Abstract
Infection during pregnancy can lead to activation of the maternal immune system and has been associated with an increased risk of having an offspring later diagnosed with a neurodevelopmental disorders (NDD) such as autism spectrum disorder (ASD) or Schizophrenia (SZ). Most maternal immune activation (MIA) studies to date have been in rodents and usually involve the use of lipopolysaccharide (LPS) or polyinosinic:polycytidylic acid (poly I:C). However, since NDD are based on behavioral changes, a model of MIA in non-human primates could potentially provide data that helps illuminate complex behavioral and immune outputs in human NDD. In this study twenty-one pregnant rhesus macaques were either given three injections over 72 hours of poly I:C-LC, a double stranded RNA analog (viral mimic), or saline as a control. Injections were given near the end of the first trimester or near the end of the second trimester to determine if there were differences in immune output due to the timing of MIA. An additional three non-treated animals were used as controls. The offspring were followed until 4 years of age, with blood collected at the end of their first (year 1) and fourth (year 4) years to assess dynamic cellular immune function. Induced responses from peripheral immune cells were measured using multiplex assays. At one year of age, MIA exposed offspring displayed elevated production of innate inflammatory cytokines including: interleukin (IL)-1β, IL-6, IL-12p40, and tumor necrosis factor (TNF)α at baseline and following stimulation. At four years of age, the MIA exposed offspring continued to display elevated IL-1β, and there was also a pattern of an increased production of T-cell helper type (TH)-2 cytokines, IL-4 and IL-13. Throughout this time period, the offspring of MIA treated dams exhibited altered behavioral phenotypes including increased stereotyped behaviors. During the first two years, stereotyped behaviors were associated with innate cytokine production. Self-directed behaviors were associated with TH2 cytokine production at year 4. Data from this study suggests long-term behavioral and immune activation was present in offspring following MIA. This novel non-human primate model of MIA may provide a relevant clinically translational to help further elucidate the role between immune dysfunction and complex behavioral outputs following MIA.
Keywords: Fetal priming, maternal immune activation, autism, schizophrenia, behavior, cytokines, non-human primate
1.0 Introduction
The recent Zika virus outbreaks have fostered public concerns about the impact that infections during pregnancy can have on fetal development (Rasmussen et al. 2016). Research over the last several decades has found associations between prenatal infections with an increased risk for altered neurodevelopmental trajectories. For example, epidemiological studies focusing on maternal infections during gestation showed associations of increased risk for developing a neurodevelopmental disorders (NDD), such as autism spectrum disorders (ASD) in the child (Atladottir et al. 2010, Brown 2012) or schizophrenia (Brown 2012) later in life. Furthermore, these studies suggest that this phenomenon is not specific to any particular infectious agent, but instead, is driven by the maternal immune response (Atladottir et al. 2010). Translational animal models investigating maternal immune activation (MIA) have found that in the absence of an actual infectious agent, immune stimulation alone, either by bacterial or viral products, or specific cytokines trigger an active immune response in the pregnant dam that elicits abnormal behavior, including anxiety, impaired social and repetitive behaviors in the offspring (Shi et al. 2005, Smith et al. 2007).
Most MIA studies to date have been in rodents and involve the use of lipopolysaccharide (LPS) or polyinosinic:polycytidylic acid (poly I:C); although several models have also generated MIA with live influenza virus or by injection of inflammatory cytokine, interleukin (IL)-6 to produce similar outcomes (Shi et al. 2005, Smith et al. 2007, Meyer et al. 2009, Patterson 2009, Meyer and Feldon 2010). The major findings of these studies were changes in offspring behavior, altered brain development and immune dysfunction in the offspring (Meyer et al. 2006b, Patterson 2009, Shi et al. 2009, Ito et al. 2010, Garbett et al. 2012, Hsiao et al. 2012, Malkova et al. 2012, Garay et al. 2013, Meyer 2014). Behavioral changes have varied between studies but have included such phenomena as reduced or altered ultrasonic vocalizations, reduced sociability, increased repetitive behaviors loss of latent inhibition, reduced open field exploration, deficits in reversal learning and impairments in pre-pulse inhibition (Meyer et al. 2006a, Meyer et al. 2006b, Meyer et al. 2008, Han et al. 2011, Hsiao and Patterson 2011, Malkova et al. 2012, Schwartzer et al. 2013). In human studies, immune abnormities are often observed in individuals with NDD (Ashwood et al. 2008, Potvin et al. 2008, Muller and Schwarz 2010, Ashwood et al. 2011a, Ashwood et al. 2011c, Ashwood et al. 2011b, Onore et al. 2012, Di Nicola et al. 2013, McAllister 2014, Rose and Ashwood 2014, Careaga et al. 2015). Alterations in immune function have also been observed in rodent offspring of MIA treated dams, including increased production of IL-6 and IL-17 from mononuclear cells, increased granulocyte and monocyte populations, increased production of IL-12p40 and Chemokine (C-C motif) ligand (CCL)-3 from macrophages, an increased T-helper (TH)-17 cell skewing, and altered profiles of fetal, juvenile, and adult brain cytokine and chemokine levels (Meyer et al. 2006b, Meyer et al. 2008, Mandal et al. 2011, Hsiao et al. 2012, Garay et al. 2013, Mandal et al. 2013, Onore et al. 2014, Choi et al. 2016). While rodent models of MIA provide researchers with a useful initial model to begin to investigate the intricate pathways and interactions between the developing nervous and immune systems, the disparity between human and rodent social structures limits some of the translational aspects of this model in regard to certain complex human behaviors. Moreover, since NDD are, so far at least, based solely on behavioral criteria, non-human primates may be better suited to explore the complexities of behavioral driven disorders due to their closer relationship to humans (Watson and Platt 2012, Chang et al. 2013, Meyer 2014).
To date few studies have explored MIA in non-human primates (Short et al. 2010, Willette et al. 2011, Bauman et al. 2014, Machado et al. 2015, Weir et al. 2015). In one study, maternal influenza infection during early third trimester, led to offspring with smaller brain volume and reduced gray matter, particularly in the cingulate and parietal areas (Short et al. 2010). Prenatal LPS exposure during early third trimester led to increases in global white matter in the brains of the offspring and a trend for larger brain volume, which was accompanied by altered behaviors including reduced response to prepulse inhibition acoustic startle (Willette et al. 2011). However, we do not know of any study that has looked at immune responses in the MIA model in non-human primates.
In this study we sought to examine the effects of MIA on offspring immune activation in a non-human primate model. Previously we reported finding increased repetitive behaviors, motor stereotypies, decreased affiliative vocalizations, and abnormal social behaviors in offspring of non-human primate dams injected with poly I:C to induce MIA (Bauman et al. 2014); offspring also had abnormal gaze patterns when presented with various rhesus monkey facial expressions (Machado et al. 2015). In addition, we also reported findings of altered dendritic morphology of increased number of oblique dendrites and narrower apical dendritic diameter, in MIA offspring compared to saline controls. (Weir et al. 2015). In the present study, we examined plasma cytokine concentrations and dynamic induced cellular responses of peripheral immune cells from offspring of MIA treated dams during the first (year 1) and fourth (year 4) year of life and found elevated production of innate cytokines and chemokines at year 1, and a pattern of elevated TH2 cytokines during year 4. Moreover, many of the measured cytokines correlated with the emergence of repetitive behaviors in MIA exposed offspring and may provide insight into observations of increased immune activation and increased impairment in symptoms of NDD (Ashwood et al. 2011a, Ashwood et al. 2011c).
3.0 Methods and Materials
The experimental methods used were developed in consultation with the California National Primate Research Center veterinary staff. The University of California, Davis Institutional Animal Care and Use Committee approved all protocols used. All attempts were made (in terms of social housing, enriched diet, use of positive reinforcement strategies, and minimizing the duration of daily training/testing sessions) to promote the psychological well-being of the animals that participated in this research. Detailed methods on the administration of polyinosinic:polycytidylic acid stabilized with poly-L-lysine (poly I:C-LC), rearing conditions and behavioral observations have been previously published (Bauman et al. 2014) and are described below:
2.1 Maternal Administration of Poly ICLC
A total of 24 rhesus monkeys with timed pregnancies were placed into two main treatment groups, namely; controls or MIA and each dam gave birth to a single infant (supplemental Table 1). Maternal injection of poly I:C-LC or saline took place at 8 am on gestational days 43, 44, and 46; a total of 10 dams received injections of either poly I:C (n=6) or saline (n=4), in the first trimester group. Second trimester dams were injected at 8 am on gestational days 100, 101 and 103 where 11 dams received injections of poly I:C (n=7) and saline (n=4). For animals that were in the MIA cohort, three injections of 0.25 mg/kg of poly IC-LC (Oncovir, Inc., Washington, DC) were administered. Three untreated (no administration of saline or poly I:C) animals were also included to determine whether there was an effect of saline on behavioral and immune outcomes.
2.2 Rearing conditions
Mother and infant were housed in individual cages with continual visual access to other animals. Enrichment and species-typical social development was facilitated by placing four mother-infant pairs and an adult male in large chain link enclosures for three hours a day. Each of these socialization groups consisted of both control and treatment males and females. Infants were weaned at 6 months of age, but continued daily peer group interactions through approximately 2 years of age. At the time of the current study, all animals were housed indoors in social pairs 24 hours per day, 7 days per week.
2.3 Behavioral Observations
Offspring participated in a series of behavioral and cognitive experiments from birth through four years of age, as described in our previous publications (Bauman et al. 2014, Machado et al. 2015). Here we focus specifically on the emergence of stereotypic behaviors observed in captive macaques, including whole body motor stereotypies (i.e., pacing, bouncing, swaying, circling, and somersaulting) and self-directed or self-injurious behaviors (i.e., saluting, self-biting, self-hitting, and head-banging). Stereotypic behaviors were quantified at three timepoints (i) Post-weaning (10 months of age), (ii) Juvenile (22 months of age) and (iii) Subadult (46 months of age). Animals were placed in a large, unfamiliar cage alone and observed for two 5 minute focal samples on 2 separate days. Observations were collected and analyzed by trained observers, blind to the experimental conditions of the animals. At 10 and 22 months of age, the MIA animals displayed an array of abnormal, repetitive behaviors (i.e., whole body, self-directed etc.), thus we initially utilized a composite score of all repetitive behaviors, rather than sub-categories for these analyses (Bauman et al. 2014). As the animals aged, the presence of district categories of repetitive behaviors became apparent. Thus, the four year old data is presented as whole body (i.e., repetitive pacing, bouncing, flipping, spinning, swinging) versus self-directed (i.e, self-clasp, salute, self-bite and other abnormal self-directed behavioral patterns) behaviors as defined previously by our laboratory (Bauman et al. 2008) and others (Pomerantz et al. 2012, Spinelli et al. 2012).
2.4 Blood Collection and Peripheral Blood Mononuclear Cell (PBMC) stimulation
Peripheral blood was collected via an intravenous arm draw at 1 year of age and again at 4 years of age (see Supplemental Table 2 for a timeline of Behavioral assays and blood draws). 4 mLs of peripheral blood was collected into acid-citrate-dextrose Vacutainers. Blood was centrifuged at 2100 rpm for 10 mins and plasma was collected and aliquoted into 2 mL cyrovials and stored in −80 °C until cytokine analysis was performed. The remaining blood cells were layered on lymphocyte separation medium (Corning; Manassas, VA) and PBMC were separated by gradient centrifugation. PBMC were washed twice with 50 mLs each with Hanks Balanced Salt Solution (Corning; Manassas, VA). Cells were counted, excluding dead cells based on trypan blue staining, and PBMC concentrations were adjusted to a concentration of 1 × 106 cells/mL in complete media (RPMI 1640 (Invitrogen; Carlsbad, CA) with 10% Fetal Bovine Serum (Corning; Manassas, VA), 100 IU/ml penicillin (Invitrogen; Carlsbad, CA) and 100 IU/ml streptomycin (Invitrogen; Carlsbad, CA), 1% L-glutamine (Invitrogen; Carlsbad, CA)). Cells were stimulated with TLR4 agonist (LPS; 50 mg/mL) (Sigma-Aldrich; St. Louis, MO) or TLR3 agonist (Poly I:C; 100 mg/mL) (Sigma-Aldrich; St. Louis, MO). After 48 hours, supernatants were collected and stored at −80 °C until analysis.
2.5 Cytokine Analysis
Cytokine concentrations in the supernatants of stimulated cells were determined by a multiplexing bead immunoassays assay designed specifically to analyze non-human primate cytokines (Millipore, Billerica, MA). Quantification of Granulocyte Colony Stimulating Factor (G-CSF), Granulocyte Macrophage Colony Stimulating Factor (GM-CSF), Interleukin (IL)-1β, IFNγ, IL-2, IL-4, IL-5, IL-6, chemokine (C-X-C motif) ligand (CXCL)-8, IL-10, IL-12p40, IL-13, IL-17, chemokine (C-C motif) ligand (CCL)-2, CCL-3,CCL-4 and tumor necrosis factor(TNF)α was analyzed in the plasma and collected supernatants. Samples were run according to manufacturer’s protocol and guidelines. 25 μL of sample was incubated with antibody-immobilized beads, following incubation wells were washed and then samples were incubated with detection antibodies, followed by the addition of streptavidin-phycoerythrin. After streptavidin-phycoerthrin incubation, wells were washed again and beads were resuspended with sheath fluid. Analysis of the bead sets was run on a Bio-Plex 200 system (Bio-Rad Laboratories, Inc.). Unknown sample cytokine concentrations were determined by Bio-Plex Manager software; calculations were based on a standard curve of known concentration provided in the kit by the manufacturer. Values of sample cytokines are expressed in pg/mL and have the following detection range: IL-4: (4.9 – 20,000 pg/mL); IL-10 (12.2 – 50,000 pg/mL); GM-CSF, G-CSF, IFNγ, IL-1β, IL-2, IL-5, IL-6, CXCL-8, IL-12p40, IL-13, IL-17, CCL-2, CCL-3, CCL-4, TNFα (2.4 – 10,000 pg/mL). Sample concentrations that were below the limit of detection were given a proxy value as half the limit of detection for statistical comparisons.
2.6 Statistical Analysis
Analysis of the data using a Shapiro-Wilk test indicated that distribution of the cytokine data was not normally distributed. Therefore, the Kruskal-Wallis rank sum test was used to compare cytokine levels between groups. Data are expressed as median values (interquartile ranges). A Mann-Whitney non-parametric U-test (with a Holm step down procedure to correct for multiple comparisons) was used in post-hoc analyses to compare cytokine levels between groups and adjusted p values < 0.05 were considered statistically significant. Outliers were removed using Robust regression and outlier removal (ROUT). Correlations were analyzed using Spearman’s Rank-Order Correlation, p values <0.05 were considered statistically significant. Initial analysis of data revealed no differences in cytokine measures between first trimester injected MIA offspring and second trimester MIA offspring, or between the first and second trimester saline offspring and untreated controls; therefore, the MIA offspring where combined into one group (MIA) and the saline and untreated animals were combined into one group (control).
4.0 Results
3.1 Year 1
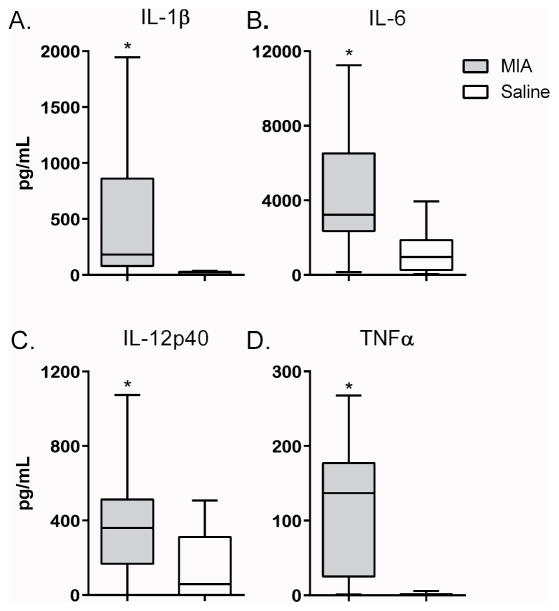
Analysis of plasma cytokine levels for the year 1 offspring revealed elevated TNFα (p = 0.046), IL-2 (p = 0.047), IFNγ (p = 0.017), IL-13 (p = 0.046) and G-CSF (p = 0.006) in MIA offspring compared to controls (Table 1). We also observed elevated cytokine production by PBMC in the MIA group following cell culture compared to controls (Table 2). Cultured PBMC from offspring of MIA treated dams produced significantly more cytokines associated with the innate immune response at baseline (media alone), including: IL-6 (p = 0.043), IL-12p40 (p = 0.026) and TNFα (p = 0.015) and chemokines: G-CSF (p = 0.002), GM-CSF (p = 0.007), CCL-3 (p = 0.035) and CCL-4 (p = 0.007). The cytokines IL-2 (p = 0.034), IFNγ (p = 0.022) and IL-10 (p = 0.007) were also elevated in the MIA offspring compared to controls (Table 2). Furthermore, stimulation of cultured PBMC from MIA offspring with a TLR3 agonist induced a significant increase in cytokine production including IL-1β (p = 0.001) (Figure 1A), IL-6 (p = 0.005) (Figure 1B), IL-2 (p = 0.002), IL-10 (p = 0.023), IL-12p40 (p = 0.023) (Figure 1C) and TNF-α (p = 0.001) (Figure 1D), as well as the chemokines G-CSF (p = 0.007), GM-CSF (p = 0.007), CXCL-8 (p = 0.010), CCL-2 (p = 0.030), CCL-3 (p = 0.006) and CCL-4 (p = 0.001) compared to controls. Following TLR4 stimulation, cultured PBMC from MIA offspring produced significantly more G-CSF (p = 0.0004), GM-CSF (p = 0.0016), IL-12p40 (p = 0.0121) and IL-2 (p = 0.004) compared to controls.
Table 1. Year 1 and year 4 plasma cytokine concentrations.
Plasma cytokine concentrations (pg/ml) measured at 1 and 4 years of age in offspring of non-human primates exposed to maternal immune activation (MIA) during gestation. IL-6, IL-17, CCL3 and CCL4 were undetectable in plasma for year 2. IL-4 was undetectable in plasma at both time points. Data presented as median with interquartile range; * denotes p-values less than 0.1 and ** denotes p-value less than 0.05.
| Plasma Cytokine Concentrations
|
|||||
|---|---|---|---|---|---|
| Year 2 | Year 4 | ||||
| MIA offspring | Saline Offspring | MIA offspring | Saline Offspring | ||
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | ||
|
| |||||
| Innate | IL-1β | 0.7 (0.7–0.7) | 0.7 (0.7–0.7) | 0.87 (0.66–1.71) | 0.56 (0.21–1.31) |
|
| |||||
| IL-6 | - | - | 1.62* (0.8–5.1) | 0.8 (0.8–1.07) | |
|
| |||||
| IL-12p40 | 45.37 (36.39–81.25) | 70.27 (41.31–75.15) | 29.9 (16.02–46.15) | 26.75 (0.75–69.99) | |
|
| |||||
| TNFα | 1.05** (1.05–34.64) | 1.05 (1.05–1.05) | 62.99 (41.32–83.75) | 45.45 (28.88–58.33) | |
|
| |||||
| TCell | IL-2 | 9.99** (4.58–21.23) | 2.4 (1.68–6.94) | 9.64 (2.2–27.44) | 11.33 (2.48–15.72) |
|
| |||||
| TH1 | IFNγ | 4.77** (1.1–10.21) | 1.1 (1.1–2.35) | 25.68* (15.72–32.73) | 13.8 (6.43–22.99) |
|
| |||||
| TH2 | IL-4 | - | - | - | - |
|
| |||||
| IL-5 | 1.22 (0.52–2.54) | 1.03 (0.2–1.32) | 4.85 (3.3–13.45) | 3.225 (2.1–6.77) | |
|
| |||||
| IL-13 | 1.75** (36.39–81.25) | 1.75 (41.31–75.15) | 26.98 (15.81–35.7) | 18.93 (15.61–26.43) | |
|
| |||||
| TH17 | IL-17 | - | - | 1.64 (1.17–3.9) | 1.64 (0.73–1.97) |
|
| |||||
| TReg | IL-10 | 29.66 (3.1–71.6) | 8.99 (3.1–16.31) | 40.92* (31.46–82.87) | 31.46 (15.6–49.72) |
|
| |||||
| Growth Factors | G-CSF | 3.62** (1.05–22.83) | 1.05 (1.05–1.05) | 130.3 (76.88–234.8) | 116.2 (62.41–134.4) |
|
| |||||
| GM-CSF | 0.5 (0.31–1.10) | 0.36 (0.31–0.42) | 1.62 (1.3–2.07) | 1.09 (0.81–1.65) | |
|
| |||||
| Chemokines | CXCL8 | 113.1 (30.89–510) | 48.14 (33.16–122) | 151.6* (126.2–279.6) | 110.5 (20.86–230.9) |
|
| |||||
| CCL2 | 144.7 (130.2–181.6) | 143.6 (97.32–186.1) | 179.8** (111.1–208.4) | 79.24 (35.44–120.5) | |
|
| |||||
| CCL3 | - | - | 28.01 (20.2–53.08) | 33.16 (23.35–45.36) | |
|
| |||||
| CCL4 | - | - | 5.74 (0.8–17.52) | 0.85 (0.8–6.83) | |
p <0.1;
p < 0.05;
IL=interleukin; G-CSF=Granulocyte Colony Stimulating Factor; GM-CSF-Granulocyte Macrophage Colony Stimulating Factor; CCL= Chemokine (C-C Motif) Ligand; CXCL= Chemokine (C-X-C Motif) Ligand
Table 2. Year 1 induced cytokine production.
Comparison of cytokine production (pg/ml) from peripheral blood mononuclear cell culture after 48 hours of stimulation with either TLR-4 agonist, TLR-3 agonist or in media alone, in offspring at year 1 of non-human primates exposed to maternal immune activation (MIA) during gestation. Data presented as Median with interquartile range; * denotes p-values less than 0.1 and ** denotes p-value less than 0.05.
| Year 2 Induced Cytokines
|
|||||||
|---|---|---|---|---|---|---|---|
| Media | TLR-4 | TLR-3 | |||||
| MIA offspring | Saline Offspring | MIA offspring | Saline Offspring | MIA offspring | Saline Offspring | ||
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | ||
|
| |||||||
| Innate | IL-1β | 26.63 (1.68–150) | 0.7 (0.7–64.51) | 2,736 (2,388–8,557) | 2,604 (1,035–7,809) | 181.9** (80–861.2) | 6.16 (0.7–28.1) |
|
| |||||||
| IL-6 | 1,035** (75.61–10,000) | 4.16 (0.8–1,693) | 9,488 (6,917–23,596) | 9,142 (4,258–10,342) | 3,226** (2,357–6,527) | 964.6 (265.6–1,866) | |
|
| |||||||
| IL-12p40 | 206.6** (31.82–1,003) | 2.89 (0.6–372.6) | 630.8** (306.1–763.2) | 263.3 (106–429.1) | 360.2** (167.4–512.4) | 58.31 (0.6–311.4) | |
|
| |||||||
| TNFα | 22.45** (2.73–96) | 1.05 (1.05–12.16) | 310.3 (6.68–621.7) | 378.6 (1.05–565.5) | 136.8** (25.24–177) | 1.05 (1.05–1.4) | |
|
| |||||||
| TCell | IL-2 | 3.47** (3.3–4.73) | 3.3 (3.3–3.3) | 51.86** (3.3–66.89) | 3.3 (3.3–3.3) | 13.08** (3.3–17.47) | 3.3 (3.3–3.3) |
|
| |||||||
| TH1 | IFNγ | 2.31** (1.1–17.74) | 1.1 (1.1–1.1) | 7.6 (1.87–73.76) | 8.61 (1.1–37.95) | 24.24 (2.92–45.64) | 4.72 (1.1–23.58) |
|
| |||||||
| TH2 | IL-4 | 4.37** (1.35–18.25) | 1.35 (1.35–1.35) | 1.35* (1.35–39.62) | 1.35 (1.35–1.35) | 1.35** (1.35–20.99) | 1.35 (1.35–1.35) |
|
| |||||||
| IL-5 | 1.15 (1.15–1.15) | 1.15 (1.15–1.15) | 1.15 (1.15–1.15) | 1.15 (1.15–1.15) | 1.15 (1.15–1.15) | 1.15 (1.15–1.15) | |
|
| |||||||
| IL-13 | 1.75 (1.75–1.75) | 1.75 (1.75–1.75) | 1.75 (1.75–1.75) | 1.75 (1.75–1.75) | 1.75 (1.61–2.85) | 1.75 (1.75–1.75) | |
|
| |||||||
| TH17 | IL-17 | 0.85 (0.85–0.85) | 0.85 (0.85–0.85) | 0.85 (0.85–0.85) | 0.85 (0.85–0.85) | 0.85 (0.22–0.85) | 0.85 (0.85–0.85) |
|
| |||||||
| TReg | IL-10 | 35.75** (3.1–64.83) | 3.1 (3.1–3.1) | 3,420 (2,890–5,725) | 4,338 (1,139–7,468) | 94.75** (3.1–656.3) | 3.1 (3.1–13.07) |
|
| |||||||
| Growth Factors | G-CSF | 5.98** (1.91–9.15) | 1.1 (1.1–1.1) | 5,854** (637.9–7,863) | 1.1 (1.1–135.8) | 69.54** (268–717.9) | 1.1 (1.1–1.1) |
|
| |||||||
| GM-CSF | 1.31** (0.7–1.79) | 0.7 (0.7–0.7) | 20.68** (3.61–127.2) | 0.7 (0.7–0.7) | 7.9** (0.7–15.47) | 0.7 (0.7–0.7) | |
|
| |||||||
| Chemokines | CXCL8 | 3,024 (747–10,976) | 469.3 (125.4–9,223) | 46,498 (37,590–59,898) | 34,844 (23,255–42,839) | 10,243** (6,315–14,499) | 279 (130.4–5,274) |
|
| |||||||
| CCL2 | 387.1 (71.98–8,063) | 62.24 (0.8–7,108) | 25,420 (22,919–27,856) | 26,531 (25,332–31,097) | 6238** (4,551–7,468) | 507.9 (157.4–6,689) | |
|
| |||||||
| CCL3 | 9.79** (1.85–4,289) | 1.85 (1.85–1.85) | 14,498 (5,442–21,740) | 11,374 (5,323–18,447) | 1,532** (34.98–4,485) | 17.41 (1.85–83.94) | |
|
| |||||||
| CCL4 | 12.83** (2.55–121.6) | 2.55 (2.55–2.55) | 168.5 (87.1–266.6) | 35.46 (2.55–262.5) | 58.94** (26.21–164.3) | 2.55 (2.55–18.05) | |
p <0.1;
p < 0.05;
CCL= Chemokine (C-C Motif) Ligand; CXCL= Chemokine (C-X-C Motif) Ligand; G-CSF=Granulocyte Colony Stimulating Factor; GM-CSF- Granulocyte Macrophage Colony Stimulating Factor; IFN= interferon; IL=interleukin; T cell= T lymphocyte; TH= T helper; TLR= toll like receptor; TNF= tumor necrosis factor; Treg= regulatory T cell
Figure 1. Year 1 Induced cytokine production.
Induced cytokine production from year 1 offspring after stimulation with TLR-3 agonist poly IC. Graphs depict innate cytokine levels of IL-1α (a), IL-6 (b), IL-12p40 (c) and TNFα (d) between MIA offspring (grey bars) and control offspring (white bars). Concentrations shown in pg/mL. Data depicted as box showing 25–75 percentiles and whisker percentiles and 5–95percentiles. *denotes p-value < 0.05.
3.2 Year 4
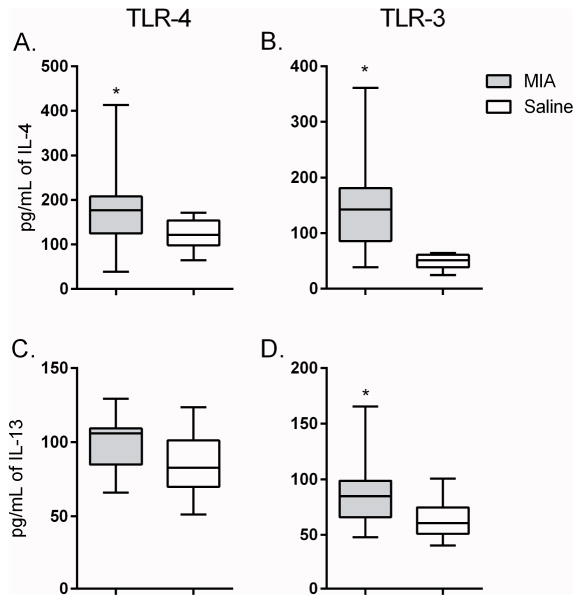
The analysis of plasma at year 4 showed that several cytokines were again elevated from MIA offspring compared to controls, although this profile differed from that observed at year 1 (Table 1). Plasma concentrations of CCL2 (p = 0.005) and IL-6 (p = 0.050) were higher in MIA offspring compared to controls. In addition, there were trends for increased IFNγ (p = 0.087), CXCL8 (p = 0.091) and IL-10 (p = 0.094) in MIA compared to controls that did not reach statistical significance after correction for multiple comparisons. Cytokines were increased in cultured PBMC, at baseline (media alone) in MIA offspring compared to controls. These included increased G-CSF (p=0.022) IL-2 (p = 0.029) and IL-10 (p = 0.060) (which were also increased at year 1), as well as increased CXCL-8 (p = 0.001) and CCL-2 (p = 0.025). There was also a trend for increased IL-1β (p=0.085) when compared to saline controls that did not reach significance (Table 3). Following stimulation with a TLR-3 agonist, there was increased production of G-CSF (p = 0.003) and a trend for elevated CCL-2 (p = 0.083) in MIA offspring compared to controls. Notably in year 4, following TLR3 stimulation of PBMC isolated from MIA offspring there was increased production of TH2 associated cytokines, including elevated production of the canonical TH2 cytokines, IL-4 (p = 0.006) (Figure 2A) and IL-13 (p = 0.041)Figure 2C), compared to control offspring. In cell cultures stimulated with a TLR-4 agonist, increased production of the TH2 cytokine IL-4 (p = 0.022) (Figure 2B) was also observed in MIA offspring compared to controls. Increased levels of G-CSF (p = 0.009), IL-1β (p = 0.036) and IL-17 (p = 0.023), as well as a trend for elevated CCL-2 (p = 0.076) and IFNγ (p = 0.078) were also noted in MIA offspring compared to controls after TLR-4 stimualtion.
Table 3. Year 4 induced cytokine production.
Comparison of cytokine production (pg/ml) from peripheral blood mononuclear cell culture after 48 hours of stimulation with either TLR-4 agonist, TLR-3 agonist or in media alone, in offspring at year 4 of non-human primates exposed to maternal immune activation (MIA) during gestation. Data presented as Median with interquartile range; * denotes p-values less than 0.1 and ** denotes p-value less than 0.05.
| Year 4 Induced Cytokines
|
|||||||
|---|---|---|---|---|---|---|---|
| Media | TLR-4 | TLR-3 | |||||
| MIA offspring | Saline Offspring | MIA offspring | Saline Offspring | MIA offspring | Saline Offspring | ||
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | ||
| Innate | IL-1β | 125.7* (97.99–208.3) | 63.85 (24.57–138) | 1,916** (1,145–2,856) | 1,086 (992.4–1,523) | 690.6 (314.2–1,382) | 541.3 (302.8–874.4) |
|
| |||||||
| IL-6 | 1,053 (763.8–1,473) | 1,112 (479.9–1,345) | 5,200 (4,270–6,505) | 5,515 (4,693–6,117) | 3,467 (2,430–5,447) | 3,693 (2,802–6,518) | |
|
| |||||||
| IL-12p40 | 29.53 (22.17–38.98) | 22.17 (13.74–32.21) | 147.6 (88.51–164.1) | 98.18 (80.31–123.3) | 101.4 (60.22–144.6) | 58.51 (13.19–110.7) | |
|
| |||||||
| TNFα | 161.4 (83.18–201.7) | 108.8 (78.16–128) | 1,314 (999.9–2,174) | 1,540 (1,188–2,169) | 1,255 (697.5–1,913) | 1,413 (728.1–1,809) | |
|
| |||||||
| TCell | IL-2 | 7.89** (5.74–10.13) | 5.12 (3.26–8.57) | 50.36 (39.16–59.83) | 41.71 (34.32–53.05) | 33.56 (21.89–40.88) | 24.33 (14.91–35.64) |
|
| |||||||
| TH1 | IFNγ | 5.31 (2.06–12.79) | 2.64 (1.29–4.38) | 147.1* (134.9–183.5) | 134.9 (122.6–165.3) | 192.5 (128.5–299.8) | 159.2 (116.2–302.2) |
|
| |||||||
| TH2 | IL-4 | 22.64 (17.83–38.76) | 21.45 (12.23–27.92) | 176.9** (125–207.8) | 122.1 (98.22–154) | 142.4** (85.95–181.2) | 51.61 (38.51–61.18) |
|
| |||||||
| IL-5 | 1.02 (0.22–1.74) | 1.27 (0.36–1.27) | 5.08 (1.77–11.57) | 6.33 (1.08–6.93) | 3.83 (1.08–8.72) | 2.455 (1.08–3.83) | |
|
| |||||||
| IL-13 | 21.52 (18.81–29.92) | 20.12 (17.26–22.52) | 105.9 (84.96–109.3) | 82.8 (69.97–101.2) | 84.96** (65.92–98.74) | 60.58 (51.16–74.82) | |
|
| |||||||
| TH17 | IL-17 | 1.56 (1.36–2.47) | 1.48 (1.25–1.86) | 8.17** (7.11–9.88) | 5.84 (3.96–7.78) | 3.86 (3.05–8.36) | 3.05 (2.23–4.46) |
|
| |||||||
| TReg | IL-10 | 184.2* (116.2–306.4) | 95.33 (67.54–172.8) | 2,359 (1,514–2,714) | 1,871 (994.9–2,356) | 785 (383.3–933.4) | 591 (437.9–705.1) |
|
| |||||||
| Growth Factors | G-CSF | 217.2** (101.5–490.4) | 82.5 (56.16–118.9) | 5,492** (921.3–7,129) | 269.5 (207.1–366.1) | 1,198** (248.3–1,392) | 176.8 (111.7–324.6) |
|
| |||||||
| GM-CSF | 5.31 (2.06–12.79) | 2.64 (1.293–4.375) | 54.69 (32.07–130.8) | 44.97 (31.29–73.2) | 19.11 (11–50.94) | 19.3 (12.15–45.3) | |
|
| |||||||
| Chemokines | CXCL8 | 6,634** (3,701–10,233) | 1,764 (1,143–3,134) | 40,834 (18,878–62,749) | 25,855 (20,162–36,203) | 18,352 (11,489–21,108) | 8,696 (7,890–16,700) |
|
| |||||||
| CCL2 | 1,497** (463.7–5,276) | 416.7 (164.4–775.5) | 19,253* (6,309–27,919) | 7,262 (5,259–9,319) | 10,051* (2,812–23,877) | 2,688 (1,920–6,144) | |
|
| |||||||
| CCL3 | 232.4 (45.74–593.3) | 245.9 (54.64–444.6) | 3,257 (2,206–4,588) | 3,164 (2,438–3,747) | 1,469 (611.2–2,151) | 1,390 (1,106–3,111) | |
|
| |||||||
| CCL4 | 96.12 (64.6–135.1) | 89.26 (61.76–113.5) | 337.2 (215.8–432.2) | 282 (211.8–483.6) | 296.8 (179.6–372.2) | 222.5 (133.1–355.6) | |
p <0.1;
p < 0.05;
CCL= Chemokine (C-C Motif) Ligand; CXCL= Chemokine (C-X-C Motif) Ligand; G-CSF=Granulocyte Colony Stimulating Factor; GM-CSF- Granulocyte Macrophage Colony Stimulating Factor; IFN= interferon; IL=interleukin; T cell= T lymphocyte; TH= T helper; TLR= toll like receptor; TNF= tumor necrosis factor; Treg= regulatory T cell
Figure 2. Year 4 induced cytokine production.
Induced cytokine production from year 4 offspring after stimulation with either TLR-3 agonist (a. and c.) or TLR-4 agonist (b. and d.). Graphs depict classical TH2 cytokine levels of IL-4 (a. and b.) and IL-13 (c. and d.) between MIA offspring (grey bars) and control offspring (white bars). Concentrations shown in pg/mL. Data depicted as box showing 25–75 percentiles and whisker percentiles and 5–95 percentiles. *denotes p-value < 0.05.
3.3 Associations between behavioral and immune responses
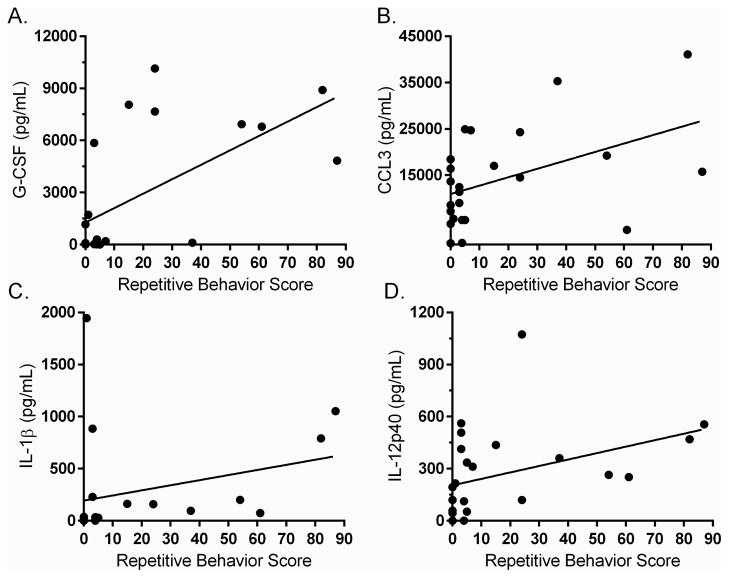
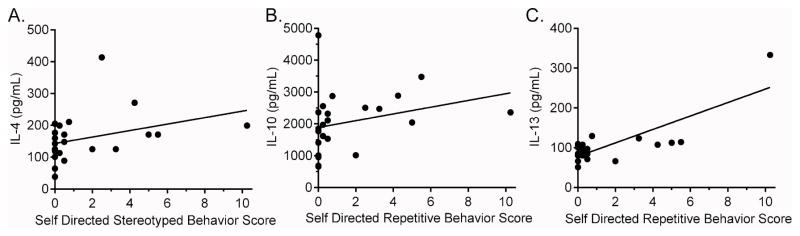
As previously reported, the MIA treated offspring exhibit stereotyped behaviors more frequently than controls during the first two years of life (Bauman et al. 2014). Here we first carried out an exploratory analysis of immune profiles from blood samples collected at 12 months of age with individual stereotpy data from each animal collected before, at 10 months of age (labeled as postweaned) (Table 4), and after, at 22 months of age (labeled as junenile) (Supplemental Table 2) the first year blood collection/processing. Positive correlalations were found between several innate immune cell cytokines and chemokines and stereotyped behaviors observed at 10 months of age, including TLR-4 induced production of G-CSF (Spearman = 0.642; p=0.001) (Figure 3A), CCL3 (r = 0.438; p = 0.032) (Figure 3B) and CXCL8 (r = 0.461; p = 0.023). TLR-3 induced innate immune cytokines, including IL-1β (r = 0.618; p = 0.004) (Figure 3C), IL-6 (r = 0.448; p = 0.032) and IL-12p40 (r = 0.524; p = 0.015) (Figure 3D) were found to have a positve association with stereotyped behaviors. Year 1 cytokines were also found to associate with stereotyped behaviors observed at the juvenile (22 months) age. A positive association was observed between the production of G-CSF (r = 0.490; p = 0.015) and IL-6 (r = 0.408; p = 0.048) in media cultures and stereotyped behaviors (Supplemental Table 3). Induced IL-12p40 cytokine production following TLR-3 stimulation was also associated with increased stereotypy (r = 0.425; p = 0.038). Following TLR-4 stimulation, increased G-CSF production was associated with increased juvenile stereotyped behaviors (r = 0.531; p = 0.007). Behavioral observations at 4 years of age were expanded to include subcategories of self-directed and whole body stereotyped behaviors. We found associations in year 4 blood analysis, primarily between increased TH2 cytokines and more impaired behaviors (Table 5). Of note, following TLR-3 stimulation, the PBMC production of G-CSF (r = 0.544; p = 0.009), IL-4 (r = 0.445; p = 0.033), IL-5 (r = 0.645; p=0.016) and IL-12p40 (r=0.499; p=0.015) at year 4, was associated with increased self-directed behaviors. Analysis of whole body stereotypies also revealed an association with increased IL-6 production following TLR3 stimulation (r=−0.415; p=0.049). Following TLR-4 stimulation, PBMC production of IL-4 (r=0.447; p=0.032) (Figure 4A), IL-10 (r = 0.516; p = 0.012) (Figure 4B) and IL-13 (r=0.581; p=0.004) (Figure 4C) was associated with increased self-directed behaviors.
Table 4. Year 1 cytokine and behavior associations.
Associations between cell culture cytokine production after 48 hours of stimulation with TLR-4, TLR-3 agonist or in media and stereotyped behaviors measured around 1 year of age. Data presented as Spearman’s rank correlation coefficient and p-value. Only significant correlations are shown.
| Postweaned Stereotyped Behaviors
| |||
|---|---|---|---|
| Cytokine | Spearman’s r | p-value | |
| Media | IL-1β | 0.735 | 0.001 |
| IL-6 | 0.601 | 0.002 | |
| IL-10 | 0.570 | 0.014 | |
| IL-12p40 | 0.590 | 0.003 | |
| TNFα | 0.584 | 0.006 | |
| CCL2 | 0.547 | 0.006 | |
| CCL4 | 0.603 | 0.005 | |
| CXCL8 | 0.508 | 0.011 | |
| G-CSF | 0.656 | 0.003 | |
| GM-CSF | 0.500 | 0.035 | |
|
| |||
| TLR3 | IL-1β | 0.618 | 0.004 |
| IL-6 | 0.448 | 0.032 | |
| IL-10 | 0.524 | 0.015 | |
| IL-12p40 | 0.583 | 0.003 | |
| CCL2 | 0.461 | 0.023 | |
| CCL4 | 0.534 | 0.010 | |
| CXCL8 | 0.524 | 0.009 | |
| G-CSF | 0.452 | 0.046 | |
|
| |||
| TLR4 | IL-1β | 0.524 | 0.010 |
| IL-6 | 0.539 | 0.010 | |
| IL-10 | 0.471 | 0.031 | |
| IL-12p40 | 0.465 | 0.025 | |
| CCL3 | 0.438 | 0.032 | |
| CXCL8 | 0.461 | 0.023 | |
| G-CSF | 0.642 | 0.001 | |
| GM-CSF | 0.441 | 0.046 | |
CCL= Chemokine (C-C Motif) Ligand; CXCL=Chemokine (C-X-C Motif) Ligand; G-CSF=Granulocyte Colony Stimulating Factor; GM-CSF- Granulocyte Macrophage Colony Stimulating Factor; IL=interleukin; TLR= toll like receptor; TNF= tumor necrosis factor
Figure 3. Year 1 cytokine and behavior associations.
Year 1 cytokine correlations to repetitive behaviors. Top row (a–c) illustrate relationship between TLR-4 induced chemokine production and post-weaned repetitive behavior scores. Bottom row illustrates relationship between TLR-3 induced innate cytokines and post-weaned repetitive behavior score (d–f). Cytokines concentration is shown as pg/mL.
Table 5. Year 4 cytokine and behavior associations.
Associations between cell culture cytokine production after 48 hours of stimulation with TLR-4 agonist, TLR-3 agonist or in media and self-directed stereotyped behaviors measured around 4 years of life. Data presented as Spearman’s rank correlation coefficient and p value. Only significant correlations are shown.
| Sub-Adult Stereotyped Behaviors
| |||
|---|---|---|---|
| Self Directed Stereotypies
| |||
| Cytokine | Spearman r | p-value | |
| TLR3 | IL-4 | 0.445 | 0.034 |
| IL-5 | 0.645 | 0.016 | |
| IL-12 | 0.499 | 0.015 | |
| IL-17 | 0.482 | 0.023 | |
| G-CSF | 0.543 | 0.009 | |
|
| |||
| TLR4 | IL-4 | 0.447 | 0.032 |
| IL-10 | 0.516 | 0.012 | |
| IL-13 | 0.581 | 0.004 | |
G-CSF=Granulocyte Colony Stimulating Factor; IL=interleukin; TLR= toll like receptor; TNF=tumor necrosis factor
Figure 4. Year 4 cytokine and behavior associations.
Year 4 cytokine correlations to self-directed repetitive behaviors. Graphs illustrate relationship between TLR-4 induced cytokine production and Sub-Adult repetitive behavior scores. Cytokines concentration is shown as pg/mL.
4.0 Discussion
Maternal immune activation studies in rodents have reported findings of aberrant behaviors, changes in brain volume, altered neuron morphologies, increases in immune cell populations and immune mediators that have added support to epidemiological findings that associate immune activation during gestation as a potentially contributing risk factor for NDD in a subset of individuals. However, the complexity of human behaviors makes it difficult to directly translate many rodent behaviors to those observed in humans, necessitating a better translational animal model, one whose behavior and social structure more closely resembles that of humans. We have demonstrated that MIA induced by poly I:C-LC in rhesus monkeys leads to long-term behavioral changes in the offspring, including behaviors relevant to both ASD and SZ (Bauman et al. 2014, Machado et al. 2015), evidence of altered dendritic morphology (Weir et al. 2015), and in the current study, evidence of elevated plasma cytokine concentrations and increased functional immune responses following immune cell activation. These findings add further validity that this novel non-human model of MIA may have translational relevance to complex human NDD. Long-term immune alterations were observed in the MIA offspring. Assessment of cytokines in plasma showed elevated levels in the MIA group for both years 1 and 4 compared to controls. More specifically, MIA offspring displayed elevated chemokines and IFNγ at both time-points. Dynamic immune cellular responses were also altered over time with increased production of innate immune cell cytokines predominating in MIA offspring at year 1, and with a more skewed TH2 phenotype at year 4. Moreover, several innate immune cell and TH2 associated cytokines correlated with emergence of stereotyped behaviors measured at several different time-points. The findings of long-term altered immune responses in the MIA group supports previous findings of immune changes in rodent models of MIA, and perhaps more importantly from studies of immune function in humans with NDD.
In a murine model of MIA, we previously showed increased production of IL-12(p40) and CCL3 from stimulated macrophages derived from adult offspring of poly I:C injected dams (Onore et al. 2014). Hsiao et al. reported findings of persistently altered immune responses in adult offspring of dams treated with poly I:C, including increased production of IL-6 and IL-17 from stimulated T cells and increased numbers of leukocyte subsets, most notably granulocytes (Hsiao et al. 2012). In the present non-human primate model of MIA, we report findings of hyper-responsive leukocytes with elevated production of innate cytokines during year 1. While we continued to see some innate driven responses during year 4, MIA offspring displayed a shift towards a TH2 phenotype with elevated production of IL-4 and IL-13. These shifts in cytokine production in response to various immune cell stimuli suggest that while innate cytokines may be driving inflammation early on in life for MIA offspring, TH2 cytokines may play a more important role later on.
Both innate and adaptive cellular responses have been implicated in human studies of ASD and SZ (MÜller et al. 2000, Onore et al. 2012). We, and others, have reported altered antibody profiles, as well as dysregulated monocyte, NK cell and T cell responses in children with ASD (Singh 1996, Okada et al. 2007, Ashwood et al. 2008, Ashwood et al. 2011a, Suzuki et al. 2011, Al-Ayadhi and Mostafa 2012, Ricci et al. 2013, Careaga et al. 2015). Studies investigating plasma cytokine concentration in children with ASD have generally reported findings of elevated pro-inflammatory but reduced anti-inflammatory cytokine levels (for review see (Rose and Ashwood 2014)). Our laboratory has previously found increased cytokines associated with the innate immune response including IL-1β, IL-6, and IL-12 in plasma of children with ASD compared to typically developing (TD) children and children with developmental delays (Ashwood et al. 2011a). A number of studies have replicated these findings, showing elevated IL-1β, IL-6 and/or IL-12 in individuals with ASD (Emanuele et al. 2010, Suzuki et al. 2011, Ricci et al. 2013). In the current study, at year 1, MIA offspring were also found to produce elevated levels of the innate cytokines IL-1β, IL-6, IL-12(p40) and TNFα at baseline (media alone) and after stimulation with a TLR-3 agonist compared to controls. All four of these cytokines play an important role in innate immunity (Trinchieri 1995, Fearon and Locksley 1996, Jones et al. 2005, Arend et al. 2008) and expression of these cytokines are directly downstream of TLR signaling (Medzhitov 2001). Increased production of IL-1β, IL-6 and TNFα after TLR-2 or TLR-4 stimulation is not only frequently reported cytokine in ASD, but has also been connected with worsening behaviors (Enstrom et al., 2010, Jyonouchi et al. 2014). Interestingly, IL-1β production after stimulation was elevated at both year 1 and year 4 time points in MIA offspring compared to controls, suggesting that this inflammatory cytokine may play an important contributing role to altered behaviors and/or pathology.
In addition to the innate immune cell cytokines, TH2 cytokines have also been associated with worse behavioral and cognitive outcome in individuals with ASD in multiple studies (Enstrom et al. 2010, Ashwood et al. 2011a, Ashwood et al. 2011c, Al-Ayadhi and Mostafa 2013, Jyonouchi et al. 2014). For example, IL-4, was found to be associated with impairments in nonverbal communication and IL-13, another TH2 associated cytokine, showed a trend associating with social impairment (Ashwood et al. 2011a). In the current study, elevated innate immune cell cytokines were associated with increased stereotyped behaviors, although we did see a trend for year 1 offspring, TLR-4 induced production of IL-13 positively correlate with stereotyped behaviors at the juvenile (22 months) age. In year 4, there were associations with more impaired behavior and TH2 cytokines, including IL-4, IL-5 and IL-13. These data are intriguing, and the potential role for TH2 and innate immune cell cytokines play in behaviors associated with NDD need further study.
Immune perturbations in SZ have also been reported, with both innate and TH2 processes implicated (Muller and Schwarz 2010, Al-Amin et al. 2013). Studies in patients with SZ have reported an increase in innate immune cell populations including monocytes, neutrophils and γδCD+8 T cells (Wilke et al. 1996, Müller et al. 1998), elevated levels of serum or plasma IL-6 (Maes et al. 1995, Frommberger et al. 1997, Lin et al. 1998) and increased IL-4 concentrations in cerebral spinal fluid (CSF) (Mittleman et al. 1997). Furthermore, similar to ASD, cytokine levels in SZ also seem to be associated with worsening behaviors. For example, one study found plasma IL-6 to be elevated during acute phases of illness but found no difference when the patients were in remission (Frommberger et al. 1997). Another study reported an association between negative behaviors and CSF levels of IL-10, but only in non-medicated patients (van Kammen et al. 1997). Behavioral state, medication, age and duration of illness all contribute to the extent and most likely, the type of immune dysfunction experienced in individuals with SZ making it difficult to clearly elucidate the role of the immune system in this complicated neurodevelopmental disorder.
Immune findings in NDD literature have been mixed with regard to cellular phenotypes or T cell skewing. For instance, some, studies emphasizeTH1 cell phenotypes while others demonstrated a more TH2 phenotype. What may seem like inconsistencies in the field most likely reflect the heterogeneity of NDDs that consist of potential multiple subtypes. In a recent study, utilizing a large cohort of subjects from the Autism Phenome Project (APP), evidence suggests that there was a significant subgroup of individuals within the ASD population that had elevated production of inflammatory cytokines following immune cell stimulation, in contrast to a group of children with ASD that did not. The children with enhanced immune activation had more severe core ASD features with more impaired behaviors. Further, cluster analysis suggested that the type of cellular immune response also varied within the group of children with enhanced immune cell activation with, one subgroup exhibiting an innate cytokine profile, a second with a TH1 cell profile of upregulated production of IFNγ and a third subgroup exhibiting a TH2 profile with elevated IL-13 production (Careaga et al. 2015). Multiple etiologies (known and unknown) may contribute to NDD or a more central regulatory mechanism controlling all immune responses may be occurring but that certain individuals display different inflammatory profiles in the absence of this regulation. Our current findings of increased innate and T cell responses in the non-human primate MIA model are consistent with the findings of distinct subgroups within NDD that exhibit evidence of immune dysfunction.
The relationship between immune dysfunction and behavioral outcome is still unclear. While there is evidence from the literature as well as our own findings that indicate increased inflammation is associated with increased severity of aberrant behavior, the exact role that immune dysfunction plays remains unknown; it could potentially be additive, synergistic, causal or independent of behaviors. Genetic studies evaluating differential gene expression in both ASD and SZ have found differences in immune related genes (Garbett et al. 2008, Muller and Schwarz 2010). Furthermore, several implicated genes contribute to both neural and immune pathways, and could affect both independently of one another, or combined play an additive or synergistic role. There is also evidence that changes to the current immune state can alter behavior. For example one study reported improved behaviors in children with ASD when they had a fever (Curran et al. 2007). Immune intervention therapies in ASD have reported improvements in behaviors (Asadabadi et al. 2013). However, while we know that inflammation can contribute to/cause behavioral change, such as with sickness behaviors and assoicated loss of appetite, lethargy and social withdrawal (Dantzer et al. 2008), we currently do not know the exact role that cytokines and immune dysfunction play in ASD or SZ associated behaviors. It is highly likely that there are many factors contributing to NDD behaviors, with the role of the immune system varying based on the individual etiology and subtype of NDD.
The findings presented here add further support to the potential of maternal immune activation during gestation having lifelong effects on the offspring, including altered immune function. The non-human primate model of MIA, due to the sophisticated cognitive and social behavior exhibited by non-human primates, may provide us with new insights into human behavioral disorders. One major limitation of this study was the small sample sizes in each group. Due to the limited offspring numbers we combined offspring from both the first and second trimesters in order to generate more statistical power. While we did not see any significant differences between the first and second trimester MIA offspring, larger studies are needed to probe specific effects of MIA at different exposure time points during gestation. Future studies should also focus on the relationship between immune dysfunction and behaviors and the potential mechanisms that govern them, and ultimately, begin to explore potential immune therapies/interventions.
4.1 Conclusions
Activation of the maternal immune system during gestation can lead to persistent immune dysfunction in the offspring. Dynamic cellular responses from PBMC revealed offspring of MIA treated damsdisplayed elevated production of innate immune cell associated cytokines early in life, shifting to a more TH2 type response as the animals aged. Furthermore, this study supports the findings of increase inflammation associated with abnormal behaviors seen in previous rodent models of MIA. Taken together with our previous findings of increased ASD and SZ relevant behaviors and altered dendritic morphology, evidence of an altered immune response adds further validity for the use of non-human primate models of MIA to better assess neurodevelopmental and behavioral disorders such as ASD and SZ.
Supplementary Material
Supplementary Table 1. Subject demographics:
Number of offspring born to Poly I:C-LC injected dams or Control dams, during either the end of the first or second trimester.
Supplemental Table 2. Timeline
Timeline of blood collection and behavioral assays
Supplemental Table 3. Year 1 cytokine association to juvenile behaviors:
Associations between cell culture cytokine production after 48 hours of stimulation with TLR-4 agonist, TLR-3 agonist or in media and repetitive behaviors measured at 1 year of life. Data presented as Spearman’s rank correlation coefficient and p value. Only significant (p < 0.05) and trending (p < 0.1) correlations are shown.
Highlights.
Maternal immune activation in a non-human primate model leads to juvenile offspring that exhibit elevated innate immune cell responses at 2 years of age
At 4 years of age, older offspring exhibit a more TH2 cytokine phenotype with elevated production of IL-4 and IL-13
Innate cytokines positively correlate with stereotyped behaviors at year 2, while TH2 associated cytokines positively correlate with self-directed stereotypies at year 4.
Acknowledgments
The authors of this study would like to thank Oncovir, Inc. for providing the stabilized poly IC-LC. We would also like to thank the late Dr. Paul Patterson for helping initiate this project and his guidance throughout these collaborations. The study was supported by NIH R15HD082638, U54HD079125, Peter Emch Foundation, and Autism Speaks Foundation (#7567), the Research Investments in the Sciences and Engineering (RISE [I-CAN-SZ]); Simons Foundation (SFARI [9900060]) and NARSAD foundation (Separate awards to P.A. and M.B.). Additional support was provided by the Center for Neuroscience at the University of California, Davis and the base grant (RR00169) of the California National Primate Research Center (CNPRC).
Footnotes
Conflicts of interest
All authors declare they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Amin MM, Uddin MMN, Reza HM. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clinical Psychopharmacology and Neuroscience. 2013;11(3):144. doi: 10.9758/cpn.2013.11.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ayadhi LY, Mostafa GA. Elevated serum levels of interleukin-17A in children with autism. J Neuroinflammation. 2012;9:158. doi: 10.1186/1742-2094-9-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ayadhi LY, Mostafa GA. Elevated serum levels of macrophage-derived chemokine and thymus and activation-regulated chemokine in autistic children. J Neuroinflammation. 2013;10:72. doi: 10.1186/1742-2094-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunological Reviews. 2008;223(1):20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- Asadabadi M, Mohammadi MR, Ghanizadeh A, Modabbernia A, Ashrafi M, Hassanzadeh E, Forghani S, Akhondzadeh S. Celecoxib as adjunctive treatment to risperidone in children with autistic disorder: a randomized, double-blind, placebo-controlled trial. Psychopharmacology. 2013;225(1):51–59. doi: 10.1007/s00213-012-2796-8. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Enstrom A, Krakowiak P, Hertz-Picciotto I, Hansen RL, Croen LA, Ozonoff S, Pessah IN, Van de Water J. Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. J Neuroimmunol. 2008;204(1–2):149–153. doi: 10.1016/j.jneuroim.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011a;25(1):40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Altered T cell responses in children with autism. Brain Behav Immun. 2011b;25(5):840–849. doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol. 2011c;232(1–2):196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40(12):1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Iosif AM, Smith SE, Bregere C, Amaral DG, Patterson PH. Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol Psychiatry. 2014;75(4):332–341. doi: 10.1016/j.biopsych.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Babineau BA, Mason WA, Amaral DG. Emergence of stereotypies in juvenile monkeys (Macaca mulatta) with neonatal amygdala or hippocampus lesions. Behav Neurosci. 2008;122(5):1005–1015. doi: 10.1037/a0012600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol. 2012;72(10):1272–1276. doi: 10.1002/dneu.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga M, Rogers S, Hansen RL, Amaral DG, Van de Water J, Ashwood P. Immune Endophenotypes in Children with Autism Spectrum Disorder. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SW, Brent LJ, Adams GK, Klein JT, Pearson JM, Watson KK, Platt ML. Neuroethology of primate social behavior. Proc Natl Acad Sci U S A. 2013;110(Suppl 2):10387–10394. doi: 10.1073/pnas.1301213110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016 doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran LK, Newschaffer CJ, Lee LC, Crawford SO, Johnston MV, Zimmerman AW. Behaviors associated with fever in children with autism spectrum disorders. Pediatrics. 2007;120(6):e1386–1392. doi: 10.1542/peds.2007-0360. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nicola M, Cattaneo A, Hepgul N, Di Forti M, Aitchison KJ, Janiri L, Murray RM, Dazzan P, Pariante CM, Mondelli V. Serum and gene expression profile of cytokines in first-episode psychosis. Brain, Behavior, and Immunity. 2013;31:90–95. doi: 10.1016/j.bbi.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele E, Orsi P, Boso M, Broglia D, Brondino N, Barale F, di Nemi SU, Politi P. Low-grade endotoxemia in patients with severe autism. Neurosci Lett. 2010;471(3):162–165. doi: 10.1016/j.neulet.2010.01.033. [DOI] [PubMed] [Google Scholar]
- Enstrom AM, Onore CE, Van de Water JA, Ashwood P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immun. 2010;24(1):64–71. doi: 10.1016/j.bbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272(5258):50. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- Frommberger UH, Bauer J, Haselbauer P, Fraulin A, Riemann D, Berger M. Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission. Eur Arch Psychiatry Clin Neurosci. 1997;247(4):228–233. doi: 10.1007/BF02900219. [DOI] [PubMed] [Google Scholar]
- Garay PA, Hsiao EY, Patterson PH, McAllister AK. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun. 2013;31:54–68. doi: 10.1016/j.bbi.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbett K, Ebert PJ, Mitchell A, Lintas C, Manzi B, Mirnics K, Persico AM. Immune transcriptome alterations in the temporal cortex of subjects with autism. Neurobiol Dis. 2008;30(3):303–311. doi: 10.1016/j.nbd.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbett KA, Hsiao EY, Kalman S, Patterson PH, Mirnics K. Effects of maternal immune activation on gene expression patterns in the fetal brain. Transl Psychiatry. 2012;2:e98. doi: 10.1038/tp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Li N, Meng Q, Shao Q, Wang W. Maternal immune activation impairs reversal learning and increases serum tumor necrosis factor-α in offspring. Neuropsychobiology. 2011;64(1):9–14. doi: 10.1159/000322455. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci U S A. 2012;109(31):12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011;25(4):604–615. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito HT, Smith SE, Hsiao E, Patterson PH. Maternal immune activation alters nonspatial information processing in the hippocampus of the adult offspring. Brain Behav Immun. 2010;24(6):930–941. doi: 10.1016/j.bbi.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Richards PJ, Scheller J, Rose-John S. Review: IL-6 transsignaling: the in vivo consequences. Journal of interferon & cytokine research. 2005;25(5):241. doi: 10.1089/jir.2005.25.241. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Geng L, Davidow AL. Cytokine profiles by peripheral blood monocytes are associated with changes in behavioral symptoms following immune insults in a subset of ASD subjects: an inflammatory subtype? Journal of Neuroinflammation. 2014;11:187. doi: 10.1186/s12974-014-0187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyonouchi H, Sun S, Itokazu N. Innate immunity associated with inflammatory responses and cytokine production against common dietary proteins in patients with autism spectrum disorder. Neuropsychobiology. 2002;46(2):76–84. doi: 10.1159/000065416. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Sun S, Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J Neuroimmunol. 2001;120(1–2):170–179. doi: 10.1016/s0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- Lin A, Kenis G, Bignotti S, Tura GJB, De Jong R, Bosmans E, Pioli R, Altamura C, Scharpé S, Maes M. The inflammatory response system in treatment-resistant schizophrenia: Increased serum interleukin-6. Schizophrenia Research. 1998;32(1):9–15. doi: 10.1016/s0920-9964(98)00034-6. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Whitaker AM, Smith SEP, Patterson PH, Bauman MD. Maternal Immune Activation in Nonhuman Primates Alters Social Attention in Juvenile Offspring. Biological Psychiatry. 2015;77(9):823–832. doi: 10.1016/j.biopsych.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Bosmans E, Calabrese J, Smith R, Meltzer HY. Interleukin-2 and interleukin-6 in schizophrenia and mania: effects of neuroleptics and mood stabilizers. J Psychiatr Res. 1995;29(2):141–152. doi: 10.1016/0022-3956(94)00049-w. [DOI] [PubMed] [Google Scholar]
- Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26(4):607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal M, Donnelly R, Elkabes S, Zhang P, Davini D, David BT, Ponzio NM. Maternal immune stimulation during pregnancy shapes the immunological phenotype of offspring. Brain Behav Immun. 2013;33:33–45. doi: 10.1016/j.bbi.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Mandal M, Marzouk AC, Donnelly R, Ponzio NM. Maternal immune stimulation during pregnancy affects adaptive immunity in offspring to promote development of TH17 cells. Brain Behav Immun. 2011;25(5):863–871. doi: 10.1016/j.bbi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- McAllister AK. Major Histocompatibility Complex I in Brain Development and Schizophrenia. Biological Psychiatry. 2014;75(4):262–268. doi: 10.1016/j.biopsych.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1(2):135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Meyer U. Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry. 2014;75(4):307–315. doi: 10.1016/j.biopsych.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol. 2010;90(3):285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 2009;33(7):1061–1079. doi: 10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Immunological stress at the maternal–foetal interface: a link between neurodevelopment and adult psychopathology. Brain, behavior, and immunity. 2006a;20(4):378–388. doi: 10.1016/j.bbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Meyer U, Murray PJ, Urwyler A, Yee BK, Schedlowski M, Feldon J. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol Psychiatry. 2008;13(2):208–221. doi: 10.1038/sj.mp.4002042. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. The Journal of neuroscience. 2006b;26(18):4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittleman BB, Castellanos FX, Jacobsen LK, Rapoport JL, Swedo SE, Shearer GM. Cerebrospinal fluid cytokines in pediatric neuropsychiatric disease. J Immunol. 1997;159(6):2994–2999. [PubMed] [Google Scholar]
- MÜLler N, Riedel M, Gruber R, Ackenheil M, Schwarz MJ. The Immune System and Schizophrenia: An Integrative View. Annals of the New York Academy of Sciences. 2000;917(1):456–467. doi: 10.1111/j.1749-6632.2000.tb05410.x. [DOI] [PubMed] [Google Scholar]
- Müller N, Schlesinger B, Hadjamu M, Riedel M, Schwarz M, Ackenheil M, Wank R, Gruber R. Increased frequency of CD8 positive gamma/delta T-lymphocytes (CD8+ γ/δ+) in unmedicated schizophrenic patients: relation to impairment of the blood–brain barrier and HLA-DPA* 02011. Schizophrenia Research. 1998;32(1):69–71. doi: 10.1016/s0920-9964(98)00036-x. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ. Immune System and Schizophrenia. Curr Immunol Rev. 2010;6(3):213–220. doi: 10.2174/157339510791823673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Hashimoto K, Iwata Y, Nakamura K, Tsujii M, Tsuchiya KJ, Sekine Y, Suda S, Suzuki K, Sugihara G, Matsuzaki H, Sugiyama T, Kawai M, Minabe Y, Takei N, Mori N. Decreased serum levels of transforming growth factor-beta1 in patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):187–190. doi: 10.1016/j.pnpbp.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26(3):383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onore CE, Schwartzer JJ, Careaga M, Berman RF, Ashwood P. Maternal immune activation leads to activated inflammatory macrophages in offspring. Brain Behav Immun. 2014;38:220–226. doi: 10.1016/j.bbi.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204(2):313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Pomerantz O, Paukner A, Terkel J. Some stereotypic behaviors in rhesus macaques (Macaca mulatta) are correlated with both perseveration and the ability to cope with acute stressors. Behav Brain Res. 2012;230(1):274–280. doi: 10.1016/j.bbr.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biological psychiatry. 2008;63(8):801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects — Reviewing the Evidence for Causality. New England Journal of Medicine. 2016;374(20):1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- Ricci S, Businaro R, Ippoliti F, Lo Vasco VR, Massoni F, Onofri E, Troili GM, Pontecorvi V, Morelli M, Rapp Ricciardi M, Archer T. Altered cytokine and BDNF levels in autism spectrum disorder. Neurotox Res. 2013;24(4):491–501. doi: 10.1007/s12640-013-9393-4. [DOI] [PubMed] [Google Scholar]
- Rose D, Ashwood P. Potential cytokine biomarkers in autism spectrum disorders. Biomark Med. 2014;8(9):1171–1181. doi: 10.2217/bmm.14.39. [DOI] [PubMed] [Google Scholar]
- Schwartzer JJ, Careaga M, Onore CE, Rushakoff JA, Berman RF, Ashwood P. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl Psychiatry. 2013;3:e240. doi: 10.1038/tp.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Smith SEP, Malkova N, Tse D, Su Y, Patterson PH. Activation of the Maternal Immune System Alters Cerebellar Development in the Offspring. Brain, behavior, and immunity. 2009;23(1):116–123. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int J Dev Neurosci. 2005;23(2–3):299–305. doi: 10.1016/j.ijdevneu.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Short SJ, Lubach GR, Karasin AI, Olsen CW, Styner M, Knickmeyer RC, Gilmore JH, Coe CL. Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol Psychiatry. 2010;67(10):965–973. doi: 10.1016/j.biopsych.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK. Plasma increase of interleukin-12 and interferon-gamma. Pathological significance in autism. J Neuroimmunol. 1996;66(1–2):143–145. doi: 10.1016/0165-5728(96)00014-8. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27(40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli S, Schwandt ML, Lindell SG, Heilig M, Suomi SJ, Higley JD, Goldman D, Barr CS. The serotonin transporter gene linked polymorphic region is associated with the behavioral response to repeated stress exposure in infant rhesus macaques. Dev Psychopathol. 2012;24(1):157–165. doi: 10.1017/S0954579411000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Matsuzaki H, Iwata K, Kameno Y, Shimmura C, Kawai S, Yoshihara Y, Wakuda T, Takebayashi K, Takagai S, Matsumoto K, Tsuchiya KJ, Iwata Y, Nakamura K, Tsujii M, Sugiyama T, Mori N. Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PLoS One. 2011;6(5):e20470. doi: 10.1371/journal.pone.0020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- van Kammen DP, McAllister CG, Kelley ME. Relationship between immune and behavioral measures in schizophrenia. In: Wieselmann G, editor. Current Update in Psychoimmunology. Vienna: Springer Vienna; 1997. pp. 51–55. [Google Scholar]
- Watson KK, Platt ML. Of mice and monkeys: using non-human primate models to bridge mouse- and human-based investigations of autism spectrum disorders. J Neurodev Disord. 2012;4(1):21. doi: 10.1186/1866-1955-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir RK, Forghany R, Smith SE, Patterson PH, McAllister AK, Schumann CM, Bauman MD. Preliminary evidence of neuropathology in nonhuman primates prenatally exposed to maternal immune activation. Brain Behav Immun. 2015;48:139–146. doi: 10.1016/j.bbi.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke I, Arolt V, Rothermundt M, Weitzsch C, Hornberg M, Kirchner H. Investigations of cytokine production in whole blood cultures of paranoid and residual schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 1996;246(5):279–284. doi: 10.1007/BF02190280. [DOI] [PubMed] [Google Scholar]
- Willette AA, Lubach GR, Knickmeyer RC, Short SJ, Styner M, Gilmore JH, Coe CL. Brain enlargement and increased behavioral and cytokine reactivity in infant monkeys following acute prenatal endotoxemia. Behav Brain Res. 2011;219(1):108–115. doi: 10.1016/j.bbr.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Subject demographics:
Number of offspring born to Poly I:C-LC injected dams or Control dams, during either the end of the first or second trimester.
Supplemental Table 2. Timeline
Timeline of blood collection and behavioral assays
Supplemental Table 3. Year 1 cytokine association to juvenile behaviors:
Associations between cell culture cytokine production after 48 hours of stimulation with TLR-4 agonist, TLR-3 agonist or in media and repetitive behaviors measured at 1 year of life. Data presented as Spearman’s rank correlation coefficient and p value. Only significant (p < 0.05) and trending (p < 0.1) correlations are shown.