Abstract
Objective
The surgical transfer of skin, fat, and/or muscle from a donor site to a recipient site within the same patient, is a widely performed procedure in reconstructive surgeries. A surgical pre-treatment strategy that is intended to increase perfusion in the flap, termed “flap delay”, is a commonly employed technique by plastic surgeons prior to flap transplantation. Here, we explored whether CD68+/CD206+ macrophages are required for arteriogenesis within the flap by performing gain-of-function and loss-of-function studies in a previously published flap delay murine model.
Methods and Results
Local injection of M2-polarized macrophages into the flap resulted in an increase in collateral vessel diameter. Application of a thin biomaterial film loaded with a pharmacological agent (FTY720), which has been previously shown to recruit CD68+/CD206+ macrophages to remodeling tissue, increased CD68+/CD206+ cell recruitment and collateral vessel enlargement. Conversely, when local macrophage populations were depleted within the inguinal fat pad via clodronate liposome delivery, we observed fewer CD68+ cells accompanied by diminished collateral vessel enlargement.
Conclusions
Our study underscores the importance of macrophages during microvascular adaptations that are induced by flap delay. These studies suggest a mechanism for a translatable therapeutic target that may be used to enhance the clinical flap delay procedure.
Keywords: arteriogenesis, macrophage, adipose, collateral vessel
INTRODUCTION
Microvascular growth and remodeling are adaptive processes that have been widely studied in skeletal muscle5,13,16, heart40, and brain18, among many other organs. Arteriogenesis and angiogenesis represent two compensatory mechanisms that are crucial to restore proper blood flow and maintain tissue health in response to biomechanical and biochemical cues. Arteriogenesis is the process by which collateral vessels increase their diameter19,21,41, while angiogenesis is the formation of new capillaries from pre-existing blood vessels9,51,57. Far less is known about these microvascular remodeling responses in adipose tissue, despite the fact that plastic surgeons have attempted to invoke these microvascular adaptations using a surgical technique called “flap delay”26,34, which is intended to improve autologous soft tissue, or adipose flap, vascularization. Flap delay involves the ligation of the main vascular pedicle into the flap 10–14 days prior to tissue transfer. It is believed that the induction of altered blood flow into the flap elicits a vascular remodeling response within the flap, which leads to better flap survival following transplantation.
Previously, we published a murine model of flap delay51 and showed that the enlargement of pre-existing collateral vessels (arteriogenesis) predominated over angiogenesis in the inguinal fat pad following ligation of the main feeding artery. Moreover, arteriogenesis was accompanied by recruitment and proliferation of anti-inflammatory, CD68+/CD206+ macrophages43 (historically designated as “M2” macrophages24,31,33). In order to evaluate the necessity for CD68+/CD206+ macrophages in arteriogenesis during flap delay, we conducted a series of gain-of-function and loss-of-function studies using our published murine model of flap delay by altering macrophage populations within the inguinal fat pad via: 1) delivery of exogenous macrophages via injection into the inguinal fat pad (gain-of-function), 2) pharmacological treatment with locally delivered FTY720, a S1P receptor-1 and -3 agonist (gain-of-function), which has previously been shown to elevate numbers of M2 macrophages and enhance collateral vessel diameter enlargement in skeletal muscle and skin2, and 3) delivery of clodronate liposomes, which has previously been shown to deplete circulating monocytes and tissue-resident macrophages11,17,38,52 (loss-of-function).
MATERIALS AND METHODS
Bone marrow isolation for macrophage injection study
All animal procedures were approved by the University of Virginia Institutional Animal Care and Use Committee. We followed a previously published protocol24 to isolate bone marrow from four twelve week-old male C57BL/6 mice (Charles River Laboratories, Wilmington, MA). Briefly, mice were euthanized via carbon dioxide asphyxiation and the skin of the back legs was removed using sterile iris scissors. Muscle, fat, and fascia surrounding the fibula, tibia, and femur were removed with sterile forceps and No. 11 scalpel blades. After removing the majority of muscle, fat, and fascia, the back legs were excised from the animal and stored in a sterile container containing PBS. Under a biosafety cabinet, the femur was dissociated from the tibia/fibula at the knee joint and complete removal of the fascia, fat, and muscle was accomplished using a No. 11 scalpel blade while submerged in sterile PBS. After the bones (tibia/fibula and femur) were void of surrounding tissue, the ends of the bones were cut with sterile iris scissor to expose the bone marrow compartment. A 23-gauge needle was used to poke two holes in the bottom of sterile 200 microliter PCR tubes. Open-ended bones (one fibula and one femur) were placed in the PCR tubes and closed and these closed PCR tubes were placed into sterile two milliliter microcentrifuge tubes (two total tubes per mouse). The tubes were spun at 3000 RPM in a microcentrifuge for 10 seconds to extract the bone marrow compartment via centrifugal force. Successful extraction was confirmed by observation of a dark red pellet at the bottom of the two milliliter microcentrifuge tube and bones that were no longer red in color. Repeated centrifugation was necessary in some trials to entirely extract bone marrow. The bone marrow pellet was resuspended in one milliliter of erythrocyte lysis buffer (eBioscience, San Diego, CA) and incubated at room temperature for eight minutes. After incubation, samples from each mouse were combined into a 15 milliliter centrifuge tube and spun at 1100 RPMs for five minutes. The supernatant was discarded, and the pellet from one mouse was suspended in 50 milliliters of media (RPMI media with 1% penicillin/streptomycin, 20 mM HEPES, 10% fetal bovine serum, and 10% L929 cell conditioned media). Ten milliliters of cell suspension were added to each 100 mm cell culture dish (total of 5 dishes per mouse), which were placed in an incubator that maintained 37°C and 5% CO2.
Culture of bone marrow-derived macrophages
Bone marrow-derived cells were inspected daily for contamination, and the cell culture media (RPMI media with 1% penicillin/streptomycin, 20 mM HEPES, 10% fetal bovine serum, and 10% L929 cell conditioned media) was changed every four days. The bone marrow cells were differentiated into macrophages after seven days in culture following a previously published protocol24. After seven days of differentiation in the L929 conditioned media, the media was switched to RPMI media without L929 conditioned media (1% penicillin/streptomycin, 20 mM HEPES, 10% fetal bovine serum). Cells were maintained in culture in this RPMI media for no more than two weeks and were not passaged.
Differentiation of macrophages into M2 phenotype
Media was supplemented with IL-4 (R&D Systems, Minneapolis, MN) to polarize bone marrow derived macrophages (passage zero) into the M2 phenotype24. Ten microliters of 10 µg/mL IL-4 was added to ten milliliters of fresh RPMI media (without L929 conditioned media) for a final concentration of IL-4 of 10 ng/mL. Spent media was removed, and the IL-4 containing media was added and incubated for 24 hours prior to use of the cells.
Vybrant DiI labeling of M2 macrophages prior to injection
After polarization with IL-4 supplemented media, the cells were labeled with Vybrant DiI cell-labeling solution (ThermoFisher Scientific, Waltham, MA) in order to visualize the cells using confocal microscopy after injection into the inguinal fat pad. Cells were removed from dishes with a 15 minute incubation of Accutase (Innovative Cell Technologies, San Diego, CA) and resuspended in RPMI media (without L929 conditioned media) at a concentration of one million cells per milliliter. Five microliters of Vybrant DiI cell-labeling solution per milliliter of cell suspension was added directly to the suspension and mixed well via gentle pipetting. The cells were incubated on ice for 15 minutes protected from light. Cells were centrifuged at 1100 RPM for five minutes and washed with sterile PBS. This process was repeated three times for a total of three PBS washes and centrifugations. After the final centrifugation, the supernatant was discarded and cells were suspended at a final concentration of 300,000 cells/mL.
Injection of Vybrant DiI labeled and unlabeled cells into inguinal fat pad
Wild type C57BL/6 mice were anesthetized and a one-centimeter incision was made over the left and right epigastric artery. The collateral vessel entering the inguinal fat pad was found on both sides by undermining fascia and surrounding adipose tissue. Please see our previously published study43 for an anatomical schematic of the inguinal fat pad and collateral vessel. Using a 27G syringe (BD Soloshot™, Franklin Lakes, NJ), 100 microliters of the DiI-labeled cell solution (300,000 cells/mL) was injected directly into the left inguinal fat pad. 100 microliters of unlabeled cells (no Vybrant DiI labeling, 300,000 cells/mL) were injected directly in the right inguinal fat pad. Incisions were sutured closed with 8-0 nylon suture (Ethicon, Somerville, NJ).
Harvest and immunostaining of adipose tissue following direct cell injection
Mice were euthanized via CO2 asphyxiation three days post-injection to quantify the retention of injected Vybrant DiI labeled cells and the total number of macrophages present post injection. The area of the inguinal fat pad surrounding the collateral vessel was harvested as previously described43. Samples of the tissues that had been injected with Vybrant DiI labeled cells from each mouse (n = 3) were divided into three, small (~1 mm3) pieces for immunostaining. All three samples were submerged in 50 microliters of 4% PFA solution and incubated overnight at 4°C protected from light. All samples were washed with PBS three times. Sample 1 was mounted on a gelatin-coated slide and imaged immediately after PBS washing as described previously43. Samples 2 and 3 were submerged in 100 microliters of 5% mouse serum in 0.3% (volume/volume) Triton X-100/PBS for 3 hours at room temperature to permeabilize and block the tissue. The permeabilization and blocking solution was removed. Sample 2 was submerged in 100 microliters of Alexa Fluor 488 conjugated isolectin (1:300, GS-IB4, ThermoFisher Scientific, Waltham, MA) and Alexa Fluor 647 anti-mouse CD68 (1:200, Clone FA-11, AbD Serotec, Raleigh, NC) diluted in 5% mouse serum in 0.3% Triton X-100/PBS. Sample 3 was submerged in 100 microliters of Alexa Fluor 488 conjugated isolectin (1:300, GS-IB4, ThermoFisher Scientific, Waltham, MA) and Alexa Fluor 647 anti-mouse CD206 (1:200, clone C068C2, BioLegend, San Diego, CA) diluted in 5% mouse serum in 0.3% Triton X-100/PBS. Samples 2 and 3 were incubated overnight on a rocker at 4°C protected from light. Samples were washed five times for five minutes each wash with 0.3% Triton X-100/PBS and then mounted on gelatin-coated slide. Samples were imaged using a Nikon TE 2000-E2 microscope equipped with a Melles Griot Argon Laser System and a Nikon D-Eclipse C1 confocal attachment (Nikon Instruments, Melville, NY). Four unique FOVs were used for macrophage quantification.
Inguinal fat pad ligation surgery
A previously published murine model of flap delay43 was used for the following “ligated” and “sham” surgeries. Briefly, the epigastric artery, which feeds the subcutaneous fat pad, was isolated via blunt dissection. In “ligated” surgeries, the epigastric artery was severed following suture ligation. In contralateral “sham” surgeries, a 10-0 nylon suture (Ethicon, Somerville, NJ) was simply passed underneath the epigastric artery but the artery was not severed. Samples of the inguinal fat pad were harvested and immunofluorescently labeled at specific time points designated below. For a more thorough description of the model used please see our previous study43.
Collateral vessel harvest and diameter quantification
Collateral vessel diameters were quantified as previously described43. Briefly, collateral vessels entering the inguinal fat pad were carefully removed to maintain structural integrity. After removal, vessels were maximally dilated using an adenosine solution and immediately fixed with 4% PFA. Tissue samples were stained with monoclonal anti-actin, α-smooth muscle Cy3 to visualize arterioles and quantify arteriole diameter. Confocal microscopy was used to acquire images of the feeding branch into the inguinal fat pad, the first branch in the fat pad, and the second branch in the fat pad. ImageJ was used to calculate vessel diameters at each of these branch points. For a more detailed protocol of this process, please see our previous study43.
Injection of Vybrant DiI labeled cells during ligation surgery
In a separate study, ligation surgeries were performed on the left and right side of C57BL/6 mice as described above and previously43. Immediately after severing the epigastric artery on the left side, 30,000 Vybrant DiI labeled M2-polarized cells (100 microliters, concentration of 300,000 cells/mL) were injected directly into the inguinal fat pad surrounding the area where the collateral vessel enters. To serve as a vehicle control for the same mouse, 100 microliters of sterile PBS was injected directly into the right inguinal fat pad where the collateral vessel enters immediately after severing the epigastric artery. Incisions on both sides were closed with 8-0 nylon suture (Ethicon, Somerville, NJ). This procedure was repeated in three mice (n = 3).
Harvest and immunostaining of collateral vessel and surrounding adipose tissue after ligation and DiI labeled cell injection
Collateral vessels and the surrounding adipose tissue was harvested three days post-surgery and stained for α-smooth muscle actin FITC (1:200, clone 1A4, Sigma, St. Louis, MO), and Alexa Fluor 647 anti-mouse CD206 (1:200, clone C068C2, BioLegend, San Diego, CA) as previously described43. Vessel diameters were quantified for the feeding vessel, the first branch, and the second branch, and CD206+ cells were quantified as previously described43. Data presented include vessel diameter measurements of the feeding collateral vessel, the first branch of the collateral vessel, and the second branch of the collateral vessel. Three mice were repeated in three mice (n = 3).
Fabrication of FTY720 loaded and unloaded PLAGA films
A previously published solvent-casting technique was used to fabricate PLAGA thin films loaded with FTY7202. Briefly, 350 mg of PLAGA (50:50 lactide:glycolide, 0.59 dL/g inherent viscosity, Sigma, St. Louis, MO) was mixed with 2 mL of dichloromethane in a glass scintillation vial until the polymer was completely dissolved. For drug loaded thin films, 1.75 mg of FTY720 (Cayman Chemical, Ann Arbor, MI) was mixed into the polymer/solvent solution prior to casting. For unloaded thin films, this step was omitted. The polymer/solvent/drug solution was quickly poured into a Bytac Teflon lined P35 glass Petri dish. The Petri dish was sealed with Parafilm and was stored and allowed to dry at −20°C for seven days. After drying, films were stored at room temperature in a desiccator until ready to use.
Film preparation for in vivo use
Prior to implantation into mice, the PLAGA thin films were sterilized. Thin films were extracted using a one millimeter biopsy punch (Integra, Plainsboro, NJ) and had an approximate area of 0.785 mm2. Films were soaked in 70% ethanol for 30 seconds followed by a soak in sterile PBS for 30 seconds. It is important to note that complete degradation of PLAGA films this size takes several weeks and significant degradation will not occur during our three day studies45,54.
Inguinal ligation and sham surgeries and thin film placement
Ligation surgeries were performed on left and right side of C57BL/6 mice as described above and previously43. Immediately after severing the epigastric artery, a one millimeter FTY720-loaded or unloaded PLAGA thin film was placed immediately adjacent to the collateral vessel entering the inguinal fat pad. The thin film was placed underneath the fascial layer so as to minimize displacement away from the inguinal fat pad and region of interest (Figure 4A,B). The left side of the mouse had the FTY720 loaded PLAGA film while the right side had an unloaded PLAGA film. Sham surgeries were performed on separate mice on both the left and right side of C57BL/6 mice. Again, the left side had the drug loaded thin film and the right side had the unloaded thin polymer film. Incisions were closed with 8-0 nylon suture (Ethicon, Somerville, NJ). The process described above was also repeated with sham surgeries on the left and right side in a separate set of experiments. These procedures were repeated in three mice (n = 3).
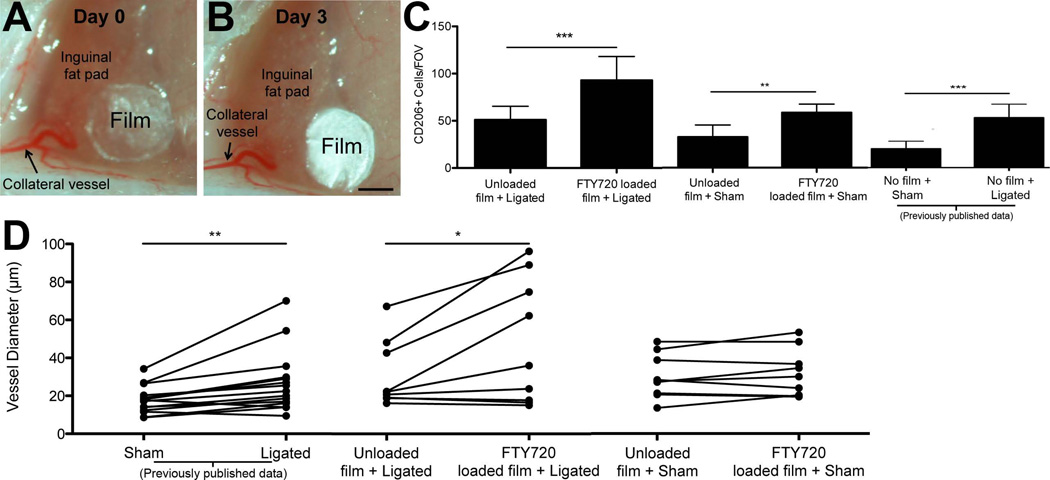
Figure 4. FTY720 delivery to inguinal fat pad.
FTY720-loaded PLAGA films were applied to inguinal fat pads, resulting in enhanced arteriogenesis. A.) Representative images of PLAGA film placement during ligation surgery and (B.) three days post-surgery. Scale bar = 500 µm C.) Quantification of CD206+ cells in unloaded and FTY720-loaded PLAGA films three days after sham or ligation surgery. Quantification reveals a significant increase in the number of CD206+ cells in ligated tissue treated with FTY720-loaded films vs. unloaded films. After sham surgery, a significant increase in the number of CD206+ cells was observed in FTY720 treated tissue. Student’s t-test, * = p-value < 0.05, ** = p-value < 0.01. *** = p-value < 0.001 D.) Individual diameter measurements for collateral vessels in inguinal fat pads treated with FTY720-loaded film were paired with corresponding branch diameter measurements for collateral vessels in fat pads treated with unloaded film (in the same mouse). A significant increase in the diameters of collateral vessels was observed in fat pads treated with FTY720-loaded film when compared to fat pads treated with unloaded films. No significant increase was seen in the sham surgery trials between FTY720-loaded and unloaded films. Sham and ligated data from a previously published study43 are presented for comparison purposes. Paired t-test, * = p-value < 0.05. n = 3 mice for all experiments, 4 unique FOVs for CD206+ cell quantification.
Harvest and immunostaining of collateral vessel and surrounding adipose tissue after ligation and PLAGA film placement
Collateral vessel and surrounding adipose tissue around the PLAGA thin film was harvested three days post-surgery and stained for Alexa Fluor 488 anti-mouse CD206 (1:200, Clone C068C2, BioLegend, San Diego, CA), α-smooth muscle actin Cy3 (1:200, Clone 1A4, Sigma, St. Louis, MO), and Alexa Fluor 647 anti-mouse CD68 (1:200, Clone FA-11, AbD Serotec, Raleigh, NC) as described above. Vessel diameters were quantified for the feeding vessel, the first branch, and the second branch, and CD206+ cells were quantified. Data presented include vessel diameter measurements of the feeding collateral vessel, the first branch of the collateral vessel, and the second branch of the collateral vessel.
Injection of clodronate and control liposomes into inguinal fat pad
To assess the efficacy of clodronate liposome-mediated depletion of macrophages within the inguinal fat pad, we performed direct injection of clodronate loaded liposomes and control liposomes (PBS-loaded liposomes) into the inguinal fat pad. A one centimeter incision was made above the epigastric artery and blunt dissection was used to locate the collateral vessel entering the side of the inguinal fat pad. Using a 27G syringe (BD Soloshot™ 301793, Franklin Lakes, NJ), 100 microliters of clodronate-loaded liposomes and control liposomes (ClodronateLiposomes.org, Haarlem, The Netherlands) were injected directly into the inguinal fat pad at the insertion site of the collateral vessel. Successful injection was confirmed by visual observation of a bubble formed within the inguinal fat pad. Clodronate liposomes were injected into the right inguinal fat pad of the mouse, and control liposomes were injected into the left inguinal fat pad. After injection the incision was closed with 8-0 nylon suture (Ethicon).
Immunostaining of adipose tissue and macrophage quantification following liposome injection
To determine the temporal effect of clodronate liposome injection on macrophage depletion, adipose tissue surrounding the collateral vessel was harvested as described previously43 at 6 hours, 12 hours, and 24 hours for three mice per time point. Harvested adipose tissue was stained with Alexa Fluor 568 conjugated isolectin (1:300, GS-IB4 ThermoFisher Scientific, Waltham, MA) and Alexa Fluor 647 anti-mouse CD68 (1:200) as described above. Three unique FOVs were acquired for each sample as 40 µm z-stacks and the number of CD68+ cells were quantified using ImageJ to assess macrophage depletion.
Ligation of epigastric artery coupled with liposome injections
After discerning the temporal effect of clodronate liposomes, we performed a separate set of experiments to evaluate the effect of macrophage depletion on the vascular remodeling observed within the inguinal fat pad. Briefly, following ligation of the epigastric artery in C57BL/6 mice, 100 microliters of clodronate loaded liposomes (left side of mouse) or control liposomes (right side of mouse) were injected directly into the inguinal fat pad at the insertion site of the collateral vessel. Surgical incisions were closed with 8-0 nylon suture and mice were allowed to recover for three days post-surgery prior to tissue harvest. This procedure was repeated in three mice (n = 3).
Harvest and immunostaining of collateral vessel and surrounding adipose tissue after ligation and liposome injection
The collateral vessel was carefully removed to retain structural integrity in order to evaluate vessel diameter. Adipose tissue surrounding the collateral vessel was also harvested during the removal process and was used for macrophage quantification. Harvested collateral vessels and surrounding adipose tissue samples were stained concurrently with α-smooth muscle actin Cy3 (1:200, Clone 1A4, Sigma, St. Louis, MO) and Alexa Fluor 647 anti-mouse CD68 (1:200, Clone FA-11, AbD Serotec, Raleigh, NC) as described previously43. Four unique FOVs were acquired for each sample as 40 µm z-stacks and the number of CD68+ cells were quantified using ImageJ. Data presented include vessel diameter measurements of the feeding collateral vessel, the first branch of the collateral vessel, and the second branch of the collateral vessel.
Statistical analysis
Student’s t-tests were used for statistical analyses between groups with statistical significance asserted at p-values < 0.05. All data are presented as average + standard deviation.
RESULTS
DiI labeling allows for visualization of M2-polarized macrophages
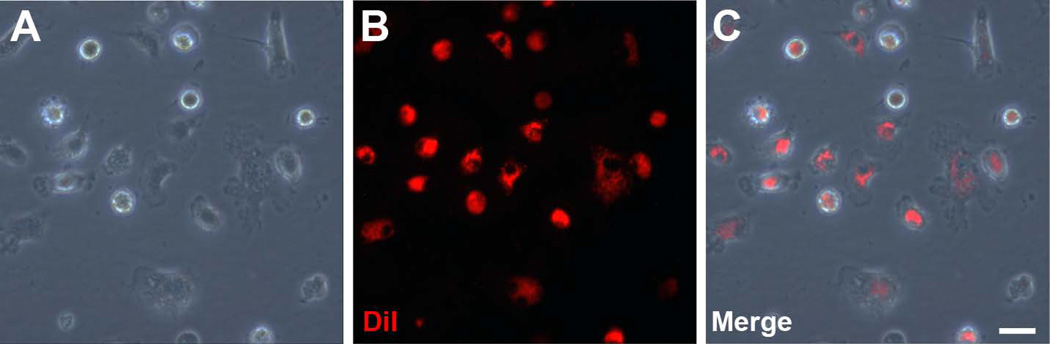
Murine bone marrow was harvested, and bone marrow-derived macrophages were cultured for seven days in macrophage differentiation media, as described above. After polarization to an M2 phenotype by IL-4 supplementation, cells were stained with DiI, and fluorescence microscopy was used to visually confirm successful staining. While in culture, the in vitro M2-polarized macrophages displayed the typical “fried egg” appearance (Figure 1A). Labeling the cells with DiI allowed for visualization of the cell membrane via florescence microscopy (Figure 1B). We observed near complete labeling of the culture bone marrow-derived cells with the DiI stain (Figure 1C).
Figure 1. Fluorescent labeling of bone marrow-derived macrophages.
In vitro culture of bone marrow-derived macrophages can be fluorescently labeled with DiI. A.) Bone marrow-derived macrophages display prototypical “fried egg” morphology in culture. B.) DiI, a membrane dye, labels macrophages prior to injection. C.) Nearly all bone marrow-derived macrophages are labeled with DiI. Scale bar = 50 µm.
DiI labeled cells remain in the inguinal fat pad three days post-injection
To assess the retention capability of the directly injected DiI-labeled macrophages, we injected 30,000 labeled cells into the inguinal fat pad at the site where the collateral vessel enters. On the contralateral side, we injected unlabeled macrophages to serve as a fluorescence control. We aimed to determine if the injected cells were retained in the tissue for three days post-injection, which is the time frame during which we observed the arteriogenic remodeling response in our previous study43. Inguinal fat pad tissue that had been injected with either unlabeled cells or DiI-labeled cells was excised three days post-injection. After fixation with 4% PFA and without permeabilizing the tissue (Sample 1 above), we mounted the tissue and visualized a substantial number of DiI+ cells that had been retained in the region of interest. We did not observe any fluorescence signal in the tissues that were injected with unlabeled cells (Figure 2A), which indicates that the fluorescent signal observed in the tissue injected with DiI-labeled cells (Figure 2B) was generated by the injected cells.
Figure 2. In vivo injection of bone marrow-derived macrophages.
Fluorescent immunohistochemical labeling reveals an increase in CD68+ and CD206+ cells in inguinal fat pads injected with DiI labeled, M2-polarized macrophages. A.) No fluorescent signal was detectable in inguinal fat pads that were injected with unlabeled cells (scale bar = 50 µm), whereas (B.) DiI labeled cells were visible in inguinal fat pads that were injected with DiI labeled, M2-polarized macrophages (scale bar = 50 µm). C.,D.) Fluorescent immunohistochemical labeling of inguinal fat pad revealed increase in CD68+ cells in (C) DiI+ cell injected tissue when compared to (D) uninjected tissue (scale bar = 20 µm). E.,F.) Fluorescent immunohistochemical labeling of inguinal fat pad revealed increase of CD206+ cells in (E) DiI+ cell injected tissue when compared to (F) uninjected tissue (scale bar = 20 µm) G.) Number of CD68+ cells in injected inguinal fat pads was increased compared to uninjected inguinal fat pads. n = 6 mice. Student’s t-test, * = p-value < 0.05. H.) Number of CD206+ cells was increased in injected inguinal fat pads when compared to uninjected inguinal fat pads. n = 5 mice. Student’s t-test, * = p-value < 0.05. Data are mean ± standard deviation.
DiI signal in injected macrophages is lost after tissue processing
We analyzed pieces of adipose tissue from cell-injected inguinal fat pads to determine if the cells that had been injected could be co-labeled with the macrophage markers CD68 and CD206 (Samples 2 and 3, respectively). This co-labeling of injected cells with macrophage markers (CD68 and CD206) would allow us to visualize if the injected DiI-labeled cells were still expressing surface markers indicative of macrophage phenotypes in vivo. Prior to staining with macrophage antibodies, a permeabilization step was required to facilitate antibody binding within the adipose tissue. Although we did not observe DiI signal (red channel) in tissues that had been injected with DiI-labeled macrophages, we observed robust CD68, CD206, and lectin staining in these tissues (Figure 2C–F). The lack of DiI signal in the tissue may have been due to the permeabilization step and the ensuing egress of the DiI dye from the cellular membrane49. Nonetheless, the fact that we observed many DiI+ cells within tissues that were not subjected to permeabilization and immunostaining supports the presence of DiI-labeled cells in these immunostained tissues.
Number of CD68+ and CD206+ cells is elevated in macrophage-injected tissue without ligation surgery
We quantified the number of CD68+ and CD206+ cells in cell-injected and uninjected tissue to determine if injection of in vitro differentiated macrophages increased the total number of macrophages three days post-injection. We observed an increase in both CD68+ cells and CD206+ cells in injected inguinal fat pads when compared to uninjected (PBS-injected) inguinal fat pads (Figure 2C&D, E&F,G&H).
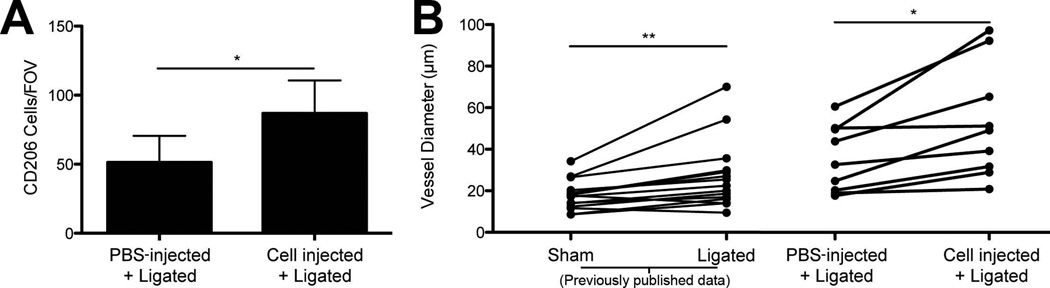
Injection of M2 macrophages increases CD206+ cell counts and collateral vessel diameters within inguinal fat pad after ligation surgery
Immediately after the main feeding arterioles to both the left and right inguinal fat pads were ligated, 30,000 M2-polarized macrophages (100 microliters) were injected into the left inguinal fat pad, while 100 microliters of sterile PBS was injected into the right inguinal fat pad. Three days after ligation surgery, we observed a significant increase in the number of CD206+ cells in the tissue injected with M2-polarized cells when compared to PBS-injected fat pads (p-value < 0.05) (Figure 3A). Within cell-injected fat pads, we counted an average of 87 ± 23 (S.D.) CD206+ cells, while in PBS-injected fat pads we counted an average of 51 ± 10 (S.D.) CD206+ cells (Figure 3A). Of note, the CD206+ cell counts within PBS-injected fat pads were in close agreement with CD206+ cell counts observed in our previous ligation surgeries with no injections43. The retention of the DiI dye taken together with the injected cell retention studies described above, the fact that CD206+ cell counts were elevated in the fat pads that were injected with M2-polarized macrophages suggests that injected M2-polarized macrophages are retained within the fat pad three days after the ligation surgery.
Figure 3. Injection of bone marrow-derived macrophages into inguinal fat pad.
Injection of bone marrow-derived macrophages increased the number of CD206+ cells and increased collateral vessel diameters when compared to PBS-injected inguinal fat pads. A.) Quantification of the number of CD206+ cells/FOV. Student’s t-test, * = p-value < 0.05. Data are mean + standard deviation. B.) Individual diameter measurements for collateral vessels in PBS-injected tissues were paired with corresponding collateral branch diameter measurements in cell-injected tissues (in the same mouse). Collateral vessels in fat pads receiving cell injections had increased diameters when compared to corresponding collateral vessels in PBS-injected fat pads. Data from fat pads experiencing either sham surgery or ligation surgery (without PBS or cell injection) from a previously published study43 are presented for comparison purposes. Paired t-test, ** = p-value < 0.01, * = p-value < 0.05. n = 3 mice for all experiments, 4 unique FOVs for CD206+ cell quantification.
We also quantified collateral vessel diameters and observed a significant diameter increase upon pairing each vessel branch with its corresponding branch in the contralateral fat pad, whose feeding artery also underwent surgical ligation but received vehicle control (PBS) injection instead of M2-polarized macrophages (Figure 3B). In comparing these data to our previously published data43, we observed similar collateral vessel diameters for PBS-injected tissues and tissues that had been ligated but received no injection (Figure 3B). Relative to PBS-injected tissues or tissues that did not receive any injection following ligation, direct injection of M2-polarized macrophages in the inguinal fat pad led to an enhanced enlargement of collateral vessel diameters following ligation surgery (Figure 3B).
FTY720-loaded and unloaded films remain in place post-implantation
We confirmed that the FTY720-loaded thin film remained in place after it was surgically placed in the inguinal fat pad. A thin fascial layer is present above the mouse inguinal fat pad, and this connective tissue structure was used to hold the thin film in place, as described in the methods. After three days post-implantation, the film was located within a few microns of where it was implanted (Figure 4A,B) in all mice receiving this treatment.
FTY720-loaded thin films increase CD206+ cell recruitment and enhance collateral vessel enlargement in ligated tissue
FTY720-loaded thin films significantly increased the number of CD206+ cells in the ligated tissue compared to unloaded films three days after ligation surgery (p-value < 0.001). FTY720-loaded films had an average of 93 ± 25 (S.D.) CD206+ cells/FOV, while unloaded films had an average of 51 ± 14 (S.D.) CD206+ cells/FOV (Figure 4C). In comparison to our prior ligation study with no implanted film43, the unloaded films had approximately the same number of CD206+ cells/FOV, suggesting that the unloaded film does not alter the CD206+ cell recruitment (Figure 4C). The FTY720-loaded films had a much greater number of CD206+ cells/FOV compared to our prior study (93 vs. 45 CD206+ cells/FOV). Taken together, these data suggest that FTY720 aids in the recruitment of CD206+ macrophages to the remodeling fat pad, similar to what has been observed in skeletal muscle2.
Comparing collateral vessels of inguinal fat pads treated with unloaded versus FTY720-loaded films revealed that FTY720-loaded films significantly increased collateral vessel diameters following ligation surgeries (Figure 4D). Data from our previous study showed an increase in the diameter of collateral vessels in ligated fat pads when compared to sham-treated fat pads, indicative of arteriogenesis in the collateral vessel in response to the ligation surgery (Figure 4B). Importantly, the diameters of the collateral vessels in the ligated tissues that were treated with unloaded film were not significantly different than those that were ligated but did not receive any film, suggesting that the thin fllm, by itself, did not significantly affect collateral vessel remodeling (Figure 4D).
As another control study, FTY720-loaded or unloaded thin films were applied to the inguinal fat pad immediately following sham surgeries. Collateral vessel diameters in inguinal fat pads that were treated with FTY720-loaded or unloaded films following the sham procedure were statistically similar to one another (Figure 4D), despite the fact that the number of CD206+ cells in FTY720-loaded tissue (average 59 ± 9 (S.D.) CD206+ cells/FOV) was elevated compared to those measured in tissues treated with unloaded films (33 ± 13 (S.D.) CD206+ cells/FOV) (p-value < 0.05) (Figure 4C). These data suggest that while FTY720 recruits CD206+ cells to the tissue in the absence of ligation, collateral vessel enlargement is contingent on ligation.
Clodronate liposomes deplete local macrophage populations 24 hours after injection, reduce CD68+ cell counts after ligation surgery, and restrict collateral vessel diameter enlargement following ligation surgeries
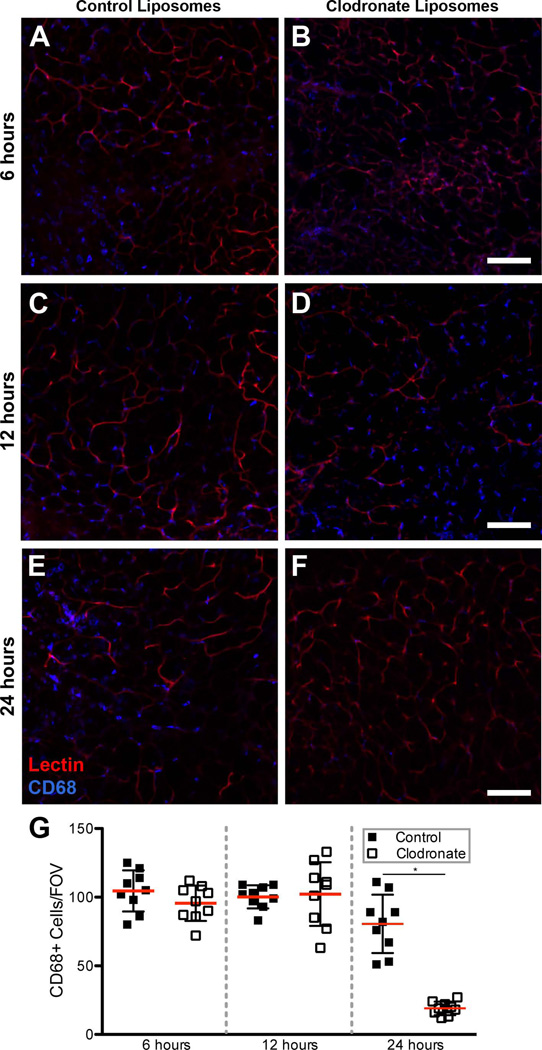
Following injection of either clodronate-loaded liposomes or control liposomes, we quantified the number of CD68+ cells within the region of interest surrounding the collateral vessel in the unoperated inguinal fat pad. After 6 hours and 12 hours, there was no significant difference in the number of CD68+ cells/FOV between control liposomes and clodronate liposomes (Figure 5A–D,G). Twenty-four hours after injection of clodronate liposomes, we observed a reduced number of CD68+ cells in the tissue relative to tissue injected with control liposomes (Figure 5E,F). The number of CD68+ cells/FOV was significantly reduced to an average of 19 ± 5 (S.D.) CD68+ cells/FOV when compared to control liposomes average of 80 ± 19 (S.D.) CD68+ cells/FOV (Figure 5G). After six and twelve hours, there was no significant difference in the number of CD68+ cells between control and clodronate liposome treated inguinal fat pads suggesting that clodronate liposome macrophage depletion is time-dependent. Taken together, these data suggest that clodronate liposome injection significantly reduces the CD68+ macrophage population in the inguinal fat pad, and that control liposomes do not significantly affect CD68+ macrophage counts.
Figure 5. Clodronate depletion of CD68+ macrophages within inguinal fat pad.
Direct injection of clodronate liposomes into the inguinal fat pad significantly depleted local CD68+ macrophage populations after 24 hours. A.,B.) Representative image of control liposome-injected inguinal fat pad (A) and clodronate liposome-injected inguinal fat pad (B) at 6 hours. C.,D.) Representative image of control liposome-injected inguinal fat pad (C) and clodronate liposome-injected inguinal fat pad (D) at 12 hours. E.,F.) Representative image of control liposome-injected inguinal fat pad (E) and clodronate liposome-injected inguinal fat pad (F) at 6 hours. G.) Quantification of CD68+ cells at 6 hours, 12 hours, and 24 hours following either control liposome or clodronate liposome injection. A significant decrease in the number of CD68+ cells was observed 24 hours post-injection in clodronate liposome-injected inguinal fat pads (Student’s t-test, * = p-value < 0.05) relative to control liposome-injected fat pads. Scale bar = 100 µm, n = 3 mice for each time point, 3 unique FOVs for CD68+ cell quantification.
Tissue treated with either control or clodronate-loaded liposomes were harvested three days after the ligation surgery was performed. Fat pads that had been injected with clodronate liposomes experienced a significant reduction in the number of CD68+ cells (average 28 ± 7 (S.D.) CD68+ cells/FOV) when compared to control liposome-injected fat pads (average 90 ± 13 (S.D.) CD68+ cells/FOV) (p-value < 0.01) (Figure 6A). These data support that clodronate liposomes effectively reduce macrophage counts in the inguinal flap delay model.
Figure 6. Injection of clodronate liposomes within remodeling inguinal fat pad.
Clodronate liposomes depleted the local macrophage population after ligation surgery and affected the arteriogenic response of collateral vessels. A.) Quantification of CD68+ cells reveals a significant decrease in the number of CD68+ cells within clodronate liposome-treated tissue compared to control liposome-treated tissue. Student’s t-test, ** = p-value < 0.01 B.) Collateral vessel diameters in the clodronate liposome-injected tissues were paired with the diameter measurements from the corresponding collateral vessel branches in the control liposome-injected tissues in the same mouse. Collateral vessels in control liposome-injected fat pads had larger diameters compared to corresponding collateral vessels in clodronate liposome-injected fat pads. Sham and ligated data from a previously published study43 is presented for comparison purposes. Paired t-test, ** = p-value < 0.01. n = 3 mice for all experiments, 4 unique FOVs for CD68+ cell quantification.
The diameters of collateral vessels in clodronate liposome-treated, ligated fat pads were significantly less than those in the paired collateral vessel branches of control liposome-treated, ligated fat pads (Figure 6B). This macrophage depletion study suggests that macrophages play an important role in the enlargement of collateral vessel diameters, and that this enlargement may be restricted by depletion of the macrophage population within the adipose tissue.
Further, comparison between adipose tissues that only underwent surgical ligation of the feeding artery and those that underwent clodronate liposome treatment in addition to feeding artery ligation reveals a relative decrease in the overall diameters of the collateral vessels of tissues that received clodronate liposomes (Figure 6B). While this comparison spans two trials that were conducted independently, it further suggests that depletion of macrophages within the fat pad reduces the arteriogenic response of collateral vessels.
DISCUSSION
The microvasculature rapidly adapts its structure and function in response to biochemical and biomechanical cues6,25,51,57. The plastic surgery community has exploited microvascular growth and remodeling responses by utilizing a surgical pre-treatment technique to enhance the repair of soft tissue defects with autologous flaps. “Flap delay” involves the surgical restriction of blood flow into the tissue flap several days prior to its transfer to the recipient site, and this alteration leads to better flap survival after final surgical transfer for reconstruction. In our previous study43, we examined a murine model of flap delay and observed significant arteriogenesis (i.e. enlargement of collateral vessel diameters) in the adipose flap in response to the ligation of the main vascular pedicle. Preceding collateral diameter enlargement, we observed an increase in the recruitment of circulating monocytes and local increases in the number of CD206+ cells (historically defined as M2 macrophages24,31,33). We also observed local proliferation of recruited macrophages surrounding the collateral vessels, as evidenced by positive Ki-67 staining. Based on these findings, we hypothesized that M2 macrophages play a significant role in the arteriogenic response observed within the flap.
Herein, we aimed to test the hypothesis that the recruitment of M2 macrophages is necessary for arteriogenesis following the flap delay procedure. Our gain-of-function studies successfully increased M2 macrophage numbers, which was accompanied by more extensive diameter enlargement in the collateral vessels feeding the inguinal fat pad. Conversely, loss-of-function studies that effectively reduced the macrophage population significantly attenuated the arteriogenic response. Together, these studies confirm that M2 macrophages are instrumental in driving vascular remodeling in fat and help elucidate the interplay between the microvasculature and immune system in remodeling adipose tissue. More broadly, these findings support the therapeutic targeting of macrophages to enhance and/or accelerate the desirable vascular adaptations invoked by the flap delay procedure.
Although this study is the first, to our knowledge, to confirm a role for macrophages in orchestrating collateral vessel remodeling in adipose tissue, the process of collateral vessel enlargement has been studied extensively in skeletal muscle. Our group, as well as others, have demonstrated the importance of M2 macrophages in the arteriogenic response following an alteration in blood flow in skeletal muscle2,5,53. In these studies (as well as our previous study43) we observed a similar process in adipose tissue – the recruitment of circulating monocytes and an increase in the number of M2 macrophages. In skeletal muscle and adipose tissue, we have observed an increase in collateral vessel diameter which is likely contingent on the increase of M2 macrophages5. Indeed, collateral vessel diameter enlargement, the hallmark of arteriogenesis, has been shown in other tissues to be reliant on the recruitment of circulating monocytes followed by their extravasation into the interstitial tissue5,32. Extravasated monocytes differentiate into macrophages and then secrete potent growth factors such as FGF-2, which stimulate smooth muscle cell and endothelial cell proliferation41,42. This study, as well as other studies previously conducted in our lab2,30, have reported the potential therapeutic potential of increasing monocyte recruitment to remodeling tissue. Increasing monocyte recruitment to adipose tissue as reported in this study may have potentially exciting therapeutic potential. Localized proliferation of the vascular cells leads to the expansion of the collateral vessel diameter, causing an increase in blood flow39,42. Lasch et al. reported that inhibition of arginase attenuates collateral vessel arteriogenesis in skeletal muscle by reducing M2 macrophage accumulation and recruitment28. While our study did not delineate between recruited and tissue-resident macrophage populations or assess local growth factor levels, it is a first step toward these logical follow-on studies.
In our first gain-of-function experiment, local injection of M2-polarized macrophages into the inguinal fat pad enhanced the arteriogenic response of the collateral vessels, but there are several caveats that should be considered. Throughout these studies (and the other gain-of-function and loss-of-function studies), immunofluorescent labeling of adipose tissue was used for cellular quantification and visualization. Because of the limited number of channels available for confocal imaging and the need to immunofluorescently visualize microvascular changes, we were not able to use more than one antibody to label macrophages in our quantification studies. For our studies, we used CD68 to immunofluorescently label all macrophages and CD206 to immunofluorescently label M2 macrophages for quantification purposes. Although CD68 is a well-accepted marker of macrophages3, other cell types also express CD68 including mast cells10, endothelial cells15, fibroblasts15, and osteoclasts1. CD206 is also used frequently in the literature to label M2 macrophages50,55, but other cell types can also express CD206 such as dendritic cells56. Because we were limited to one marker for macrophage identification and thus did not use a panel of markers to identify the macrophages, the conclusions of this study may be tempered because we did not rule out the other possible cell types that could be expressing CD68 or CD206. The use of flow cytometry with a panel of multiple macrophage markers for macrophages (i.e. CD68, CD163, F4/80) and M2 macrophages (i.e. CD206, Arginase-1, Ym136) may help eliminate this potential caveat to our studies. Importantly, we believe that we are able to distinguish macrophages from other cell types based on their morphometry and spatial location within the adipose tissue. We were not able to co-label injected cells (DiI) with M2 macrophage markers (CD206) due to our staining protocol which included tissue permeabilization, but, in the future, it may be possible to utilize a different cellular stain (i.e. PKH-2629, calcein-AM8) that is more resilient to permeabilization techniques. Because of the absence of DiI signal in the tissue, we are not able to say definitively that we are increasing the local M2 macrophage population via direct injection. Indeed, the CD206+ cells depicted in Figures 2E,F were not positive for DiI, but we believe that the lack of DiI signal is due to the permeabilization step prior to immunofluorescently labeling the samples, which is consistent with other reports in the literature49. We were encouraged by the finding that many DiI+ cells were observed in samples not subjected to permeabilization (Figure 2B) and the finding of increased numbers of CD206+ cells in the DiI-injected tissues. The use of flow cytometry to confirm the observed increase in CD206+ cells within the tissue would allow for the quantification of cells expressing DiI (or other dyes) and M2 macrophage markers and would allow us to determine the effect of local M2 macrophage populations more exactly. It is unknown whether the injected macrophages maintained an M2 phenotype after they were injected into the inguinal fat pad. Macrophages are plastic and can polarize across phenotypes based on different environmental cues23,46. An interesting and important follow-on experiment would be to inject non-polarized macrophages (M0) or M1-polarized into the remodeling fat pads and see if there is an effect on the remodeling collateral vessels. If these proposed studies do not elicit the same effect as injected M2 macrophages, this may provide confidence that the enhancement in collateral vessel enlargement is due to the M2 macrophages that are retaining their phenotype in vivo. Additionally, we injected 30,000 M2-polarized macrophages per inguinal fat pad, but we believe that it would be important to determine their dose effect and titrate the number of injected cells. It is possible that we injected a supraphysiological number of M2 macrophages into the fat pad and the enhanced arteriogenic response observed would not be observed in a normal inflammatory response within the fat pad. In our previous study, we observed increased numbers of proliferating macrophages within remodeling fat pads specifically in the localized region around the remodeling collateral vessels43. Studies in skeletal muscle have also shown that local proliferation of macrophages within an ischemic tissue is, in part, responsible for the observed arteriogenic response2,5. Therefore, it would be important to evaluate whether injected macrophages also proliferate within the fat pad and whether proliferation of injected macrophages is required for an enhanced arteriogenic remodeling response.
Recent studies conducted in collaboration with our group have found that application of an FTY720-loaded PLAGA film increased M2 monocyte/macrophage recruitment2, as well as arteriogenesis in ischemic skeletal muscle44. Here, we applied an FTY720-loaded film as a gain-of-function intervention to study how enhanced recruitment of M2 macrophages affects arteriogenesis in adipose tissue. In these gain-of-function studies, we found an increase in the arteriogenic effects observed coupled with an increase in the number of M2 macrophages recruited to the remodeling fat pad. While we did not quantify local FTY720 concentrations in the tissue, we are confident that the drug exhibited bioactivity based on previous release studies that have confirmed drug release for several weeks and the long half-life (7 days) of FTY72022,45. In the future, it is important to consider the dose of FTY720, as we only evaluated one concentration of drug. It is also possible that FTY720 acted on other cell types within the adipose tissue, which contributed to the arteriogenic response. Indeed, it is well known that a number of various other cell types (e.g. mast cells27, neutrophils12,14, and mural cells22), many of which have been implicated in arteriogenesis, also express S1P receptor-1 and -3, which are activated by FTY720. Taken together, both gain-of-function methods for expanding the M2 macrophage population led to increased collateral vessel diameters in response to ligation surgery, corroborating the role that M2 macrophages play in arteriogenesis.
In our loss-of-function study, we used injections of clodronate-containing liposomes in our pre-clinical model of flap delay as a means to locally deplete macrophage populations within the inguinal fat pad. Injection of clodronate liposomes successfully reduced the local macrophage population within inguinal fat pads 24 hours after injection and restricted the arteriogenic response observed previously. Clodronate liposomes serve as a useful tool to transiently and locally deplete macrophage populations within a tissue, and they have been used extensively in the literature7,11,52. However, there are several caveats to their usage. First, there are numerous phagocytic cells types other than macrophages (e.g. mast cells, neutrophils, dendritic cells) within adipose tissue that would be subjected to the phagocytic, apoptotic mechanism enacted by clodronate liposomes. While we demonstrate the ability of clodronate liposomes to deplete macrophage populations, there is also a possibility that the clodronate liposomes depleted other cell populations within the inguinal fat pad, some of which may also be implicated in the arteriogenic response. For example, Heissig et al. showed that mast cells are necessary for the revascularization of skeletal muscle in a hindlimb ischemia model and proposed that the secretion of MMP-9 and VEGF is central to this process20. The role of neutrophils in arteriogenesis is not well known, but a study by Soehnlein et al. suggested that neutrophils play a role in recruitment of circulating monocytes47,48. Ohki et al. report that G-CSF administration aids in revascularization through protein secretion by neutrophils35. So, while it is possible that other phagocytic cells in addition to macrophages were depleted in our studies, the use of control liposomes (PBS loaded) served as an important contralateral control. We injected the liposomes during the ligation surgeries, and based on our previous findings we expected that the macrophage population in clodronate liposome-treated tissues would be significantly depleted at 24 hours (Figure 5). It is important to note that clodronate and the liposomes used are non-toxic themselves so side effects (local and systemic) are limited. Free clodronate drug, which can be released from dead macrophages, has an extremely short half-life in vivo (15 minutes) and will not easily pass the phospholipid bilayers of the cellular membrane4,37. Clodronate liposome-mediated macrophage depletion is a transient effect38, and it is unclear how long the depletion lasts. The literature reports ranging durations of macrophage depletion (from 1 week37 to 3 weeks4) depending on application technique, tissue type, and volume of liposomes delivered. We are unaware of any other studies in which clodronate liposomes have been directly injected into adipose tissue, and as such we cannot be certain that the macrophage depletion persisted for three days post-surgery when tissue was harvested, although the existing literature would support this. Further, we injected clodronate liposomes during surgery rather than 24 hours prior to surgery, and thus during the first 24 hours after surgery there was a population of macrophages present within the tissue. Future studies are needed to determine whether and to what extent injection of clodronate liposomes prior to surgery would affect diameter enlargement of collateral vessels in the inguinal fat pad.
CONCLUSIONS
This study confirms our hypothesis that macrophages, and specifically M2-polarized macrophages, are necessary for the collateral vessel remodeling response observed in the murine model of flap delay. We demonstrated that both local administration of M2 macrophages into the inguinal fat pad and pharmacological recruitment of M2 macrophages to the inguinal fat pad increased the arteriogenic response in collateral vessels following ligation of the main feeding artery to the inguinal fat pad. Further, we performed a loss-of-function macrophage depletion study, in which the arteriogenic response was significantly restricted. Taken together, our studies underscore the importance of macrophages in collateral vessel arteriogenesis within adipose tissue, and may implicate macrophages as a therapeutic target that can be manipulated to enhance the flap delay procedure.
PERSPECTIVES.
Surgical tissue reconstruction is a widely practiced technique to reconstruct soft tissue defects but survival of the tissue flap is often low because of improper vascularization. Our data suggest that M2-polarized macrophages are necessary for the arteriogenic remodeling response within adipose tissue flaps. We hope that this paper may suggest a mechanism for a translatable therapeutic target that may be used to enhance the clinical flap delay procedure and improve reconstructive surgeries.
Acknowledgments
The authors would like to thank Anthony Bruce for the management of the mouse colony. The authors also thank Dr. Song Hu, Dr. Patrick Cottler, and Dr. Norbert Leitinger for helpful discussion and comments in planning and analyzing these studies.
SOURCES OF FUNDING
NIH T32GM008715 to S.A.S.; NIH EY022063, NIH HL082838, and The Hartwell Foundation to S.M.P.
LIST OF ABBREVIATIONS
- VEGF
Vascular endothelial growth factor
- FGF-2
Fibroblast growth factor 2
- PBS
Phosphate buffered saline
- PFA
Paraformaldehyde
- FOVs
Fields of view
- G-CSF
Granulocyte-colony stimulating factor
- MMP-9
Matrix metallopeptidase 9
- PCR
Polymerase chain reaction
- IL-4
Interleukin 4
- PLAGA
Poly(lactide-co-glycolide acid)
- S1P
Sphingosine-1-phosphate
REFERENCES
- 1.Ashley JW, Shi Z, Zhao H, Li X, Kesterson RA, Feng X. Genetic Ablation of CD68 Results in Mice with Increased Bone and Dysfunctional Osteoclasts. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awojoodu AO, Ogle ME, Sefcik LS, Bowers DT, Martin K, Brayman KL, Lynch KR, Peirce-Cottler SM, Botchwey E. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc. Natl. Acad. Sci. U. S. A. 2013;110:13785–13790. doi: 10.1073/pnas.1221309110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bain CC, Bravo-blas A, Scott CL, Perdiguero EG, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, Mowat AM. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Imm. 2014;15 doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biewenga J, van der Ende MB, Krist LF, Borst A, Ghufron M, van Rooijen N. Macrophage depletion in the rat after intraperitoneal administration of liposome-encapsulated clodronate: depletion kinetics and accelerated repopulation of peritoneal and omental macrophages by administration of Freund’s adjuvant. Cell Tissue Res. 1995;280:189–196. doi: 10.1007/BF00304524. [DOI] [PubMed] [Google Scholar]
- 5.Bruce AC, Kelly-Goss MR, Heuslein JL, Meisner JK, Price RJ, Peirce SM. Monocytes are recruited from venules during arteriogenesis in the murine spinotrapezius ligation model. Arterioscler. Thromb. Vasc. Biol. 2014:1–11. doi: 10.1161/ATVBAHA.114.303399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corliss BA, Azimi MS, Munson J, Peirce SM, Murfee WL. Macrophages: An Inflammatory Link between Angiogenesis and Lymphangiogenesis. Microcirculation November. 2015 doi: 10.1111/micc.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danenberg HD. Macrophage Depletion by Clodronate-Containing Liposomes Reduces Neointimal Formation After Balloon Injury in Rats and Rabbits. Circulation. 2002;106:599–605. doi: 10.1161/01.cir.0000023532.98469.48. [DOI] [PubMed] [Google Scholar]
- 8.Denholm EM, Stankus GP. Differential effects of two fluorescent probes on macrophage migration as assessed by manual and automated methods. Cytometry. 1995;19:366–369. doi: 10.1002/cyto.990190412. [DOI] [PubMed] [Google Scholar]
- 9.Egami K. Ischemia-induced angiogenesis: role of inflammatory response mediated by P-selectin. J. Leukoc. Biol. 2006;79:971–976. doi: 10.1189/jlb.0805448. [DOI] [PubMed] [Google Scholar]
- 10.Ehg JEER, Ush DOB, Ard JEMW. Continuing Education CE3 - Histopathology of the Rodent Lymphoid and Hematopoietic Systems The Utility of Immunohistochemistry for the Identification of Hematopoietic and Lymphoid Cells in Normal Tissues and Interpretation of Proliferative and Inflammator. Toxicol. Pathol. 2012:345–374. doi: 10.1177/0192623311430695. [DOI] [PubMed] [Google Scholar]
- 11.Ferenbach DA, Sheldrake TA, Dhaliwal K, Kipari TMJ, Marson LP, Kluth DC, Hughes J. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int. 2012;82:928–933. doi: 10.1038/ki.2012.207. [DOI] [PubMed] [Google Scholar]
- 12.Frink M, Kaudel CP, Hildebrand F, Pape H-C, Klempnauer J, Winkler M, Krettek C, van Griensven M. FTY720 improves survival after transient ischemia and reperfusion of the hind limbs. J. Trauma. 2007;63:263–267. doi: 10.1097/TA.0b013e3180d0a6fc. [DOI] [PubMed] [Google Scholar]
- 13.Mac Gabhann F, Peirce SM. Collateral Capillary Arterialization following arteriolar ligation in murine skeletal muscle. Microcirculation. 2010;17:333–347. doi: 10.1111/j.1549-8719.2010.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorlino CV, Ranocchia RP, Harman MF, García IA, Crespo MI, Morón G, Maletto BA, Pistoresi-Palencia MC. Neutrophils exhibit differential requirements for homing molecules in their lymphatic and blood trafficking into draining lymph nodes. J. Immunol. 2014;193:1966–1974. doi: 10.4049/jimmunol.1301791. [DOI] [PubMed] [Google Scholar]
- 15.Gottfried E, Weber A, Rehli M, Peuker A, Mu A. Expression of CD68 in Non-Myeloid Cell Types. Basic Immunol. 2008:453–463. doi: 10.1111/j.1365-3083.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- 16.Gruionu G, Hoying JB, Pries AR, Secomb TW. Structural Remodeling of the Mouse Gracilis Artery: Coordinated Changes in Diameter and Medial Area Maintain Circumferential Stress. Microcirculation. 2012;19:610–618. doi: 10.1111/j.1549-8719.2012.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hays PL, Kawamura S, Deng X-H, Dagher E, Mithoefer K, Ying L, Rodeo Sa. The role of macrophages in early healing of a tendon graft in a bone tunnel. J. Bone Joint Surg. Am. 2008;90:565–579. doi: 10.2106/JBJS.F.00531. [DOI] [PubMed] [Google Scholar]
- 18.Hecht N, He J, Kremenetskaia I, Nieminen M, Vajkoczy P, Woitzik J. Cerebral hemodynamic reserve and vascular remodeling in C57/BL6 mice are influenced by age. Stroke. 2012;43:3052–3062. doi: 10.1161/STROKEAHA.112.653204. [DOI] [PubMed] [Google Scholar]
- 19.Heil M, Schaper W. Influence of Mechanical, Cellular, and Molecular Factors on Collateral Artery Growth (Arteriogenesis) Circ. Res. 2004;95:449–458. doi: 10.1161/01.RES.0000141145.78900.44. [DOI] [PubMed] [Google Scholar]
- 20.Heissig B, Rafii S, Akiyama H, Ohki Y, Sato Y, Rafael T, Zhu Z, Hicklin DJ, Okumura K, Ogawa H, Werb Z, Hattori K. Low-dose irradiation promotes tissue revascularization through VEGF release from mast cells and MMP-9-mediated progenitor cell mobilization. J. Exp. Med. 2005;202:739–750. doi: 10.1084/jem.20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helisch A, Schaper W. Arteriogenesis: the development and growth of collateral arteries. Microcirculation. 2003;10:83–97. doi: 10.1038/sj.mn.7800173. [DOI] [PubMed] [Google Scholar]
- 22.Huang C, Das A, Barker D, Tholpady S, Wang T, Cui Q, Ogle R, Botchwey E. Local delivery of FTY720 accelerates cranial allograft incorporation and bone formation. Cell Tissue Res. 2012;347:553–566. doi: 10.1007/s00441-011-1217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MPJ, Donners MMPC. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17:109–118. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
- 24.Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, Kensler T, Ravichandran KS, Isakson BE, Wamhoff BR, Leitinger N. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ. Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly-Goss MR, Sweat RS, Stapor PC, Peirce SM, Murfee WL. Targeting Pericytes for Angiogenic Therapies. Microcirculation. 2014;21:345–357. doi: 10.1111/micc.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerrigan C. Skin flap failure: pathophysiology. Plast. Reconstr. Surg. 1983;72:766–774. doi: 10.1097/00006534-198312000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Kurashima Y, Kunisawa J, Higuchi M, Gohda M, Ishikawa I, Takayama N, Shimizu M, Kiyono H. Sphingosine 1-Phosphate-Mediated Trafficking of Pathogenic Th2 and Mast Cells for the Control of Food Allergy. J. Immunol. 2007;179:1577–1585. doi: 10.4049/jimmunol.179.3.1577. [DOI] [PubMed] [Google Scholar]
- 28.Lasch M, Caballero-martinez A, Troidl K, Schloegl I, Lautz T, Deindl E. Arginase inhibition attenuates arteriogenesis and interferes with M2 macrophage accumulation. 2016;96:830–838. doi: 10.1038/labinvest.2016.62. [DOI] [PubMed] [Google Scholar]
- 29.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic Switching of Adipose Tissue Macrophages With Obesity Is Generated by Spatiotemporal Differences in Macrophage Subtypes. Diabetes. 2008;57:3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin K, Kegelman C, Virgilio K, Passipieri J, Christ G, Blemker S, Peirce S. In Silico and In Vivo Experiments Reveal M-CSF Injections Accelerate Regeneration Following Muscle Laceration. Ann. Biomed. Eng. 2016 doi: 10.1007/s10439-016-1707-2. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Santibañez G, Nien-Kai Lumeng C. Macrophages and the Regulation of Adipose Tissue Remodeling. Annu. Rev. Nutr. 2014;34:57–76. doi: 10.1146/annurev-nutr-071812-161113. [DOI] [PubMed] [Google Scholar]
- 32.Meisner JK, Price RJ. Spatial and Temporal Coordination of Bone Marrow-Derived Cell Activity during Arteriogenesis: Regulation of the Endogenous Response and Therapeutic Implications. Microcirculation. 2010;17:583–599. doi: 10.1111/j.1549-8719.2010.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray PJ, Allen JE, Biswas SK, et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers B, Cherry G. Causes of necrosis in pedicle flaps. Plast. Reconstr. Surg. 1968;42:43–50. [PubMed] [Google Scholar]
- 35.Ohki Y, Heissig B, Sato Y, Akiyama H, Zhu Z, Hicklin DJ, Shimada K, Ogawa H, Daida H, Hattori K, Ohsaka A. Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB. J. 2005;19:2005–2007. doi: 10.1096/fj.04-3496fje. [DOI] [PubMed] [Google Scholar]
- 36.Raes G, Van den Bergh R, De Baetselier P, Ghassabeh GH, Scotton C, Locati M, Mantovani a, Sozzani S. Arginase-1 and Ym1 Are Markers for Murine, but Not Human, Alternatively Activated Myeloid Cells. J. Immunol. 2005;174:6561–6562. doi: 10.4049/jimmunol.174.11.6561. [DOI] [PubMed] [Google Scholar]
- 37.van Rooijen N, Kors N, Kraal G. Macrophage subset repopulation in the spleen: differential kinetics after liposome-mediated elimination. J. Leukoc. Biol. 1989;45:97–104. doi: 10.1002/jlb.45.2.97. [DOI] [PubMed] [Google Scholar]
- 38.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 39.van Royen N, Piek JJ, Buschmann I, Hoefer I, Voskuil M, Schaper W. Stimulation of arteriogenesis; a new concept for the treatment of arterial occlusive disease. Cardiovasc. Res. 2001;49:543–553. doi: 10.1016/s0008-6363(00)00206-6. [DOI] [PubMed] [Google Scholar]
- 40.Schaper J, König R, Franz D, Schaper W. The endothelial surface of growing coronary collateral arteries. Intimal margination and diapedesis of monocytes - A combined SEM and TEM study. Virchows Arch. A Pathol. Anat. Histol. 1976;370:193–205. doi: 10.1007/BF00427580. [DOI] [PubMed] [Google Scholar]
- 41.Schaper W, Scholz D. Factors Regulating Arteriogenesis. Arterioscler. Thromb. Vasc. Biol. 2003;23:1143–1151. doi: 10.1161/01.ATV.0000069625.11230.96. [DOI] [PubMed] [Google Scholar]
- 42.Schaper W, Scholz D. Factors Regulating Arteriogenesis. Arterioscler. Thromb. Vasc. Biol. 2003;23:1143–1151. doi: 10.1161/01.ATV.0000069625.11230.96. [DOI] [PubMed] [Google Scholar]
- 43.Seaman SA, Cao Y, Campbell CA, Peirce SM. Macrophage Recruitment and Polarization During Collateral Vessel Remodeling in Murine Adipose Tissue. Microcirculation. 2016:75–87. doi: 10.1111/micc.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sefcik LS, Aronin CEP, Awojoodu AO, Shin SJ, Mac Gabhann F, MacDonald TL, Wamhoff BR, Lynch KR, Peirce SM, Botchwey EA. Selective activation of sphingosine 1-phosphate receptors 1 and 3 promotes local microvascular network growth. Tissue Eng. Part A. 2011;17:617–629. doi: 10.1089/ten.tea.2010.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sefcik LS, Petrie Aronin CE, Wieghaus KA, Botchwey EA. Sustained release of sphingosine 1-phosphate for therapeutic arteriogenesis and bone tissue engineering. Biomaterials. 2008;29:2869–2877. doi: 10.1016/j.biomaterials.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114:4613–4623. doi: 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- 48.Soehnlein O, Zernecke A, Eriksson EE, Rothfuchs AG, Pham CT, Herwald H, Bidzhekov K, Rottenberg ME, Weber C, Lindbom L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112:1461–1471. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Staffend NA, Meisel RL. DiOlistic Labeling of Neurons in Tissue Slices: A Qualitative and Quantitative Analysis of Methodological Variations. Front. Neuroanat. 2011;5 doi: 10.3389/fnana.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suga H, Eto H, Aoi N, Kato H, Araki J, Doi K, Higashino T, Yoshimura K. Adipose tissue remodeling under ischemia: death of adipocytes and activation of stem/progenitor cells. Plast. Reconstr. Surg. 2010;126:1911–1923. doi: 10.1097/PRS.0b013e3181f4468b. [DOI] [PubMed] [Google Scholar]
- 52.Summan M, Warren GL, Mercer RR, Chapman R, Hulderman T, Van Rooijen N, Simeonova PP. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1488–R1495. doi: 10.1152/ajpregu.00465.2005. [DOI] [PubMed] [Google Scholar]
- 53.Takeda Y, Costa S, Delamarre E, et al. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature. 2011;479:122–126. doi: 10.1038/nature10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wieghaus KA, Nickerson MM, Petrie CE, Sefcik LS, Price RJ, Paige MA, Brown ML, Botchwey EA. Expansion of microvascular networks in vivo by phthalimide neovascular factor 1 (PNF1) Biomaterials. 2010;29:4698–4708. doi: 10.1016/j.biomaterials.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willenborg S, Lucas T, van Loo G, Knipper Ja, Krieg T, Haase I, Brachvogel B, Hammerschmidt M, Nagy A, Ferrara N, Pasparakis M, Eming Sa. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120:613–625. doi: 10.1182/blood-2012-01-403386. [DOI] [PubMed] [Google Scholar]
- 56.Wollenberg A, Mommaas M, Oppel T, Schottdorf E, Gu S. Expression and Function of the Mannose Receptor CD206 on Epidermal Dendritic Cells in Inflammatory Skin Diseases. J. Invest. Dermatol. 2002:327–334. doi: 10.1046/j.0022-202x.2001.01665.x. [DOI] [PubMed] [Google Scholar]
- 57.Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr. Opin. Investig. Drugs. 2010;11:298–308. [PMC free article] [PubMed] [Google Scholar]