Abstract
Background
Outcomes of HIV-infected children before widespread use of antiretroviral therapy (ART) for treatment and prevention of mother-to-child transmission (PMTCT) have been well characterized but less is known about children who acquire HIV infection in the context of good ART access.
Methods
We enrolled newly-diagnosed HIV-infected children ≤24 months (mos) of age at 3 hospitals and 2 clinics in Johannesburg, South Africa. We report ART initiation and mortality rates during 6 months from enrollment and factors associated with mortality.
Results
Of 272 children enrolled, median age 6.1mos, 69.5% were diagnosed during hospitalization. By 6mos post-enrollment, 53(19.5%) died and 73(26.8%) were lost-to-follow-up. Using Kaplan-Meier analysis, the probability of death by 6mos after enrollment was 23.5%. The median age of death was 9.1mos (95% confidence interval[CI]: 8.6; 12.0). Overall, 226(83%) children initiated ART which was associated with a 71% reduction in risk of death (Hazard Ratio[HR] =0.29 [95%CI: 0.15; 0.58]). In multivariable analysis of infant factors, weight-for-age z-score <-2SD (HR=2.43[95%CI: 1.03-5.73]), CD4<20% (HR=3.29[95%CI: 1.60-6.76]) and identification during hospitalization (HR=2.89[95%CI: 1.16-7.25]) were independently associated with mortality. In multivariable analysis of maternal factors, CD4≤350/no maternal ART was associated with increased mortality risk (HR=2.57[95%CI: 1.19-5.59]) vs. CD4>350/no maternal ART; exposure to maternal/infant antiretrovirals for PMTCT was associated with reduced mortality risk (HR=0.53[95%CI: 0.28-0.99]) vs. no PMTCT.
Conclusions
ART initiation is highly protective against death in young children. However, despite improved access to ART, young children remain at risk for early death; innovative approaches to rapidly diagnose and initiate treatment as early in life as possible are needed.
Introduction
South Africa has documented remarkable success preventing and treating pediatric HIV infection. In 2014 mother-to-child HIV transmission(MTCT) rates were estimated at 4.0% 1,2 and more than 150,000 children initiated antiretroviral treatment (ART) 2,3. While decreased mortality among HIV-infected adults and children was demonstrated, 4-8 there were an estimated 12,000 deaths among children < 14 years of age in 2014 2.
Over the last decade new scientific findings have been incorporated into South African guidelines. In 2008 the Option A PMTCT approach was introduced, modified in 2010, followed by adoption of Option B in 2013 and Option B+ in 2015 9-11. In parallel, early infant diagnostic testing (EID) using dried blood spots for polymerase chain reaction(PCR) testing was scaled-up 12,13. Pediatric treatment recommendations have also evolved rapidly. In response to the CHER study, treatment was recommended for all children <12 months of age, subsequently extended to include all children less than age five14. In South Africa, protease inhibitor- based therapy was always recommended for infants regardless of prior nevirapine(NVP) exposure for PMTCT 15,16.
Similar advances have been documented in many high-burden countries in Sub-Saharan Africa, but hundreds of thousands of children continue to acquire perinatal HIV infection 2,7,18. While health outcomes prior to widespread availability of ART have been described 19 we know less about children who acquire HIV in the context of good access to EID and to antiretrovirals for prevention and treatment.
In 2011 we conducted a surveillance study of all infants and young children diagnosed with HIV infection at the three hospitals and two clinics in Johannesburg/Soweto, South Africa to support their engagement in care and ART initiation. We describe mortality in these children over the first six months after study enrollment.
Methods
Study Population
We conducted a surveillance study aimed to recruit all newly-diagnosed HIV-infected children ≤2 years of age at Rahima Moosa Mother and Child Hospital, Charlotte Maxeke Johannesburg Academic Hospital, Chris Hani Baragwanath Academic Hospital, Perinatal HIV Research Unit clinic, and Witkoppen clinic, in Johannesburg, South Africa, January-December 2011. Children were identified at inpatient and outpatient services by facility health workers and study staff according to eligibility criteria: child ≤24 months with a positive HIV PCR if ≤18 months or a positive HIV antibody test if >18 months; mother/primary caregiver had not yet received test results or received results within the past two weeks. Children already on ART were enrolled if the other criteria were met. The study was approved by the Institutional Review Boards of the University of the Witwatersrand and Columbia University.
Study Procedures
At enrollment mothers completed a questionnaire detailing: socioeconomic status, HIV care and PMTCT, infant feeding, barriers to care, disclosure, alcohol consumption, stigma, and psychologic distress. Whenever possible, clinical parameters reported in the questionnaire were verified in medical records. Blood samples were collected from mother and child for plasma HIV RNA viral load (VL) (Roche Taqman HIV-1 Test v2.0) and CD4 cell count/percent. Study staff assisted families to ensure that all participants enrolled in care and initiated ART expeditiously. HIV care and ART was provided, free of charge, at the study facilities according to national guidelines, which specified ART initiation for all children < 2 years of age 11. After 6 months, clinical status and laboratory results were abstracted from medical records. For those whose status could not be determined on record review, attempts were made to reach the family telephonically.
Statistical Analysis
Enrollment characteristics were summarized using, for categorical variables, simple proportions and, for continuous variables, means, medians and interquartile range (IQR). Sex- and age-adjusted weight- and height-for-age z-scores (WAZ and HAZ) were calculated using World Health Organization norms 20. We used two sample t-test or Wilcoxon signed rank test to compare normally- and non-normally-distributed continuous variables, respectively. Categorical data were compared using the chi-squared test.
The primary outcome was child mortality within six months of enrollment. We used the Kaplan-Meier method to calculate the cumulative probability of death by 6-months after enrollment overall and stratified by key covariates. Cox Proportional Hazard models were used to analyze which enrollment characteristics were potential risk factors for mortality. Characteristics were classified into fixed covariate categories except ART initiation, which was considered as a time-dependent variable. To take loss to follow-up adequately into account in this model, we imputed ART-free survival time to 6 months post-enrollment for those lost-to-follow-up before 6 months. Unadjusted hazard ratios (HR) and respective 95% confidence intervals (CI) were reported. The role of confounding and mediation was investigated in stratified analysis and systematic multivariable Cox Proportional models. Final models accounted for biologically-meaningful relationships, potential confounders that changed main effects by more than 10%, and statistical significance (p<0.05) of retained covariates. Proportional hazards assumptions were tested by checking martingale residuals for each important covariate. Statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC).
Results
Cohort description
Overall 289 children were enrolled; 272 were included in analysis, excluding 17 children (5.9%) already on ART. Of 272 ART-naïve children, 52.2% female, the median age at enrollment was 6.2 months (Table 1). The majority (69.5%) were identified as HIV-infected during a hospital admission, 20.7% in PMTCT follow-up, and 9.8% through immunization services. The median age at HIV diagnostic testing was 3.7 months (IQR 1.6-9.1). Children identified during hospital admission were older at testing (median 6.0 months, IQR 2.6-12.9) than those tested in PMTCT follow-up (median 1.6 months, IQR 1.5-3.3). A third (33.1%) had no PMTCT antiretroviral exposure (maternal or infant), 51.5% had been exposed to antiretroviral prophylaxis (maternal, infant or both but excluding maternal ART) and 15.4% had mothers who received ART during pregnancy. Approximately half had ever breastfed (53.2%) and 15.4% were breastfeeding at enrollment.
Table 1. Characteristics of 272 newly-diagnosed HIV-infected children ≤ 2 years of age identified in Johannesburg, South Africa, January-December 2011.
| Total N (%) | Child age at enrollment N (%) | |||
|---|---|---|---|---|
| n=272* | < 6 months n=133 | 6 – 12 months n=74 | > 12 months n=65 | |
| Sex | ||||
| Male | 130 (47.8%) | 55 (41.4%) | 35 (47.3%) | 40 (61.5%) |
| Female | 142 (52.2%) | 78 (58.6%) | 39 (52.7%) | 25 (38.5%) |
| Identification site | ||||
| PMTCTˆ Follow-up | 53 (20.7%) | 31 (25.4%) | 13 (18.3%) | 9 (14.3%) |
| Hospital ward | 178 (69.5%) | 74 (60.7%) | 52 (73.2%) | 52 (82.5%) |
| Immunization services | 25 (9.8%) | 17 (13.9%) | 6 (8.5%) | 2 (3.2%) |
| Birth characteristics | ||||
| Low Birth Weight (<2500g) | 57 (23.2%) | 36 (29.0%) | 16 (24.2%) | 5 (8.9%) |
| < 37 weeks gestation | 49 (19.1%) | 29 (23.4%) | 14 (20.0%) | 6 (9.7%) |
| PMTCT status | ||||
| No PMTCT | 88 (33.1%) | 28 (21.5%) | 24 (32.4%) | 36 (58.1%) |
| Some PMTCT# | 137 (51.5%) | 74 (56.9%) | 40 (54.1%) | 23 (37.1%) |
| Maternal ARTˆ,§ | 41 (15.4%) | 28 (21.5%) | 10 (13.5%) | 3 (4.8%) |
| Feeding history | ||||
| Never breastfed | 125 (46.8%) | 77 (59.7%) | 33 (44.6%) | 15 (23.4%) |
| Breastfed & stopped | 101 (37.8%) | 32 (24.8%) | 28 (37.8%) | 41 (64.1%) |
| Still breastfeeding | 41 (15.4%) | 20 (15.5%) | 13 (17.6%) | 8 (12.5%) |
| Infant CD4 testing | ||||
| Done | 261 (96.0%) | 128 (96.2%) | 70 (94.6%) | 63 (96.9%) |
| Median CD4 percentage (IQR)ˆ | 20.0 (12.5 – 28.9) | 22.7 (13.8 – 31.8) | 17.1 (10.3 -28.7) | 18.6 (11.5 – 24.0) |
| Infant HIV-1 RNA viral load (copies/ml) | ||||
| Done | 244 (89.7%) | 120 (90.2%) | 66 (89.2%) | 58 (89.2%) |
| Median (IQR)ˆ copies/ml | 1,226,150 (393,905 – 4,890,899) | 1,610,059 (412,005 – 7,634,266) | 1,261,398 (414,831-4,058,715) | 752,867 (340,485 – 2,061,430) |
PMTCT – prevention of mother to child transmission; ART – antiretroviral treatment; IQR – interquartile range
Numbers do not add up to totals for all covariates due to missing data
This includes maternal and/or infant antiretrovirals for prophylaxis, excluding maternal antiretroviral treatment
Maternal antiretroviral treatment regardless of other antiretroviral use as infant prophylaxis
For 261 children with CD4 testing at enrollment, the median CD4 was 20.0% (IQR: 12.5% - 28.9%) (Table 1). HIV RNA VL was available for 244 children. Median VL was 1,226,150 copies/ml (IQR: 393,905 – 4,890,899 copies/ml), and was lower among children >12 months of age (752,867 copies/ml) compared with those <6 months (1,610,059 copies/ml) and 6-12 months of age (1,261,398 copies/ml) at enrollment.
Mortality
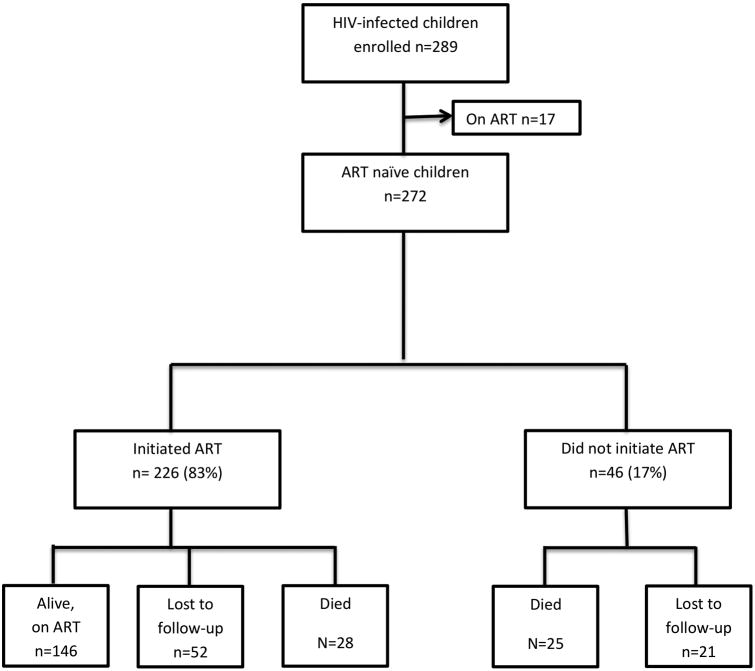
By 6 months after enrollment, 53 children (19.5%) had died and 73 children (26.8%) were no longer in care at the study facility (Figure 1). Among those not retained, 18(24.7%) were known to have relocated outside of Johannesburg, 20 (27.3%) were reported alive, and the status of the other 35 (47.9%) was unknown. Only 146 (53.7%) children were alive and known to be retained in care 6 months after enrollment. Using Kaplan-Meier analysis, the probability of death by the first week after enrollment was 4.7% (n=12), by one month 9.6% (n=24), by 3 months 18.1% (n=43), and by 6 months 23.5% (n=53). The median age of death was 9.1 months (95% CI: 8.6;12.0).
Figure 1. Enrollment, ART initiation, and 6 month outcomes for 272 newly diagnosed, ART-naïve, HIV-infected children < 24 months of age, Johannesburg, South Africa, January-December 2011.
Antiretroviral treatment initiation
Among 226 (83.1%) children who initiated ART within 6 months of study enrollment (Figure 1), the median time to initiation was 8 days after study enrollment (95% CI: 6.0-12.0); 23 days after HIV diagnostic test results were provided (95%CI: 18.0-27.0) and 36 days from time of blood draw for the most recent diagnostic test (95%CI: 31.0-40.0). All 146 children who were alive and in follow-up at 6 months initiated ART whereas only 52.8% (28/53) of those who died and 71.2% (52/73) of those who were lost to follow-up before 6 months were known to have initiated ART. Among the group of children who ever started treatment, there was little difference in the median time of ART initiation among those children who died (10 days; 95%CI: 5.0-18.0) vs. those who survived (8 days; 95%CI: 6.0- 12.0, p=0.3007).
Risk factors for mortality
Antiretroviral treatment initiation, treated as a time-dependent variable, was associated with a 71% reduction in the risk of death (HR=0.29; 95%CI: 0.15-0.58) (Table 2). Identification as HIV-infected during a hospital admission was associated with an almost 4-fold risk of death (HR=3.92; 95%CI: 1.66-9.22) compared to identification during PMTCT follow-up or immunization services. Children with more advanced HIV disease were at increased risk for mortality, including those with CD4≤20%, VL ≥1,500,000 copies/mL and WAZ ≤-2 SD. Age at enrollment was not associated with mortality risk.
Table 2. Child, maternal and social characteristics associated with child mortality by 6 months after enrollment among 272 newly-diagnosed HIV-infected children ≤ 2 years of age identified in Johannesburg, South Africa, January-December 2011.
| N | Probability of death | N Died | Logrank p-value | Hazard Ratio (95% CI) | |
|---|---|---|---|---|---|
| Child Antiretroviral Treatment | |||||
| Time-dependent covariate treatment vs not | 272 | N/A | 0.29 (0.15-0.58) | ||
| Identification site | |||||
| Hospital ward | 178 | 0.294 | 42 | 0.001 | 3.92 (1.66-9.22) |
| PMTCTˆ/ Immunization services | 78 | 0.089 | 6 | Ref | |
| Child age at enrollment | |||||
| < 6 month | 133 | 0.211 | 24 | 0.844 | 1.06 (0.52-2.17) |
| 6–12 month | 74 | 0.302 | 18 | 0.317 | 1.47 (0.69-3.11) |
| > 12 month | 65 | 0.208 | 11 | ref | |
| Sex | |||||
| Male | 130 | 0.247 | 27 | ref | |
| Female | 142 | 0.227 | 26 | 0.657 | 0.885 (0.52-1.52) |
| Child CD4 percentage | |||||
| > 20.0% | 129 | 0.116 | 11 | <0.0001 | ref |
| ≤ 20.0% | 132 | 0.327 | 37 | 3.59 (1.83-7.03) | |
| Child HIV-1 RNA viral load (copies/mL) | |||||
| ≥1,500,000 | 111 | 0.261 | 24 | 0.009 | 2.40 (1.22-4.72) |
| <1,500,000 | 133 | 0.118 | 13 | ref | |
| Weight-for-Age z-scores | |||||
| ≥ -2 SD* | 108 | 0.093 | 8 | <0.0001 | ref |
| < -2 SD | 149 | 0.314 | 38 | 4.25 (1.98-9.11) | |
| Maternal/Infant PMTCTˆ | |||||
| No PMTCT | 88 | 0.320 | 22 | 0.019 | 2.05 (1.12-3.76) |
| Some PMCT | 137 | 0.166 | 20 | ref | |
| ART | 41 | 0.218 | 7 | 0.645 | 1.23 (0.52-2.91) |
| Maternal health and treatment status | |||||
| CD4 ≤ 350 and no ARTˆ | 105 | 0.303 | 27 | 0.004 | 2.89 (1.36-6.14) |
| CD4 >350 and no ART | 93 | 0.111 | 9 | ref | |
| ART | 41 | 0.218 | 7 | 0.229 | 1.84 (0.69–4.95) |
| CD4 not done and no ART | 33 | 0.400 | 10 | 0.002 | 3.83 (1.56-9.43) |
| Maternal birth place | |||||
| South Africa | 188 | 0.199 | 31 | ref | |
| Other* | 76 | 0.328 | 20 | 0.031 | 1.84 (1.05-3.23) |
| Maternal education | |||||
| Primary Education (1-7) | 37 | 0.391 | 11 | 0.035 | 2.03 (1.04-3.98) |
| Grade 8 and above | 220 | 0.206 | 38 | ref | |
| Reported experience with antenatal counselors | |||||
| Positive | 190 | 0.180 | 28 | ref | 0.369 |
| Neutral+ | 17 | 0.427 | 6 | 0.036 | ref |
| Negative+ | 4 | 0.500 | 2 | 0.055 | |
| Reported experience with antenatal nurses | |||||
| Positive | 199 | 0.193 | 32 | ref | 0.41 (0.21–0.80) |
| Neutral+ | 30 | 0.350 | 8 | 0.077 | ref |
| Negative+ | 8 | 0.650 | 4 | 0.003 | |
| Infant feeding status at enrollment | |||||
| Breastfeeding | 41 | 0.163 | 5 | 0.450 | 0.70 (0.28-1.77) |
| Not Breastfeeding | 226 | 0.241 | 46 | ref | |
PMTCT – prevention of mother to child transmission; ART – antiretroviral treatment;
Zimbabwe, Mozambique, Zambia, Lesotho, Malawi
For Hazard Ratio calculation the neutral and the negative group are combined due to small numbers.
In multivariable analysis adjusting for all the variables shown in Table 2, treatment initiation as a time-dependent variable (HR= 0.43; 95%CI: 0.20-0.93), WAZ<-2 SD (HR=2.43; 95%CI: 1.03-5.73), CD4≤ 20% (HR=3.29; 95%CI: 1.60-6.76) and identification during hospitalization (HR=2.89; 95%CI: 1.16-7.25) remained independently associated with mortality. VL was no longer predictive of mortality in multivariable analysis (data not shown.)
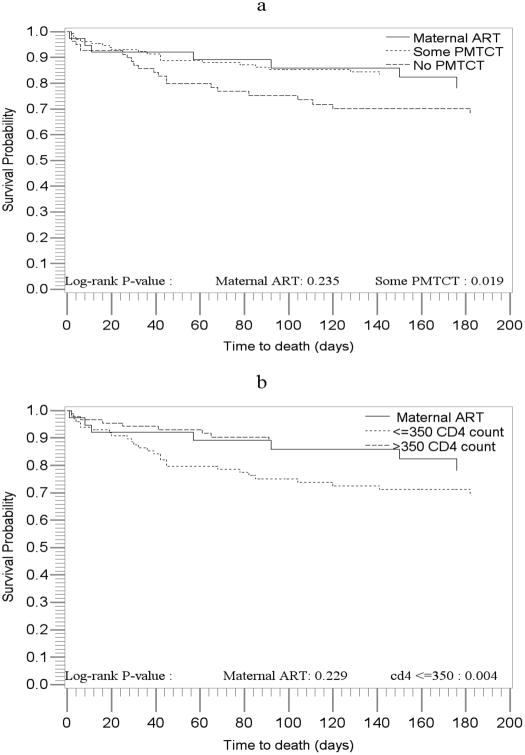
Several maternal health and social characteristics were also associated with child mortality in univariable analysis (Table 2). Receiving no PMTCT antiretrovirals (mother or infant) was associated with a doubling in mortality risk (HR=2.05; 95%CI: 1.12-3.76) compared to receipt of any PMTCT. Maternal advanced disease (CD4 count ≤350/no ART) also significantly increased child mortality compared to women with higher CD4 counts/no ART (HR=2.89; 95%CI: 1.36 – 6.14) (Table 2, figures 2a, 2b). Children born to immigrant mothers had an increased risk of death (HR=1.84; 95%CI: 1.05 - 3.23) compared to children of South African mothers. Less maternal education was associated with an increased risk of child death. Children of women reporting positive experiences with antenatal staff were at lower risk for mortality but very few women reported other than positive experiences.
Figure 2. Survival probabilities of HIV-infected children by type of PMTCT services received and by maternal health status.
In multivariable analysis including treatment initiation and all of the variables in Table 2 except experience with the nurses and counselors (which were not retained because of the small numbers), maternal CD4 ≤350/no ART remained significantly associated with increased risk of child mortality relative to maternal CD4 >350/no ART (HR=2.57; 95%CI: 1.19 - 5.59). Some PMTCT also remained significantly associated with reduced risk of child mortality relative to no PMTCT (HR=0.53; 95%CI: 0.28 - 0.99) after adjustment for these covariates (Table 3).
Table 3. Multivariable model of maternal and treatment characteristics associated with mortality by 6 months after enrollment among 272 newly-diagnosed HIV-infected children ≤ 2 years of age.
| Adjusted Hazard Ratio (95% CI) | |
|---|---|
| Child ART vs not (time-dependent) | 0.33 (0.16 - 0.68) |
| Maternal health and treatment status | |
| CD4 ≤ 350 and no ARTˆ | 2.57 (1.19 - 5.59) |
| CD4 >350 and no ART | ref |
| ART | 1.35 (0.48 - 3.78) |
| CD4 not done and no ART | 3.26 (1.14 - 9.30) |
| Maternal/Infant PMTCTˆ | |
| No PMTCT | ref |
| Some PMTCT | 0.53 (0.28 - 0.99) |
| Maternal birth place | |
| South African | ref |
| Other* | 1.80 (0.98 – 3.32) |
| Maternal Education | |
| Primary Education (1-7) | 1.55 (0.76 - 3.16) |
| High School and post high school (8 and above) | ref |
| Infant feeding status | |
| Breastfeeding at enrollment | 0.52 (0.19 – 1.48) |
| Not breastfeeding at enrollment | ref |
PMTCT – prevention of mother to child transmission; ART – antiretroviral treatment
Zimbabwe, Mozambique, Zambia, Lesotho, Malawi
Discussion
Despite broad access to early infant diagnosis and HIV prevention and treatment services in South Africa, HIV-infected infants and young children remain at high risk for poor health outcomes including early mortality. Among 272 children <2 years of age diagnosed with HIV in Johannesburg/Soweto in 2011, one third had not received any maternal or infant antiretrovirals for PMTCT and the majority was diagnosed on hospital wards, with evidence of advanced HIV disease. Most children (83%) successfully initiated ART, but even with extra support engagement provided in our project, it generally took 4-6 weeks from diagnostic testing to starting ART. While ART initiation markedly decreased the risk of death, 19.5% of children died and 26.8% were lost over six months of follow-up. These findings underscore the extreme vulnerability of perinatally-infected infants and the urgent need to better engage and retain women and their children in PMTCT and treatment services.
We aimed to identify all HIV-infected children ≤ 2 years of age at five health facilities in Johannesburg/Soweto in 2011 and facilitate enrollment and rapid ART initiation. Among the 226 children who started treatment, the median time to initiation was 8 days after enrollment and 36 days from diagnostic testing. Delays along the EID cascade have been well delineated and in comparison to many settings, 7-8 weeks from testing to treatment appears relatively efficient21. However, early in life, even the shortest delays can jeopardize the health of these vulnerable children. The introduction of EID point of care technology has the potential to accelerate diagnosis, facilitate earlier treatment initiation, and further reduce mortality 22.
We found, not surprisingly, that ART was highly effective, reducing the risk of death 4-fold. Unfortunately, despite the availability of staff to help families access services, not all children started treatment. Initiation rates were notably low among those who died (71.2%) and were lost-to-follow-up (52.8%). A quarter of the children lost to study transferred care and equally as many were reported alive when families were contacted but we believe that some proportion of children for whom we have no information likely died. Braitstein et al selected a random sample of HIV-infected children lost-to-follow-up in Kenya for home tracing23. Health workers located 36(82%) of 44 children: 7(16%) were reported dead.
Innes et al. described disease status among 403 South African infants identified 2007-201024. Treatment was initiated at a median age of 8.4 months and 62% had advanced disease at treatment start. In this cohort many had advanced disease at diagnosis 24 and, indicators of disease progression were associated with mortality: diagnosis during hospitalization, low CD4, and poor growth. Neither child age nor viral load was predictive of mortality when other factors were considered. Some children with advanced disease likely died before treatment initiation or were too compromised to respond. In Francistown, Botswana, 79 of 202 HIV-infected infants died, 56 prior to ART initiation and 23 on ART with most deaths attributed to pneumonia, sepsis and gastroenteritis25. Wagner et al, reporting outcomes of 99 early diagnosed infants in Kenya, 2005- 2007, found that children diagnosed during hospital admissions were three times more likely to die compared with those diagnosed through PMTCT programs, not dissimilar to our findings five years later 26. Other investigators have also demonstrated high mortality risk among young treated and untreated children27,28, 29.
We also delineate a relationship between maternal health and health seeking behaviors with child health outcomes. We found that children born to women with low CD4 (≤350 cells/mm3) who were not on treatment were at substantially higher risk for mortality compared to healthier women. The association between maternal and child health outcomes has been described in perinatal HIV: poor maternal health confers increased risk of MTCT, infant disease progression and death30-33. These women were eligible for ART but either did not engage in care or were not initiated on ART. In an analysis of PMTCT uptake among mothers in this cohort, we found that among women enrolled in PMTCT care only one in three meeting eligibility criteria were prescribed ART 34. Barriers to ART initiation under Option A, the PMTCT approach at the time of this study, have been well described35,36, 37.
We also found that children with any PMTCT exposure (maternal and/or infant antiretrovirals) had better outcomes than those with no PMTCT. This included reduced mortality as well as lower viral loads and higher CD4% at enrollment. We hypothesize that in utero and postnatal antiretrovirals may attenuate disease progression amongst infected infants. Antiretroviral drugs even when given in PMTCT regimens may act to lower maternal and infant viral load, thereby limiting establishment of the infant viral reservoir, lowering viral set point, and slowing disease progression. However, it is possible that non-biologic explanations may also play a role.
Our findings provide further support for the addition of diagnostic testing at delivery into the testing algorithm for EID. The majority of children in our study were identified outside of PMTCT programs. We have previously shown that failure to diagnose HIV infection during pregnancy was a primary reason for missing both PMTCT and EID34. Furthermore, MTCT among women who acquire HIV during pregnancy also contributes disproportionately to new pediatric infections18. In South Africa where the rate of institutional delivery is high, antibody testing of women with negative and/or unknown status coupled with diagnostic testing at birth of all HIV-exposed babies offers a safety net to new mothers, ensuring that they learn their diagnosis of HIV infection, and expedites the diagnosis of infected children. South Africa recently endorsed birth testing as national policy 38.
There are several limitations to our study. As we only enrolled children at five facilities in Johannesburg our findings may not be generalizable to children treated in other settings or to children born after the introduction of Option B+. However, we note that many HIV high burden countries in Sub-Saharan Africa, while endorsing the Option B+ approach, have yet to scale up both universal ART for pregnant women and well-functioning EID services for exposed infants.
Another limitation is that clinical outcomes could not be determined for 25% of children. This lost-to-follow-up rate is consistent with what is commonly reported in HIV service programs23,29. Finally, while we have one of the largest pediatric cohorts of young children diagnosed in a single calendar year, the sample size limited our ability to fully define the specific contributions of each maternal and infant factor.
Despite advances in prevention and treatment, infants with HIV infection remain at high risk for early death. Incomplete uptake and retention across the PMTCT cascade results in delayed diagnosis often only when the child is seriously ill. The implementation of Option B+ should improve maternal ART uptake but other measures such as diagnostic testing at birth will ensure timely diagnosis and successful treatment of those children who acquire HIV-infection.
Acknowledgments
The US President's Emergency Plan for AIDS Relief provided funding for this study (through the Eunice Kennedy Shriver National Institute of Child Health and Human Development—Supplement to HD 61255 Treatment Options for Protease Inhibitor Exposed Children). The reported clinical services that participants accessed were part of the South African government health care provision.
Footnotes
The authors report no conflicts of interest
References
- 1.Goga AE, Dinh TH, Jackson DJ, Lombard C, Delaney KP. First population-level effectiveness evaluation of a national programme to prevent HIV transmission from mother to child, South Africa. J Epidemiol Community Health. 2014;69(3):240–8. doi: 10.1136/jech-2014-204535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNICEF. Situation - Global Statistics Tables: Children & AIDS 2015 Statistical Update. [Accessed December 2, 2015]; http://www.childrenandaids.org/situation.
- 3.UNAIDS. How AIDS changed everything - MDG6: 15 years, 15 lessons of hope from the AIDS response. Geneva, Switzerland: UNAIDS; 2015. [Google Scholar]
- 4.Boulle A, Schomaker M, May MT, et al. Mortality in patients with HIV-1 infection starting antiretroviral therapy in South Africa, Europe, or North America: a collaborative analysis of prospective studies. PLoS medicine. 2014;11(9):e1001718. doi: 10.1371/journal.pmed.1001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson LF, Mossong J, Dorrington RE, et al. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS medicine. 2013;10(4):e1001418. doi: 10.1371/journal.pmed.1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mutevedzi PC, Newell ML. The changing face of the HIV epidemic in sub-Saharan Africa. Tropical medicine & international health : TM & IH. 2014;19(9):1015–28. doi: 10.1111/tmi.12344. [DOI] [PubMed] [Google Scholar]
- 7.Davies MA, May M, Bolton-Moore C, et al. Prognosis of children with HIV-1 infection starting antiretroviral therapy in Southern Africa: a collaborative analysis of treatment programs. The Pediatric infectious disease journal. 2014;33(6):608–16. doi: 10.1097/INF.0000000000000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerber KJ, Lawn JE, Johnson LF, et al. South African child deaths 1990-2011: have HIV services reversed the trend enough to meet Millennium Development Goal 4? AIDS (London, England) 2013;27(16):2637–48. doi: 10.1097/01.aids.0000432987.53271.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Department of Health., Republic of South Africa. Clinical Guidelines: PMTCT (Prevention of Mother-to-Child Transmission) Pretoria: National Department of Health; 2010. [Google Scholar]
- 10.National Department of Health. Policy and Guidelines for the Implementation of the PMTCT Program. 2008. Pretoria: National Department of Health; 2008. [Google Scholar]
- 11.National Department of Health., Republic of South Africa. The South African Antiretroviral Treatment Guidelines. Pretoria: National Department of Health; 2013. [Google Scholar]
- 12.Barron P, Pillay Y, Doherty T, et al. Eliminating mother-to-child HIV transmission in South Africa. Bulletin of the World Health Organization. 2013;91(1):70–4. doi: 10.2471/BLT.12.106807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherman GG, Lilian RR, Bhardwaj S, Candy S, Barron P. Laboratory information system data demonstrate successful implementation of the prevention of mother-to-child transmission programme in South Africa. South African medical journal. 2014;104(3 Suppl 1):235–8. doi: 10.7196/samj.7598. [DOI] [PubMed] [Google Scholar]
- 14.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. The New England journal of medicine. 2008;359(21):2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palumbo P, Lindsey JC, Hughes MD, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. The New England journal of medicine. 2010;363(16):1510–20. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Violari A, Lindsey JC, Hughes MD, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. The New England journal of medicine. 2012;366(25):2380–9. doi: 10.1056/NEJMoa1113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mofenson LM. Prevention of mother-to-child HIV transmission: can we meet the goal of global elimination of new pediatric infections? Current opinion in HIV and AIDS. 2013;8(5):443–6. doi: 10.1097/COH.0b013e328363d280. [DOI] [PubMed] [Google Scholar]
- 18.Dinh TH, Delaney KP, Goga A, et al. Impact of Maternal HIV Seroconversion during Pregnancy on Early Mother to Child Transmission of HIV (MTCT) Measured at 4-8 Weeks Postpartum in South Africa 2011-2012: A National Population-Based Evaluation. PloS one. 2015;10(5):e0125525. doi: 10.1371/journal.pone.0125525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet (London, England) 2004;364(9441):1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. The WHO Child Growth Standards. 2006 [Google Scholar]
- 21.Ghadrshenas A, Ben Amor Y, Chang J, et al. Improved access to early infant diagnosis is a critical part of a child-centric prevention of mother-to-child transmission agenda. AIDS (London, England) 2013;27 Suppl 2:S197–205. doi: 10.1097/QAD.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 22.Essajee S, Vojnov L, Penazzato M, et al. Reducing mortality in HIV-infected infants and achieving the 90-90-90 target through innovative diagnosis approaches. Journal of the International AIDS Society. 2015;18(7 Suppl 6):20299. doi: 10.7448/IAS.18.7.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braitstein P, Songok J, Vreeman RC, et al. “Wamepotea” (they have become lost): outcomes of HIV-positive and HIV-exposed children lost to follow-up from a large HIV treatment program in western Kenya. Journal of acquired immune deficiency syndromes (1999) 2011;57(3):e40–6. doi: 10.1097/QAI.0b013e3182167f0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Innes S, Lazarus E, Otwombe K, et al. Early severe HIV disease precedes early antiretroviral therapy in infants: Are we too late? Journal of the International AIDS Society. 2014;17:18914. doi: 10.7448/IAS.17.1.18914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motswere-Chirwa C, Voetsch A, Lu L, et al. Follow-up of infants diagnosed with HIV - Early Infant Diagnosis Program, Francistown, Botswana, 2005-2012. MMWR Morbidity and mortality weekly report. 2014;63(7):158–60. [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner A, Slyker J, Langat A, et al. High mortality in HIV-infected children diagnosed in hospital underscores need for faster diagnostic turnaround time in prevention of mother-to-child transmission of HIV (PMTCT) programs. BMC pediatrics. 2015;15:10. doi: 10.1186/s12887-015-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lilian RR, Kalk E, Technau KG, Sherman GG. Birth diagnosis of HIV infection in infants to reduce infant mortality and monitor for elimination of mother-to-child transmission. The Pediatric infectious disease journal. 2013;32(10):1080–5. doi: 10.1097/INF.0b013e318290622e. [DOI] [PubMed] [Google Scholar]
- 28.Nuwagaba-Biribonwoha H, Wang C, Kilama B, et al. Implementation of antiretroviral therapy guidelines for under-five children in Tanzania: translating recommendations into practice. Journal of the International AIDS Society. 2015;18:20303. doi: 10.7448/IAS.18.1.20303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNairy ML, Lamb MR, Carter RJ, et al. Retention of HIV-infected children on antiretroviral treatment in HIV care and treatment programs in Kenya, Mozambique, Rwanda, and Tanzania. Journal of acquired immune deficiency syndromes (1999) 2013;62(3):e70–81. doi: 10.1097/QAI.0b013e318278bcb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia PM, Kalish LA, Pitt J, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group The New England journal of medicine. 1999;341(6):394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- 31.Abrams EJ, Wiener J, Carter R, et al. Maternal health factors and early pediatric antiretroviral therapy influence the rate of perinatal HIV-1 disease progression in children. AIDS (London, England) 2003;17(6):867–77. doi: 10.1097/00002030-200304110-00012. [DOI] [PubMed] [Google Scholar]
- 32.Shearer WT, Quinn TC, LaRussa P, et al. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. Women and Infants Transmission Study Group. The New England journal of medicine. 1997;336(19):1337–42. doi: 10.1056/NEJM199705083361901. [DOI] [PubMed] [Google Scholar]
- 33.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Mwiya M, Thea DM. Potential impact of new WHO criteria for antiretroviral treatment for prevention of mother-to- child HIV transmission. AIDS (London, England) 2010;24(9):1374–7. [PMC free article] [PubMed] [Google Scholar]
- 34.Technau KG, Kalk E, Coovadia A, et al. Timing of maternal HIV testing and uptake of prevention of mother-to-child transmission interventions among women and their infected infants in Johannesburg, South Africa. Journal of acquired immune deficiency syndromes (1999) 2014;65(5):e170–8. doi: 10.1097/QAI.0000000000000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gourlay A, Birdthistle I, Mburu G, Iorpenda K, Wringe A. Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: a systematic review. Journal of the International AIDS Society. 2013;16:18588. doi: 10.7448/IAS.16.1.18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abrams EJ, Myer L. Can we achieve an AIDS-free generation? Perspectives on the global campaign to eliminate new pediatric HIV infections. Journal of acquired immune deficiency syndromes (1999) 2013;63 Suppl 2:S208–12. doi: 10.1097/QAI.0b013e3182986f55. [DOI] [PubMed] [Google Scholar]
- 37.Myer L, Phillips T, Manuelli V, McIntyre J, Bekker LG, Abrams EJ. Evolution of antiretroviral therapy services for HIV-infected pregnant women in Cape Town, South Africa. Journal of acquired immune deficiency syndromes (1999) 2015;69(2):e57–e65. doi: 10.1097/QAI.0000000000000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Department of Health, Republic of South Africa. National Consolidated Guidelines for the Prevention of Mother-to-Child Transmission of HIV (PMTCT) and the Management of HIV in Children, Adolescents and Adults. Pretoria: National Department of Health; 2015. [Google Scholar]