Abstract
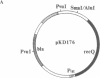
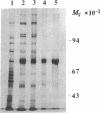
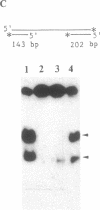
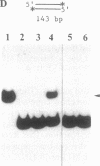
The Escherichia coli recQ gene, a member of the RecF recombination gene family, was set in an overexpression plasmid, and its product was purified to near-homogeneity. The purified RecQ protein exhibited a DNA-dependent ATPase and a helicase activity. Without DNA, no ATPase activity was detected. The capacity as ATPase cofactor varied with the type of DNA in the following order: circular single strand greater than linear single strand much greater than circular or linear duplex. As a helicase, RecQ protein displaced an annealed 71-base or 143-base single-stranded fragment from circular or linear phage M13 DNA, and the direction of unwinding seemed to be 3'----5' with respect to the DNA single strand to which the enzyme supposedly bound. Furthermore, the protein could unwind 143-base-pair blunt-ended duplex DNA at a higher enzyme concentration. It is concluded that RecQ protein is a previously unreported helicase, which might possibly serve to generate single-stranded tails for a strand transfer reaction in the process of recombination.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Monem M., Dürwald H., Hoffmann-Berling H. DNA unwinding enzyme II of Escherichia coli. 2. Characterization of the DNA unwinding activity. Eur J Biochem. 1977 Sep 15;79(1):39–45. doi: 10.1111/j.1432-1033.1977.tb11781.x. [DOI] [PubMed] [Google Scholar]
- Cohen A., Laban A. Plasmidic recombination in Escherichia coli K-12: the role of recF gene function. Mol Gen Genet. 1983;189(3):471–474. doi: 10.1007/BF00325911. [DOI] [PubMed] [Google Scholar]
- Finch P. W., Emmerson P. T. The nucleotide sequence of the uvrD gene of E. coli. Nucleic Acids Res. 1984 Jul 25;12(14):5789–5799. doi: 10.1093/nar/12.14.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist C. A., Denhardt D. T. Escherichia coli rep gene: sequence of the gene, the encoded helicase, and its homology with uvrD. Nucleic Acids Res. 1987 Jan 26;15(2):465–475. doi: 10.1093/nar/15.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Horii Z., Clark A. J. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- Irino N., Nakayama K., Nakayama H. The recQ gene of Escherichia coli K12: primary structure and evidence for SOS regulation. Mol Gen Genet. 1986 Nov;205(2):298–304. doi: 10.1007/BF00430442. [DOI] [PubMed] [Google Scholar]
- Iwasaki H., Shiba T., Makino K., Nakata A., Shinagawa H. Overproduction, purification, and ATPase activity of the Escherichia coli RuvB protein involved in DNA repair. J Bacteriol. 1989 Oct;171(10):5276–5280. doi: 10.1128/jb.171.10.5276-5280.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A. A., Morrison P. T., Kolodner R. Genetic recombination of bacterial plasmid DNA. Analysis of the effect of recombination-deficient mutations on plasmid recombination. J Mol Biol. 1982 Sep 25;160(3):411–430. doi: 10.1016/0022-2836(82)90305-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeBowitz J. H., McMacken R. The Escherichia coli dnaB replication protein is a DNA helicase. J Biol Chem. 1986 Apr 5;261(10):4738–4748. [PubMed] [Google Scholar]
- Lee M. S., Marians K. J. Escherichia coli replication factor Y, a component of the primosome, can act as a DNA helicase. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8345–8349. doi: 10.1073/pnas.84.23.8345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett S. T., Kolodner R. D. Identification and purification of a single-stranded-DNA-specific exonuclease encoded by the recJ gene of Escherichia coli. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2627–2631. doi: 10.1073/pnas.86.8.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi-DeLuca C., Lovett S. T., Kolodner R. D. Genetic and physical analysis of plasmid recombination in recB recC sbcB and recB recC sbcA Escherichia coli K-12 mutants. Genetics. 1989 Jun;122(2):269–278. doi: 10.1093/genetics/122.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdi A. A., Lloyd R. G. The recR locus of Escherichia coli K-12: molecular cloning, DNA sequencing and identification of the gene product. Nucleic Acids Res. 1989 Sep 12;17(17):6781–6794. doi: 10.1093/nar/17.17.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson S. W. Escherichia coli helicase II (urvD gene product) translocates unidirectionally in a 3' to 5' direction. J Biol Chem. 1986 Aug 5;261(22):10169–10175. [PubMed] [Google Scholar]
- Matson S. W., George J. W. DNA helicase II of Escherichia coli. Characterization of the single-stranded DNA-dependent NTPase and helicase activities. J Biol Chem. 1987 Feb 15;262(5):2066–2076. [PubMed] [Google Scholar]
- Matson S. W., Richardson C. C. DNA-dependent nucleoside 5'-triphosphatase activity of the gene 4 protein of bacteriophage T7. J Biol Chem. 1983 Nov 25;258(22):14009–14016. [PubMed] [Google Scholar]
- Merril C. R., Switzer R. C., Van Keuren M. L. Trace polypeptides in cellular extracts and human body fluids detected by two-dimensional electrophoresis and a highly sensitive silver stain. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4335–4339. doi: 10.1073/pnas.76.9.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G., Strauch K. L., Kukral A. M., Miller J. L., Wingfield P. T., Mazzei G. J., Werlen R. C., Graber P., Movva N. R. N-terminal methionine-specific peptidase in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1987 May;84(9):2718–2722. doi: 10.1073/pnas.84.9.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison P. T., Lovett S. T., Gilson L. E., Kolodner R. Molecular analysis of the Escherichia coli recO gene. J Bacteriol. 1989 Jul;171(7):3641–3649. doi: 10.1128/jb.171.7.3641-3649.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabeppu Y., Kondo H., Kawabata S., Iwanaga S., Sekiguchi M. Purification and structure of the intact Ada regulatory protein of Escherichia coli K12, O6-methylguanine-DNA methyltransferase. J Biol Chem. 1985 Jun 25;260(12):7281–7288. [PubMed] [Google Scholar]
- Nakayama H., Nakayama K., Nakayama R., Irino N., Nakayama Y., Hanawalt P. C. Isolation and genetic characterization of a thymineless death-resistant mutant of Escherichia coli K12: identification of a new mutation (recQ1) that blocks the RecF recombination pathway. Mol Gen Genet. 1984;195(3):474–480. doi: 10.1007/BF00341449. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Irino N., Nakayama H. The recQ gene of Escherichia coli K12: molecular cloning and isolation of insertion mutants. Mol Gen Genet. 1985;200(2):266–271. doi: 10.1007/BF00425434. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Date T., Gomi T., Konishi K., Pitot H. C., Cantoni G. L., Fujioka M. Molecular cloning, sequence analysis, and expression in Escherichia coli of the cDNA for guanidinoacetate methyltransferase from rat liver. Proc Natl Acad Sci U S A. 1988 Feb;85(3):694–698. doi: 10.1073/pnas.85.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H. Genetic locations of uvrD and pol genes of E. coli. Mol Gen Genet. 1970;108(4):378–381. doi: 10.1007/BF00267777. [DOI] [PubMed] [Google Scholar]
- Scott J. F., Kornberg A. Purification of the rep protein of Escherichia coli. An ATPase which separates duplex DNA strands in advance of replication. J Biol Chem. 1978 May 10;253(9):3292–3297. [PubMed] [Google Scholar]
- Smith G. R. Homologous recombination in procaryotes. Microbiol Rev. 1988 Mar;52(1):1–28. doi: 10.1128/mr.52.1.1-28.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman I., Fassler J. S., Bennett D. C. Relative map location of the rep and rho genes of Escherichia coli. J Bacteriol. 1982 Sep;151(3):1637–1640. doi: 10.1128/jb.151.3.1637-1640.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vales L. D., Rabin B. A., Chase J. W. Subunit structure of Escherichia coli exonuclease VII. J Biol Chem. 1982 Aug 10;257(15):8799–8805. [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wang T. C., Smith K. C. Postreplicational formation and repair of DNA double-strand breaks in UV-irradiated Escherichia coli uvrB cells. Mutat Res. 1986 Jan;165(1):39–44. doi: 10.1016/0167-8817(86)90007-6. [DOI] [PubMed] [Google Scholar]
- Wood E. R., Matson S. W. Purification and characterization of a new DNA-dependent ATPase with helicase activity from Escherichia coli. J Biol Chem. 1987 Nov 5;262(31):15269–15276. [PubMed] [Google Scholar]
- Wood E. R., Matson S. W. The molecular cloning of the gene encoding the Escherichia coli 75-kDa helicase and the determination of its nucleotide sequence and gentic map position. J Biol Chem. 1989 May 15;264(14):8297–8303. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yarranton G. T., Das R. H., Gefter M. L. Enzyme-catalyzed DNA unwinding. A DNA-dependent ATPase from E. coli. J Biol Chem. 1979 Dec 10;254(23):11997–12001. [PubMed] [Google Scholar]
- Yarranton G. T., Gefter M. L. Enzyme-catalyzed DNA unwinding: studies on Escherichia coli rep protein. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1658–1662. doi: 10.1073/pnas.76.4.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]