Abstract
Background
Circulating epithelioid cells (CECs), also known as circulating tumor/cancer/epithelial/non-hematologic cells, are a prognostic factor in various malignancies that can be isolated via various protocols. We analyzed the cytomorphologic characteristics of CECs isolated by size in a cohort of patients with benign and malignant pancreatic diseases to determine if cytomorphological features could predict CEC origin.
Methods
Blood samples were collected from 9 healthy controls and 171 patients with pancreatic disease presenting for surgical evaluation prior to treatment. Blood was processed with the ScreenCell size-based filtration device. Evaluable CECs were analyzed in a blinded fashion for cytomorphologic characteristics including cellularity, nucleoli, nuclear size/irregularity/variability/hyperchromasia, and nuclear-cytoplasmic ratio. Statistical differences between variables were analyzed via Fisher's exact test.
Results
No CECs were identified in normal healthy controls (n=9). Of the 115 patients with CECs (positive or suspicious), 25 had non-malignant disease and 90 had malignancy. There were no significant differences in any of the cytologic criteria between groups divided by benign versus malignant, neoplastic versus non-neoplastic, or pancreatic ductal adenocarcinoma versus neuroendocrine tumor.
Conclusions
CECs were seen in patients with malignant and non-malignant pancreatic disease, but not in healthy controls. There were no morphologic differences between cells from different pancreatic diseases. This suggests that numerous conditions may be associated with CECs in the circulation and that care must be taken not to over-interpret cells identified by cytomorphology as indicative of circulating tumor cells of pancreatic cancer. Additional studies are required to determine the origin and clinical significance of these cells.
Keywords: Circulating tumor cells, circulating epithelial cells, pancreas, CEC, CTC
Introduction
Circulating Epithelioid Cells (CECs) are defined as cells with epithelioid cytological characteristics found in the peripheral blood, often at a very low frequency. In different contexts, these are known alternatively as circulating tumor cells, circulating cancer cells, circulating epithelial cells, or circulating non-hematologic cells. We have chosen to term these cells as CECs, instead of circulating tumor cells, to refer to cells present in the circulation that appear epithelial-like on microscopy (i.e. epithelioid), but lack additional studies to confirm a tumor as the site of origin. As would be expected from the numerous ways to refer to these cells, there are several methods of enriching, isolating, and studying them.
The widely-cited first published instance of CEC observation occurred in 1869, where an Australian physician noticed atypical cells in the blood of a patient at autopsy that closely mimicked their widely metastatic carcinoma1. CellSearch, the first FDA-approved clinical assay for circulating epithelial cells was only approved in 20042. This assay utilizes antibodies for epithelial markers (normally EpCAM) attached to magnetic beads to isolate cells of epithelial origin. This isolation step is followed by labeling with fluorophore-conjugated antibodies to other epithelial markers (typically cytokeratins) for visualization, enumeration, and characterization3. CellSearch positivity has been correlated to worse prognosis in numerous carcinomas, including breast cancer4,5, non-small cell lung carcinoma (NSCLC)6, cholangiocarcinoma7, and colorectal cancer8, among others.
In recent years there has been a significant increase in research regarding CEC analysis. Flow cytometry, similar to its application in hematopathology but using antibodies for keratins and EpCAM, has been used to identify CECs. In addition, some newer flow cytometry machines allow for a degree of cell imaging and localization of markers to the individual cell locations (nuclear, cytoplasmic, membranous, etc.), possibly providing a more comprehensive picture of their phenotype9.
Extremely sophisticated microfluidic chips have been developed to both characterize and isolate CECs. These chips utilize the flow properties of the larger cells, along with antibody coated surfaces to isolate cells with particular characteristics10. Chips have also been designed that rely on intracellular cohesion to sort out cells in clusters11. In addition to allowing for very high purity isolation and characterization of CECs, these chip systems allow for collecting unmodified cells, which can be further studied by genetic or immunologic analysis. Through the isolation and sequencing of single cells, researchers have been able to demonstrate clonality between circulating tumor cells and both primary and metastatic foci in a case of widely metastatic prostate cancer12.
Another method for isolating CECs is the Isolation by Size of Epithelial Tumor cells (ISET), which uses size exclusion filters to collect cells13. One platform for this method is ScreenCell, which consists of single-use filters with 8 um pores that allow erythrocytes and leukocytes to pass through but trap cells larger than normal hematologic elements14. These filters have the advantage that they are relatively easy and inexpensive to use and have the ability to trap unmodified cells, which can be characterized with conventional stains, immunohistochemistry, or sent for molecular analysis15. ISET-positivity has been described in several gastrointestinal tumors16,17, and are associated with worse prognosis in numerous malignancies, including, colorectal cancer18, uveal melanomas19, NSCLC20, and pancreatic cancer21,22.
Although these different isolation methods are theoretically looking for the same CECs, they have somewhat different operating characteristics. For example, ISET yields circulating tumor cells in a larger proportion of cases compared to CellSearch in patients with lung cancer23, and another study found only a weak correlation in the number of CECs isolated by the two methods in metastatic lung, prostate, and breast cancer24. This difference in performance may indicate that different cells are being isolated by the disparate methods and calls into question what is actually being isolated by these techniques. We have previously published a study of a series of surgical patients with pancreatic pathology who were found to have suspicious or malignant appearing CECs when characterized by cytology alone25. In this group of patients, cells were detected in patients with malignant or non-malignant pancreatic pathology25. In this paper we will discuss the cytomorphologic characteristics of the CECs isolated in a variety of pathological conditions of the pancreas with the aim of determining features that can distinguish benign from malignant conditions. Because of its ease of use, low cost, and lack of additional requirements beyond a standard cytopathology laboratory (i.e. no additional bench machinery required), we selected ScreenCell as our test platform of choice for this study.
Material and methods
This study was performed with institutional approval from Massachusetts General Hospital (MGH). A total of 171 adult patients with pancreatic disease presenting to the MGH pancreatic surgical clinic between 10/2011 and 10/2013 were recruited prior to any surgical or medical therapy. In addition, 9 healthy adults lacking any known pancreatic pathology were recruited as controls. All patients consented to a blood draw per IRB protocol and had their samples processed by the ScreenCell (Paris, France) technique within 3 hours as described elsewhere14. In brief - 1ml of peripheral blood was added to a lysis buffer and passed through a filter with low-pressure vacuum. These filters were stained per manufacturer's instructions with Giemsa (Haem 3; Fisher Scientific, Pittsburgh, USA). Filters were evaluated by a cytopathologist experienced in pancreatic cytopathology (MBP), who was blinded to patient diagnosis. Slides were interpreted as negative (no CECs present), suspicious (atypical epithelioid cells without definitive malignant features), and positive (CECs with malignant features). CECs were also analyzed for established characteristics of pancreatic malignancy: high N/C ratio, enlarged nuclei, irregular nuclear borders, nuclear hyperchromasia, and anisonucleosis26,27. Similar criteria have been used to describe malignant appearance in ISET-isolated CECs in lung cancer patients28.
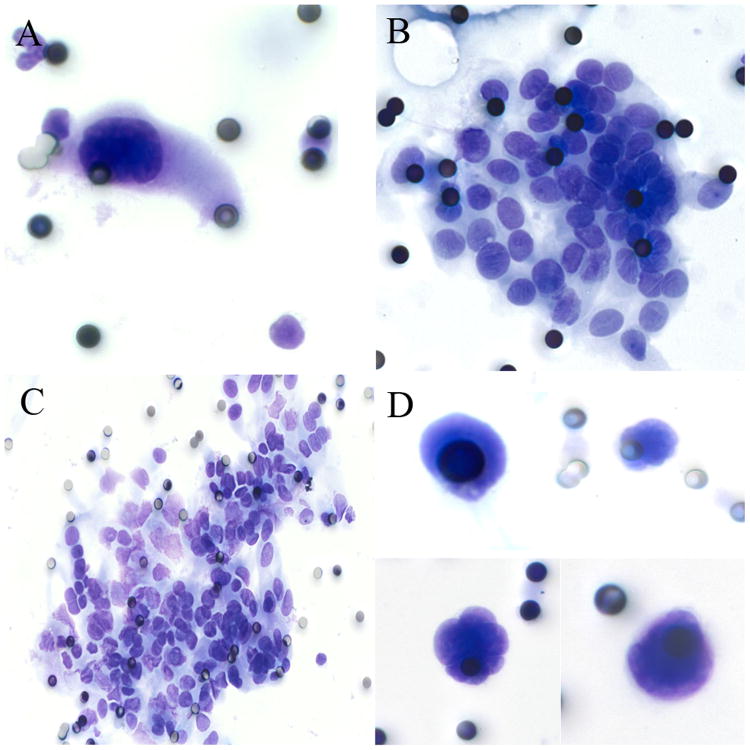
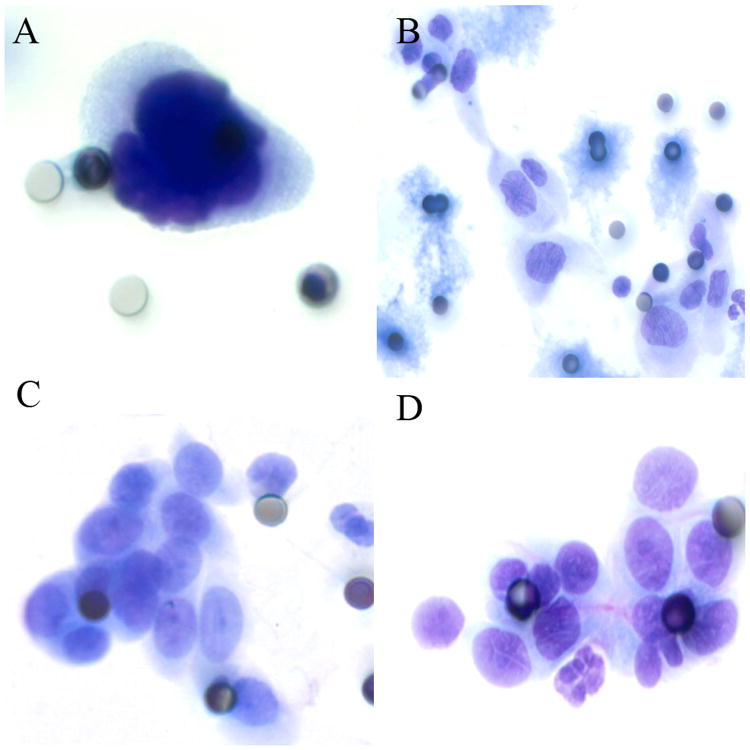
Samples with well-visualized CECs were analyzed for cellularity (low= <10 cells (Figure 1A), moderate= 10-100 cells (Figure 1B), high= >100 cells (Figure 1C), naked nuclei only (Figure 1D)). Cells were characterized by large nuclei (≥3 times pore size (8 μm); Figure 1A), cell clustering (single cells, clusters ≥5 cells (Figure 1B), or both), irregular nuclear borders (Figure 2A), nuclear hyperchromasia (Figure 2A), high N/C ratio (>0.75; Figure 2A), anisonucleosis (>2-fold variability; Figure 2B), nucleoli (not seen (Figure 2B), visible (Figure 2C), and prominent (Figure 2D)). CECs were also classified by overall impression (negative, suspicious, positive).
Figure 1.
Cytologic characteristics of circulating CECs. 1A: A paucicellular (<10 cells) specimen consisting of a single markedly enlarged cell with nuclear enlargement (>3× pore size), nuclear hyperchromasia, and nuclear membrane irregularity. 1B: A moderately cellular specimen (10-100 cells) consisting of clusters of epithelioid cells with oval nuclei and occasional nuclear grooves. 1C: A markedly cellular specimen (>100 cells) with clusters of epithelioid cells. 1D: suspicious specimens consisting of markedly enlarged, irregular nuclei but no visible cytoplasm.
Figure 2.
Nuclear and cytoplasmic characteristics of CECs. 2A: Malignant-appearing cell with nucleomegaly (>3× pore size), increased N/C ratio (>0.75), marked nuclear irregularity, and hyperchromasia. 2B: Cells demonstrating nucleomegaly, irregular nuclear borders, and marked anisonucleosis (>2× difference in nuclear size), but no visible nucleoli. 2C: Cluster of cells with increased N/C ratio and visible, but non-prominent nucleoli. 2D: groups of cells with increased N/C ratio and prominent nucleoli.
Positive CECs were enlarged (>2 times the pore size), with either irregular, hyperchromatic nuclei and scant cytoplasm (Figure 1A), or clusters of cells with round-oval nuclei with occasional grooves and visible cytoplasm (Figure 1B). Suspicious CECs were epithelioid but fell short of the criteria listed above or lacked clear cytoplasm (Figure 1D).
Differences were analyzed based on final histopathologic diagnosis of the patient at resection. Analysis compared patients with benign versus malignant lesions, neoplastic versus non-neoplastic etiologies, and patients with pancreatic ductal adenocarcinoma (PDAC) versus neuroendocrine tumors (NET) using Fisher's exact test (alpha set at p<0.05).
Results
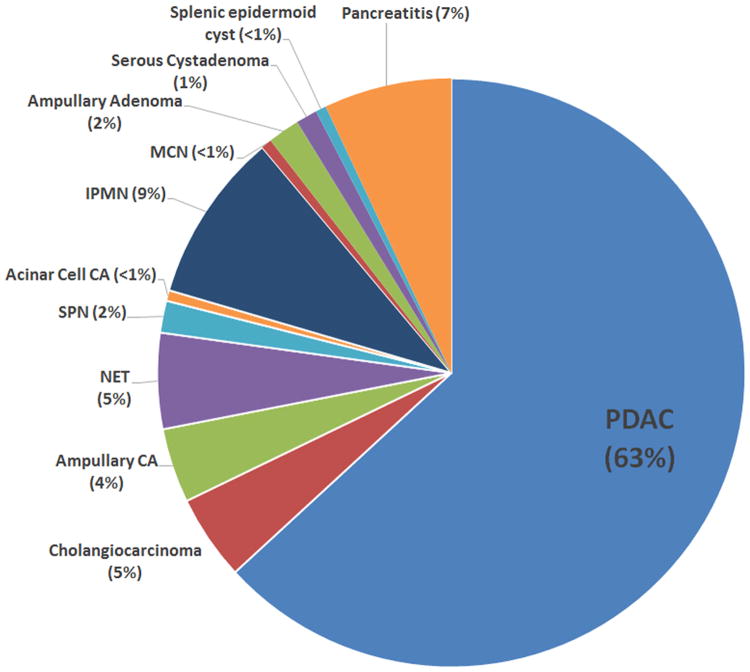
There were 171 patients in the study cohort, 115 of which had positive or suspicious CECs (67.3%). All of the nine healthy controls were negative for CECs. Malignancies in the study cohort included PDAC (n=108), cholangiocarcinoma (n=8), ampullary adenocarcinoma (n=7), NET (n=9), solid-pseudopapillary neoplasm (SPN; n=3), and acinar cell carcinoma (n=1). Benign neoplastic lesions included intraductal papillary mucinous neoplasm (IPMN; n=16), serous cystadenoma (SCA; n=2), ampullary adenoma (n=3), and mucinous cystic neoplasm (MCN; n=1). Non-neoplastic lesions included pancreatitis (n=12) and splenic epidermoid cyst (n=1) (Figure 3, Table 1). Benign lesions (IPMN, MCN, ampullary adenoma, SCA, splenic epidermoid cyst, and chronic pancreatitis) were 63% CEC positive, 9% suspicious, and 29% negative, compared to malignant lesions (PDAC, cholangiocarcinoma, ampullary adenocarcinoma, NET, SPN, and acinar cell carcinoma), which were 51% CEC positive, 15% suspicious, and 34% negative (Table 2; p=not significant (n.s.)). There were no statistically significant differences (p=n.s.) between benign and malignant diagnoses with regards to cellularity, nuclear enlargement, nuclear boarder irregularity, cell clustering, anisonucleosis, increased N/C ratio, nuclear hyperchromasia, and nucleoli (Tables 3, 4, 5, and 6).
Figure 3. Proportions of different diagnoses present in this study (n=171).
Table 1. CEC positivity by diagnosis.
| All | 171 | 91 (53.2%) | 24 (14.0%) | 56 (32.7%) |
| PDAC | 108 | 53 (49.1%) | 19 (17.6%) | 36 (33.3%) |
| Cholangiocarcinoma | 8 | 4 (50.0%) | 0 (0%) | 4 (50.0%) |
| Ampullary CA | 7 | 4 (57.1%) | 0 (0%) | 3 (42.9%) |
| NET | 9 | 6 (66.7%) | 2 (22.2%) | 1 (11.1%) |
| SPN | 3 | 2 (66.7%) | 0 (0%) | 1 (33.3%) |
| Acinar cell CA | 1 | 0 (0%) | 0 (0%) | 1 (100.0%) |
| IPMN | 16 | 12 (75.0%) | 0 (0%) | 4 (25.0%) |
| MCN | 1 | 1 (100.0%) | 0 (0%) | 0 (0%) |
| Ampullary adenoma | 3 | 1 (33.3%) | 0 (0%) | 2 (66.7%) |
| Serous cystadenoma | 2 | 2 (100.0%) | 0 (0%) | 0 (0%) |
| Splenic epidermoid cyst | 1 | 0 (0%) | 0 (0%) | 1 (100.0%) |
| Pancreatitis | 12 | 6 (50.0%) | 3 (25.0%) | 3 (25.0%) |
CEC: Circulating epithelioid cells. +: Positive. Susp: suspicious. -: Negative. PDAC: Pancreatic ductal adenocarcinoma. CA: Carcinoma. NET: Neuroendocrine tumor. SPN: Solid-pseudopapillary neoplasm. IPMN: Intraductal papillary mucinous neoplasm. MCN: Mucinous cystic neoplasm. SCA: Serous cystadenoma
Table 2. CEC positivity by diagnostic grouping.
| n | CEC+ n(%) | CEC susp. n(%) | CEC- n(%) | p | |
|---|---|---|---|---|---|
| Malignant | 136 | 69 (50.7%) | 21 (15.4%) | 46 (33.8%) | 0.44 |
| Benign | 35 | 22 (62.9%) | 3 (8.6%) | 10 (28.6%) | |
| Neoplastic | 158 | 85 (53.8%) | 21 (13.3%) | 52 (32.9%) | 0.56 |
| Non-neoplastic | 13 | 6 (46.2%) | 3 (23.1%) | 4 (30.8%) | |
| PDAC | 108 | 53 (49.1%) | 19 (17.6%) | 36 (32.7%) | 0.47 |
| NET | 9 | 6 (66.7%) | 2 (22.2%) | 1 (11.1%) |
CEC: Circulating epithelioid cells. +: Positive. Susp: suspicious. -: Negative. PDAC: Pancreatic ductal adenocarcinoma. NET: Neuroendocrine tumor.
Table 3. Specimen CEC cellularity by diagnostic grouping.
| n(%) | |
|---|---|
| Malignant <10 cells | 24 (26.7%) |
| Malignant 10-100 cells | 40 (44.4%) |
| Malignant >100 cells | 17 (18.9%) |
| Malignant Naked Nuclei only | 9 (10.0%) |
| Benign <10 cells | 7 (28.0%) |
| Benign 10-100 cells | 11 (44.0%) |
| Benign >100 cells | 6 (24.0%) |
| Benign Naked Nuclei only | 1 (4.0%) |
| p-value = 0.85 | |
| Neoplastic <10 cells | 28 (26.4%) |
| Neoplastic 10-100 cells | 48 (45.3%) |
| Neoplastic >100 cells | 21 (19.8%) |
| Neoplastic Naked Nuclei only | 9 (8.5%) |
| Non-Neoplastic <10 cells | 3 (33.3%) |
| Non-Neoplastic 10-100 cells | 3 (33.3%) |
| Non-Neoplastic >100 cells | 2 (22.2%) |
| Non-Neoplastic Naked Nuclei only | 1 (11.1%) |
| p-value = 0.77 | |
| PDAC <10 cells | 22 (30.6%) |
| PDAC 10-100 cells | 30 (41.7%) |
| PDAC >100 cells | 13 (18.1%) |
| PDAC Naked Nuclei only | 7 (9.7%) |
| NET <10 cells | 1 (12.5%) |
| NET 10-100 cells | 2 (25.0%) |
| NET >100 cells | 3 (37.5%) |
| NET Naked Nuclei only | 2 (25.0%) |
| p-value = 0.22 |
CEC: Circulating epithelioid cells. PDAC: Pancreatic ductal adenocarcinoma. NET: Neuroendocrine tumor.
Table 4. CEC clustering by diagnostic grouping.
| n (%) | |
|---|---|
| Malignant-single cells | 35 (38.9%) |
| Malignant-Clusters | 21 (23.3%) |
| Malignant- Single cells and clusters | 34 (37.8%) |
| Benign- Single cells | 8 (32.0%) |
| Benign- Clusters | 11 (44.0%) |
| Benign- Single cells and clusters | 6 (24.0%) |
| p-value = 0.14 | |
| Neoplastic- Single cells | 38 (35.8%) |
| Neoplastic- Clusters | 29 (27.4%) |
| Neoplastic- Single cells and clusters | 39 (36.8%) |
| Non-neoplastic- Single cells | 5 (55.6%) |
| Non-neoplastic- Clusters | 3 (33.3%) |
| Non-neoplastic- Single cells and clusters | 1 (11.1%) |
| p-value = 0.30 | |
| PDAC- Single cells | 29 (40.3%) |
| PDAC- Clusters | 15 (20.8%) |
| PDAC- Single cells and clusters | 28 (38.9%) |
| NET- Single cells | 3 (37.5%) |
| NET- Clusters | 3 (37.5%) |
| NET- Single cells and clusters | 2 (25.0%) |
| p-value = 0.56 |
CEC: Circulating epithelioid cells. PDAC: Pancreatic ductal adenocarcinoma. NET: Neuroendocrine tumor.
Table 5. CEC nucleolar characteristics by diagnostic grouping.
| n (%) | |
|---|---|
| Malignant-Nucleoli not seen | 66 (73.3%) |
| Malignant-nucleoli present | 17 (18.9%) |
| Malignant-prominent nucleoli present | 7 (7.8%) |
| Benign- Nucleoli not seen | 19 (76.0%) |
| Benign-nucleoli present | 5 (20.0%) |
| Benign-prominent nucleoli present | 1 (4.0%) |
| p-value = 1.00 | |
| Neoplastic-Nucleoli not seen | 77 (72.6%) |
| Neoplastic-nucleoli present | 21 (19.8%) |
| Neoplastic-prominent nucleoli present | 8 (7.5%) |
| Non-neoplastic-Nucleoli not seen | 8 (88.9%) |
| Non-neoplastic-nucleoli present | 1 (11.1%) |
| Non-neoplastic-prominent nucleoli present | 0 (0%) |
| p-value = 0.84 | |
| PDAC-Nucleoli not seen | 54 (75.0%) |
| PDAC-nucleoli present | 13 (18.1%) |
| PDAC-prominent nucleoli present | 5 (6.9%) |
| NET-Nucleoli not seen | 5 (62.5%) |
| NET-nucleoli present | 2 (25.0%) |
| NET-prominent nucleoli present | 1 (12.5%) |
| p-value = 0.52 |
CEC: Circulating epithelioid cells. PDAC: Pancreatic ductal adenocarcinoma. NET: Neuroendocrine tumor.
Table 6. Other CEC characteristics by diagnostic grouping.
| Large nuclei | Irregular nuclear boarders | Anisonucleosis | High N/C ratio | Nuclear hyperchromasia | |
|---|---|---|---|---|---|
| Malignant-Present n (%) | 42 (46.7%) | 52 (57.8%) | 41 (45.6%) | 23 (28.4%) | 32 (35.6%) |
| Malignant-Absent n (%) | 48 (53.3%) | 38 (42.2%) | 49 (54.4%) | 58 (71.6%) | 58 (64.4%) |
| Benign-Present n (%) | 15 (60.0%) | 18 (72.0%) | 13 (52.0%) | 6 (25.0%) | 11 (44.0%) |
| Benign-Absent n (%) | 10 (40.0%) | 7 (28.0%) | 12 (48.0%) | 18 (75.0%) | 14 (56.0%) |
| p= | 0.27 | 0.25 | 0.17 | 0.80 | 0.49 |
| Neoplastic-Present n (%) | 52 (49.1%) | 63 (59.4%) | 52 (49.1%) | 28 (28.9%) | 39 (36.8%) |
| Neoplastic-Absent n (%) | 54 (50.9%) | 43 (40.6%) | 54 (50.9%) | 69 (71.1%) | 67 (63.2%) |
| Non-neoplastic-Present n (%) | 5 (55.6%) | 7 (77.8%) | 2 (22.2%) | 1 (12.5%) | 4 (44.4%) |
| Non-neoplastic-Absent n (%) | 4 (44.4%) | 2 (22.2%) | 7 (77.8%) | 7 (87.5%) | 5 (55.6%) |
| p= | 0.74 | 0.32 | 0.72 | 0.44 | 0.73 |
| PDAC-Present n (%) | 34 (47.2%) | 42 (58.3%) | 33 (45.8%) | 20 (30.8%) | 26 (36.1%) |
| PDAC-Absent n (%) | 38 (52.8%) | 30 (41.7%) | 39 (54.2%) | 45 (69.2%) | 46 (63.9%) |
| NET-Present n (%) | 3 (37.5%) | 3 (37.5%) | 3 (37.5%) | 0 (0%) | 2 (25.0%) |
| NET-Absent n (%) | 5 (62.5%) | 5 (62.5%) | 5 (62.5%) | 6 (100.0%) | 6 (75.0%) |
| p= | 0.72 | 0.46 | 0.72 | 0.17 | 0.71 |
CEC: Circulating epithelioid cells. PDAC: Pancreatic ductal adenocarcinoma. NET: Neuroendocrine tumor.
Neoplastic diagnoses included PDAC, NET, cholangiocarcinoma, ampullary carcinoma, SPN, acinar cell carcinoma, IPMN, MCN, ampullary adenoma, and SCA. Non-neoplastic diseases included splenic epidermoid cysts and pancreatitis. Neoplastic lesions were 54% CEC positive, 13% suspicious, and 33% negative, compared to non-neoplastic lesions, which were 46% CEC positive, 23% suspicious, and 31% negative (p=n.s.). Similar to the comparison between malignant and benign, there was no difference between the neoplastic and non-neoplastic lesions in terms of cellularity, nuclear enlargement, nuclear boarder irregularity, cell clustering, anisonucleosis, increased N/C ratio, nuclear hyperchromasia, and nucleoli (Tables 3, 4, 5, and 6).
There was also no significant difference between PDACs, which were 49% CEC positive, 18% suspicious, 33% negative, and NETs, which were 67% CEC positive, 22% suspicious, and 11% negative (p=n.s.). As with malignant versus benign and neoplastic versus non-neoplastic, there were no significant differences between PDAC versus NET in cellularity, nuclear enlargement, nuclear boarder irregularity, cell clustering, anisonucleosis, increased N/C ratio, nuclear hyperchromasia, and nucleoli (Tables 3, 4, 5, and 6).
Discussion
We demonstrated two important points related to CECs. First, although CECs were not present in healthy volunteers, they were present in a wide variety of pancreatic diseases ranging from malignant tumors to benign, non-neoplastic pancreatitis25. Secondly, the CECs from these different diseases had identical cytologic characteristics, with similar proportions of all the features examined (cellularity, nuclear enlargement, nuclear boarder irregularity, cell clustering, anisonucleosis, increased N/C ratio, nuclear hyperchromasia, and nucleoli). These findings have significant implications for continuing research on CECs.
In this study we were unable to detect any significant differences in cytologic morphology between cells from these different diseases in any of the cytologic characteristics investigated. In a study of lung cancer patients using very similar cytologic criteria of malignancy, the authors determined that malignant cytomorphologic CEC characteristics were common, but that they did not appear to represent the final histopathology of their tumors (i.e. those from squamous carcinomas did not appear to have any evidence of squamous differentiation)28. Although they did not issue descriptive statistics of their cytomorphology, the photomicrographs of the CECs isolated from these lung cancer patients appear more or less indistinguishable from the CECs in our cohort, regardless of underlying disease28. It appears that CECs in ISET have a range of morphologic appearances, ranging from innocuous to frankly malignant, however, these appearances do not reliably distinguish benign versus malignant etiology in our cohort of pancreatic disease patients. To demonstrate this point, the malignant-appearing cells in figure 2B are actually from a patient with a benign IPMN, whereas the more banal-appearing cells of 2C are from a patient with PDAC.
Although the vast majority of studies (including the present one) do not find CECs in their control population, these patients are typically healthy adults lacking any known pathologic process5,29,30,28. In one study, the blood of 54 patients with benign colonic disease (diverticulosis, benign polyps, inflammatory bowel disease, etc.) was examined using two different antibody-based epithelial cell isolation assays31. The authors found CECs in 11.3% and 18.9% of these benign colonic disease patients and none of their healthy controls. The positive rate partially depended on the assay used, 11.3% using previously described CellSearch using anti-EpCAM and cytokeratin antibodies, and 18.9% using the Epispot assay, which isolated cytokeratin 19-positive viable cells after leukocyte depletion31. Another study examined CECs in a population of men at risk for prostate cancer. Using a differential centrifugation method followed by stains for prostate specific antigen (PSA) and racemase (which is commonly positive in prostate cancer and negative in benign prostate disease), they detected PSA-positive racemase-negative CECs in 21 of 245 patients, none of whom developed carcinoma on follow up32.
A large study using CellSearch found 1 CTC/7.5mL blood (which is not technically positive) in 5.5% of healthy patients and 7.5% of patients with benign disease (which included benign breast disease, hypertension, diabetes, arthritis, thyroid disorders, hyperlipidemia, and asthma)3. Although this also speaks to the importance of cutoffs on CEC enumeration for positive versus negative, it also implies that underlying pathology may be an important factor of CEC positivity in benign diseases. In our study, and the previously mentioned prostate and colon CEC studies, the non-neoplastic pathology underlying CEC positivity was often inflammatory (pancreatitis, chronic prostatitis, and benign colonic disease), as opposed to the benign, predominantly non-inflammatory disorders in the large CellSearch study.3,31,32 This supports that epithelial cells can be found in the circulation of patients with benign as well as malignant diseases, and that this rate of positivity may depend partially on underlying disease process as well as the assay used. However, the difficulty in parsing out these issues should not undermine the clinical importance of these CECs, which are a significant prognosticator in numerous cancers.
In many studies where sequencing is performed on CECs found in cancer patients, CECs are often clonally related to their original tumor and metastatic foci, implying that these cells are indeed tumor cells in transit12,33,34,35,36. As these may be the cells that give rise to metastatic foci, it makes their molecular characterization useful, potentially predicting the molecular features of distant disease before it becomes clinically evident. The potential of CECs to shine light on the mutational profile of these critically important cells is shown in a study where whole exome sequencing was performed on CECs, metastatic foci, and the primary tumor in a patient with metastatic prostate carcinoma12. The profile of CECs closely matched metastatic foci, implying that analysis of these cells gives insight into the true molecular profile of the most pathologic tumor cells, the ones that give rise to metastases12.
Circulating epithelioid cells have been described in many different carcinomas, and can be measured using various methods including CellSearch, flow cytometry, microfluidic chips, and ISET. All of these methods have certain strengths, and many have studies supporting their prognostic value in different carcinomas. This study is limited in that additional studies were not performed to confirm the identity of the CECs isolated. Another limitation is that, although the characteristics we analyzed were objectively defined, morphologic analysis is an intrinsically subjective process. We attempted to minimize the inherent subjectivity by having all images reviewed by a pathologist who specializes in pancreatic cytopathology (MBP), and chose characteristics like nuclear to cytoplasmic ratio, which have good reproducibility among pathologlists.37
ISET is an extremely promising modality where low cost and ease of operation allow for scalability and the ability to run ancillary studies (including immunohistochemical, in-situ hybridization, and gene sequencing) on isolated CECs. However, what this study demonstrates is that care is essential in all of these methods, which may isolate morphologically similar non-tumor cells in addition to tumor cells, especially in the context of other lesions or inflammatory conditions. The clinical context is important and ancillary testing of isolated CECs is critical for ensuring the cells of interest are truly tumor derived and pathologically significant.
Acknowledgments
Funding Sources: Dr. Sarah P Thayer was supported by NIH grant R01CA169086, and Dr. Christy E Cauley was supported by NIH grant R25CA092203
Footnotes
Statement of Disclosure: ScreenCell provided filtration devices and technical assistance for this study. All authors have nothing additional to disclose.
References
- 1.Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J. 1869;14:146–147. [Google Scholar]
- 2.Administration USF and drug. CellSearch™ Epithelial Cell Kit / CellSpotter™ Analyzer - K03158. [Accessed January 1, 2016]; http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm081239.htm. Published 2013.
- 3.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 4.Helissey C, Berger F, Cottu P, et al. Circulating tumor cell thresholds and survival scores in advanced metastatic breast cancer: The observational step of the CirCe01 phase III trial. Cancer Lett. 2015;360:213–218. doi: 10.1016/j.canlet.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Lucci A, Hall CS, Lodhi AK, et al. Circulating tumour cells in non-metastatic breast cancer: A prospective study. Lancet Oncol. 2012;13:688–695. doi: 10.1016/S1470-2045(12)70209-7. [DOI] [PubMed] [Google Scholar]
- 6.Hofman V, Ilie MI, Long E, et al. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: Comparison of the efficacy of the CellSearch Assay™ and the isolation by size of epithelial tumor cell method. Int J Cancer. 2011;129:1651–1660. doi: 10.1002/ijc.25819. [DOI] [PubMed] [Google Scholar]
- 7.Yang JD, Campion MB, Liu MC, et al. Circulating tumor cells are associated with poor overall survival in patients with cholangiocarcinoma. Hepatology. 2016;63:148–158. doi: 10.1002/hep.27944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Dalum G, de Groot MR, Stam GJ, et al. Importance of circulating tumor cells in newly diagnosed colorectal cancer. Cancer Res. 2014;74:3064–3064. doi: 10.3892/ijo.2015.2824. [DOI] [PubMed] [Google Scholar]
- 9.Dent BM, Ogle LF, O'donnell RL, et al. High-resolution imaging for the detection and characterisation of circulating tumour cells from patients with oesophageal, hepatocellular, thyroid and ovarian cancers. Int J Cancer. 2016;138:206–216. doi: 10.1002/ijc.29680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stott SL, Hsu CH, Tsukrov DI, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. October. 2010;107:18392–18397. doi: 10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarioglu AF, Aceto N, Kojic N, et al. A microfluidic device for label-free, physical capture of circulating tumor cell clusters. 2016;12:685–691. doi: 10.1038/nmeth.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohr JG, Adalsteinsson Va, Cibulskis K, et al. Whole exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat Biotechnol. 2014;32:479–484. doi: 10.1038/nbt.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vona G, Sabile A, Louha M, et al. Isolation by Size of Epithelial Tumor Cells. Am J Pathol. 2000;156:57–63. doi: 10.1016/S0002-9440(10)64706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desitter I, Guerrouahen BS, Benali-Furet N, et al. A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Res. 2011;31:427–441. [PubMed] [Google Scholar]
- 15.Zheng S, Lin H, Liu JQ, et al. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J Chromatogr A. 2007;1162:154–161. doi: 10.1016/j.chroma.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 16.Kulemann B, Pitman MB, Liss AS, et al. Circulating tumor cells found in patients with localized and advanced pancreatic cancer. Pancreas. 2015;44:547–550. doi: 10.1097/MPA.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Song P, Zou B, et al. Circulating Tumor Cell Analyses in Patients With Esophageal Squamous Cell Carcinoma Using Epithelial Marker-Dependent and -Independent Approaches. Medicine (Baltimore) 2015;94:e1565. doi: 10.1097/MD.0000000000001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaifi JT, Kunkel M, Das A, et al. Circulating tumor cell isolation during resection of colorectal cancer lung and liver metastases: A prospective trial with different detection techniques. Cancer Biol Ther. 2015;4047:699–708. doi: 10.1080/15384047.2015.1030556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzini C, Pinzani P, Salvianti F, et al. Circulating tumor cells detection and counting in uveal melanomas by a filtration-based method. Cancers (Basel) 2014;6:323–332. doi: 10.3390/cancers6010323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofman V, Bonnetaud C, Ilie MI, et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res. 2011;17(4):827–835. doi: 10.1158/1078-0432.CCR-10-0445. [DOI] [PubMed] [Google Scholar]
- 21.Poruk KE, Valero V, Saunders T, et al. Circulating Tumor Cell Phenotype Predicts Recurrence and Survival in Pancreatic Adenocarcinoma. Ann Surg. 2016;264:1073–1081. doi: 10.1097/SLA.0000000000001600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poruk KE, Blackford AL, Weiss MJ, Cameron JL, He J, Goggins MG, Rasheed Z, Wolfgang CL, W L. Circulating Tumor Cells Expressing Markers of Tumor Initiating Cells Predict Poor Survival and Cancer Recurrence in Patients with Pancreatic Ductal Adenocarcinoma. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-1467. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosokawa M, Kenmotsu H, Koh Y, et al. Size-Based Isolation of Circulating Tumor Cells in Lung Cancer Patients Using a Microcavity Array System. PLoS One. 2013;8:e67466. doi: 10.1371/journal.pone.0067466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farace F, Massard C, Vimond N, et al. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br J Cancer. 2011;105:847–853. doi: 10.1038/bjc.2011.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cauley CE, Pitman MB, Zhou J, et al. Circulating Epithelial Cells in Patients with Pancreatic Lesions: Clinical and Pathologic Findings. J Am Coll Surg. 2015;221:699–707. doi: 10.1016/j.jamcollsurg.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitman MB, Centeno BA, Ali SZ, Genevay M, Stelow E, Mino-Kenudson M, Fernandez-del Castillo C, Max Schmidt C, Brugge W LLPS of C. Standardized Terminology and Nomenclature for Pancreatobiliary Cytology: The Papanicolaou Society of Cytopathology Guidelines. Diagn Cytopathol. 2014;42:338–350. doi: 10.1002/dc.23092. [DOI] [PubMed] [Google Scholar]
- 27.Pitman MB, L L. The Papanicolaou Society of Cytopathology System for Reporting Pancreaticobiliary Cytology. New York, NY: Springer Publishing; 2015. [Google Scholar]
- 28.Hofman V, Long E, Ilie M, et al. Morphological analysis of circulating tumour cells in patients undergoing surgery for non-small cell lung carcinoma using the isolation by size of epithelial tumour cell (ISET) method. Cytopathology. 2012;23:30–38. doi: 10.1111/j.1365-2303.2010.00835.x. [DOI] [PubMed] [Google Scholar]
- 29.Hofman V, Bonnetaud C, Ilie MI, et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res. 2011;17:827–835. doi: 10.1158/1078-0432.CCR-10-0445. [DOI] [PubMed] [Google Scholar]
- 30.Cristofanilli M, Budd T, Ellis M, et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 31.Pantel K, Denève E, Nocca D, et al. Circulating epithelial cells in patients with benign colon diseases. Clin Chem. 2012;58:936–940. doi: 10.1373/clinchem.2011.175570. [DOI] [PubMed] [Google Scholar]
- 32.Murray NP, Reyes E, Badínez L, et al. Circulating prostate cells found in men with benign prostate disease are P504S negative: Clinical implications. J Oncol. 2013;77:48–51. doi: 10.1155/2013/165014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aceto N, Bardia A, Miyamoto D, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor P, Buim MME, Fanelli MF, et al. Cancer Biology & Therapy Detection of KRAS mutations in circulating tumor cells from patients with metastatic colorectal cancer. Cancer Biol Ther. 2015;16:1289–1295. doi: 10.1080/15384047.2015.1070991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ting DT, Wittner BS, Ligorio M, et al. Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep. 2014;8:1905–1918. doi: 10.1016/j.celrep.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kulemann B, Liss AS, Warshaw AL, et al. KRAS mutations in pancreatic circulating tumor cells: a pilot study. Tumor Biol. 2016;37:7547–7554. doi: 10.1007/s13277-015-4589-2. [DOI] [PubMed] [Google Scholar]
- 37.Vaickus LJ, Tambouret RH. Young investigator challenge: The accuracy of the nuclear-to-cytoplasmic ratio estimation among trained morphologists. Cancer Cytopathol. 2015;123:524–530. doi: 10.1002/cncy.21585. [DOI] [PubMed] [Google Scholar]