients with type 2 diabetes. J Clin Endocrinol Metab 2009; 94: 1689–1694. [DOI] [PubMed] [Google Scholar]
- 4. Ziemer DC, Kolm P, Weintraub WS, Vaccarino V, Rhee MK, Twombly JG et al Glucose‐independent, black–white differences in hemoglobin A1c levels: a cross‐sectional analysis of 2 studies. Ann Intern Med 2010; 152: 770–777. [DOI] [PubMed] [Google Scholar]
- 5. Bennett CM, Guo M, Dharmage SC. HbA(1c) as a screening tool for detection of Type 2 diabetes: a systematic review. Diabet Med 2007; 24: 333–343. [DOI] [PubMed] [Google Scholar]
- 6. Hare MJ, Shaw JE, Zimmet PZ. Current controversies in the use of haemoglobin A1c. J Intern Med 2012; 271: 227–236. [DOI] [PubMed] [Google Scholar]
- 7. Winslow RM. The role of hemoglobin oxygen affinity in oxygen transport at high altitude. Respir Physiol Neurobiol 2007; 158: 121–127. [DOI] [PubMed] [Google Scholar]
- 8. Naghavi M, Wang H, Lozano R, Davis A, Liang X, Zhou M et al Global, regional, and national age‐sex specific all‐cause and cause‐specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 385: 117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kent RB. Latin America: Regions and People. New York: Guilford Press, 2006. [Google Scholar]
- 10. Leon‐Velarde F, Villafuerte FC, Richalet JP. Chronic mountain sickness and the heart. Prog Cardiovasc Dis 2010; 52: 540–549. [DOI] [PubMed] [Google Scholar]
- 11. Bernardi L, Roach RC, Keyl C, Spicuzza L, Passino C, Bonfichi M et al Ventilation, autonomic function, sleep and erythropoietin. Chronic mountain sickness of Andean natives. Adv Exp Med Biol 2003; 543: 161–175. [PubMed] [Google Scholar]
- 12. Okumiya K, Fukutomi E, Kimura Y, Ishimoto Y, W‐l Chen, Ishikawa M et al Strong association between polycythemia and glucose intolerance in older adults living at high altitudes in the Andes. J Am Geriatr Soc 2011; 59: 1971–1973. [DOI] [PubMed] [Google Scholar]
- 13. Miranda JJ, Gilman RH, Garcia HH, Smeeth L. The effect on cardiovascular risk factors of migration from rural to urban areas in Peru: PERU MIGRANT Study. BMC Cardiovasc Disord 2009; 9. doi: 10.1186/1471‐2261‐9‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miranda JJ, Bernabe‐Ortiz A, Smeeth L, Gilman RH, Checkley W, Group CCS . Addressing geographical variation in the progression of non‐communicable diseases in Peru: the CRONICAS cohort study protocol. BMJ Open 2012; 2: e000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation 1993; 88: 1973–1998. [DOI] [PubMed] [Google Scholar]
- 16. Staudte RG, Sheather SJ. Robust Estimation and Testing. New York: Wiley, 2011. [Google Scholar]
- 17. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009; 32: 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen RM, Smith EP. Frequency of HbA1c discordance in estimating blood glucose control. Curr Opin Clin Nutr Metab Care 2008; 11: 512–517. [DOI] [PubMed] [Google Scholar]
- 19. Hao K, Kong F‐P, Gao Y‐Q, Tang J‐W, Chen J, Evans AM et al Inactivation of corticotropin‐releasing hormone‐induced insulinotropic role by high‐altitude hypoxia. Diabetes 2015; 64: 785–795. [DOI] [PubMed] [Google Scholar]
- 20. Windsor JS, Rodway GW. Heights and haematology: the story of haemoglobin at altitude. Postgrad Med J 2007; 83: 148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scheinfeldt LB, Tishkoff SA. Living the high life: high‐altitude adaptation. Genome Biol 2010; 11: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ng JM, Jennings PE, Laboi P, Jayagopal V. Erythropoetin treatment significantly alters measured glycated haemoglobin (HbA1c). Diabet Med 2008; 25: 239–240. [DOI] [PubMed] [Google Scholar]
- 23. Inaba M, Okuno S, Kumeda Y, Yamada S, Imanishi Y, Tabata T et al Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol 2007; 18: 896–903. [DOI] [PubMed] [Google Scholar]
- 24. Ng JM, Cooke M, Bhandari S, Atkin SL, Kilpatrick ES. The effect of iron and erythropoietin treatment on the A1C of patients with diabetes and chronic kidney disease. Diabetes Care 2010; 33: 2310–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reynafarje C, Lozano R, Valdivieso J. The polycythemia of high altitudes: iron metabolism and related aspects. Blood 1959; 14: 433–455. [PubMed] [Google Scholar]
- 26. Kim C, Bullard KM, Herman WH, Beckles GL. Association between iron deficiency and A1C Levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999‐2006. Diabetes Care 2010; 33: 780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hashimoto K, Noguchi S, Morimoto Y, Hamada S, Wasada K, Imai S et al A1C but not serum glycated albumin is elevated in late pregnancy owing to iron deficiency. Diabetes Care 2008; 31: 1945–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Samaja M, Brenna L, Allibardi S, Cerretelli P. Human red blood cell aging at 5,050‐m altitude: a role during adaptation to hypoxia. J Appl Physiol 1993; 75: 1696–1701. [DOI] [PubMed] [Google Scholar]
- 29. Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J et al Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010; 362: 800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. NCD Risk Factor Collaboration (NCD‐RisC) . Effects of diabetes definition on global surveillance of diabetes prevalence and diagnosis: a pooled analysis of 96 population‐based studies with 331 288 participants. The Lancet Diabetes & Endocrinology 2015; 3: 624–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Linear, quadratic and cubic regression models for HbA1c using glucose‐like predictor (crude and adjusted models).
Table S2. Distribution of diabetes and prediabetes at sea level and high altitude considering HbA1c or FPG standard cut‐off points and including cases of diabetes diagnosed by physician and pharmacological treatment.
Figure S1. Comparison between ROC curves at sea level (blue) and high altitude (red), for HbA1c standard cut‐off points using FPG as the gold standard of diabetes.
Figure S2. Comparison between ROC curves at sea level (blue) and high altitude (red), for HbA1c standard cut‐off points using FPG as the gold standard of prediabetes.


